ISSN: 0973-7510
E-ISSN: 2581-690X
Fungal infections have predominantly increased worldwide that leads to morbidity and mortality in severe cases. Invasive candidiasis and other pathogenic fungal infections are a major problem in immunocompromised individuals and post-operative patients. Increasing resistance to existing antifungal drugs calls for the identification of novel antifungal drug targets for chemotherapeutic interventions. This demand for identification and characterization of novel drug targets leads to the development of effective antifungal therapy against drug resistant fungi. Heat shock proteins (HSPs) are important for various biological processes like protein folding, posttranslational modifications, transcription, translation, and protein aggregation. HSPs are involved in maintaining homeostasis of the cell. A subgroup of HSPs is small heat shock proteins (sHSPs), which functions as cellular chaperones. They are having a significant role in the many cellular functions like development, cytoskeletal organization, apoptosis, membrane lipid polymorphism, differentiation, autophagy, in infection recognition and are major players in various stresses like osmotic stress, pH stress, etc. Studies have shown that fungal cells express increased levels of sHSPs upon antifungal drug induced stress responses. Here we review the important role of small heat shock proteins (sHSPs) in fungal diseases and their potential as antifungal targets.
Candida albicans, Drug resistance, Small heat shock proteins, Antifungals
Fungi are the most common human pathogen that causes severe cutaneous infections to life-threatening systemic infections. In the United States, hospital born Candida is a major pathogen and cause frequent systemic infections in the patients. It also causes frequent oral infection in immunocompromised individuals. There are several virulent factors like an expression of adhesion molecules, secretion of hydrolytic enzymes, biofilm formation and micro-environment conditions like pH, nutrients which are responsible to convert non-virulent yeast form to virulent hyphal form (Polymorphism).
In major surgeries, severe cases of superficial and systemic infections of C. albicans are reported in immunocompromised patients1. Several groups of antifungal drugs attack or disrupt cell membrane of C. albicans e.g. echinocandins, polyenes, and azoles etc.2, which act as stress to the pathogen. The severity of stress condition depends upon dose and type of drug. Various receptors on the membrane of pathogenic fungi are involved to perceive this stress by eliciting various conserved signaling pathways like MAP kinase (MAPK) and calcineurin signal transduction pathways etc.3,4
In drug resistant pathogenic fungi, several counter mechanisms exist to minimize or nullify the stress produced due to exposure of antifungal agents. Fungi static drugs that inhibit growth at the minimum growth-inhibitory concentration (MIC) did not kill fungal pathogen because of the drug tolerance phenomenon5, whereas fungicidal drugs that are been used at a higher concentration than MIC did not kill drug resistant pathogens. Prolonged exposure to different fungistatic drugs can cause genetic mutation in fungal cells that leads to various mechanisms for drug resistance like overexpression of membrane efflux pump proteins, mutations in drug target enzymes, and upregulation of stress-response genes etc.6-8.
Extensive use of these anti-fungal drugs in the clinics has increased resistance and ineffectiveness against C. albicans infections9,10. Therefore it is an emergency to come up with new therapeutic targets that can be explored for developing novel antifungal agents against C. albicans10,11. Occurrences of Heat shock proteins (HSPs) are integral to almost every organism and are also highly diverse and widely distributed among fungal groups. It is been shown that under thermal stress, these proteins are getting upregulated. Expression of the heat shock proteins under heat stress is regulated by the heat shock transcription factor 1 (Hsf1)12. In C. albicans, Hsps are important not only for the growth but also for developing infection and virulence13-15. Stress conditions are inducive to promote the expression of heat shock proteins. Hsps interact with various cellular signaling pathways like MAPK, calcium-calcineurin, cell cycle control signaling, etc. regulating homeostasis and virulence in C. albicans14,16,17. It is been reported that inhibiting or disrupting Hsps causes growth inhibition of C. albicans, which leads to reverse tolerance to available antifungal drugs. Studies have shown their involvement to confer resistance to antifungal agents by modulating HSPs associated signaling pathways in C. albicans and other pathogenic fungi.
A group of heat shock proteins known as small heat shock proteins (sHSPs) with a molecular weight ranging from 12 to 43 kDa gets upregulated upon non-thermal cues like oxidative and heavy metal stress18,19. sHSPs, not only act as chaperones but are involved in the many biological vital functions like development, cytoskeletal organization, apoptosis, membrane lipid polymorphism, differentiation, autophagy, and infection recognition20,21. These proteins can be used as therapeutic targets in the direction of the development of antifungal agents.
Heat shock proteins (HSPs) in Fungi
Hsps are integral and conserved among all living species, they respond to heat and non-thermal processes like oxidative stress and starvation that leads to stress to the organism22. These stressful conditions cause the loss of three dimensional structures of proteins and their aggregations leading to cell death. Hsps are molecular chaperones and expression of Hsps is a protective mechanism of the cell to combat these changes to ensure cell survival under the stress. There are two groups of Hsps, First is ATP-dependent high molecular weight protein Hsps, having four different families (Hsp104, Hsp90, Hsp70, and Hsp60) and a second ATP-independent family of proteins – Hsp12 and Hsp21 having low molecular weight proteins ranging between 12-42 kDa called Small Heat Shock Proteins (sHSPs) (Table 1)23. Hsps are expressed in stress and non-stress conditions while sHsps are mostly expressed under stress conditions24. It has been shown that most of the antifungal drugs are perceived as stress by the fungal cell25. Thus sHSPs can be explored as the putative targets to combat drug resistance and the development of effective therapeutics to treat fungal infections.
Table (1):
Different types of heat shock proteins (HSPs).
HSPs (ATP-Dependent High Molecular Weight Proteins) |
sHSPs (ATP-Independent Low Molecular Weight Proteins) |
|---|---|
Hsp104 |
Hsp12 |
Hsp70 |
Hsp21 |
Hsp60 |
Roles of Heat Shock Proteins (HSPs) in pathogenic fungi
High molecular weight HSPs are the major proteins that are involved in protecting cellular proteins by acting as chaperons. HSPs in pathogenic fungi are assisting in the process of making and maintaining three dimensional conformations of proteins during various stress and unfavorable conditions to counter the changes in the fungal cells. Expression and functions of different Hsps are variable with the kind of stress to the fungal pathogen.
Hsp104
Hsp104 was firstly reported in Saccharomyces cerevisiae (S. cerevisiae) and induced in elevated temperature23,26,27. It also acts as a pro-survival protein under high temperature, suggesting its role as thermal tolerance26. Mutant of hsp104Δ/Δ have shown morphological defects in hyphae28. In C. albicans, Hsp104 has a greater role in biofilm formation and virulence28. Cytosolic Hsp104 of C. albicans is not equivalent to human Hsp104, thus can be used as a promising antifungal target against C. albicans.
Hsp90
Hsp90 determines antifungal drug resistance in several diseases causing fungi like Candida albicans, A. fumigatus, and C. neoformans29-31. Hsp90 is involved in several cellular functions like development, regulation, homeostasis, and drug resistance in C. albicans16,17. A recent report showed that mutations in the region of Hsp90, responsible for post-translational modifications affect colony morphology32. Inhibitors of Hsp90 function have shown additive effects with fluconazole (FLC) against Fluconazole resistant Candida albicans33,34. In yeast, the Hsp90 function is mediated by its C-terminal phosphorylation, S-nitrosylation, and acetylation35-37. Several findings showed that Hsp90 contributes a vital function in confirming resistance to antimycotic drugs. Thus inhibiting Hsp90 function or development of pathogen specific histone deacetylase inhibitor can be the effective therapeutics to treat candidiasis.
Hsp70
Hsp70 is uniformly present from prokaryotes like bacteria to higher eukaryotes like mammals. Ssa1 and Ssa2 are two important members of the Hsp70 family on the cell surface of C. albicans38,39. C. albicans Ssa1/1 mutant showed altered virulence in vitro as well as in vivo. Ssa1 and many antimicrobial peptides have Ssa2 as receptors that also have antifungal effects.Hsp70 alone or in combination with Hsp90 plays a major role in morphogenesis and dimorphism. A report have shown that the Hsp70 works with the heat shock transcription factor 1 (Hsf1) to regulate heat shock response in the yeast40.
Hsp60
Hsp60 encodes a predictive mitochondrial heat shock protein, whose function is not known. A heterozygous mutant of Hsp60 (hsp60Δ/HSP60) has been shown to increase sensitivity with increasing temperatures. This is an indication of Hsp60 can be essential to overcome thermal stress41. Four fold increased expression of hsp60 in wild type yeast can resist oxidative stress in comparison to the mutant of hsp60. It was because iron/sulfur containing enzymes were protected from oxidative inactivation42. Another study showed an increase in the levels of Hsp60 under thermal stress and is important for differentiation, infection, and colonization43. Expression of hsp60 mRNA level increased by 5.9-6.9-foldat 40°C in A. fumigatus and A. terreus44. Hsp60 functions as an immunological trigger and play a role in fungal diseases in humans45.
Role of Small Heat shock proteins (sHSPs) in C. albicans
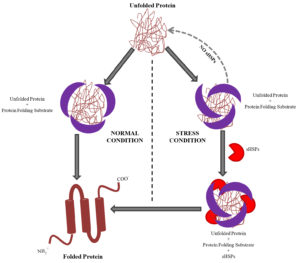
Reports have shown that sHSPs of different species function as molecular chaperones and have conserved the a-crystallin domain (ACD). sHsps are cellular chaperones, involve in the proper folding of proteins during normal and extreme conditions. Under stress conditions, sHSPs contribute in refolding of partially unfolded proteins. They bind to the irreversible aggregation of denaturing proteins and are prevented in an ATP-independent fashion46-49. Exposure to a variety of stresses like elevated temperature or oxidative stress causes unfolding of proteins and form early intermediates that can aggregates. These partially unfolding protein molecules are stabilized by the sHSPs48,50-52. Early expression of sHSPs allows rescuing proteins that are getting unfolded under stressed conditions. Completely unfolded proteins and pre-aggregated proteins are not refolded by sHSPs. Thus, sHSPs protect unfolding proteins that otherwise become irreversible aggregates under stressful conditions to maintain homeostasis within the cell (Fig. 1).
Fig 1. Model showing working of Small Heat Shock Proteins (sHSPs) system as chaperone. Protein folding is mediated by the cellular chaperon molecules(Protein folding substrate complex) which are active under the normal state of cell. sHSPs are expressed under the stress and they stabilize and assist the protein folding substrate complex to form proper confirmation and folding of the proteins. Loss of function of sHSPs in stress leads to formation of nonfunctional unfolded protein aggregates which can cause cell death, therefore sHSPs are rescuing cell under stress.
Various studies have shown a link between HSPs and virulence potential of pathogenic microorganisms18,53-58 including Hsp90 and Hsp70 in Candida albicans33,59. Biofilm formation is an important virulence phenomenon in C. albicans infection and HSPs are required for its progression14. In C. albicans have reported three sHSPs i.e. Hsp10, Hsp12, and Hsp30/Hsp31.
Hsp12
Small heat shock protein (Hsp12) contributes to heat-shock resistance and hybridization of hsp12 mRNA analysis demonstrated its co-regulation with environmental pH and CO2 in C. albicans60-63. Intracellular amount of trehalose (Protectant against cell freezing) was minimized in C. albicans by TPS1 deletion. TPS1 deletion showed overexpression of Hsp12 in C. albicans. C. albicans have shown increased cell adhesion and decrease susceptibility to the quorum sensing molecule, farnesol64,65. SSK1 mutant of C. albicans was susceptible to several oxidative agents like H2O2 and has also shown a high level of HSP1266. This shows Hsp12p has a crucial role in combating various kinds of stresses. Another study showed that increased expression of Hsp12 leads to sensitivity to itraconazole, ketoconazole, and FLC in C. albicans65.
Hsp21
Small heat shock protein 21 (Hsp21p) is another protein which plays an important role under various environmental stress for C. albicans61,67-72. It was found that Hsp21 promotes virulence of C. albicans. Tolerance to heat and oxidative stress requires Hsp21 in C. albicans73. Activation of the mitogen activated protein (MAP) kinase and normal filamentation require Hsp21. Hsp21 mutant and chemical inhibitors have shown a correlation in inhibiting germ tube formation and filamentation at the initial time point73. In-vitro, analysis of hsp21 Δ/Δ mutant strain showed an inability to damage endothelial and oral epithelial cells73. Growth of Hsp21 mutant was significantly reduced when treated with membrane perturbing agents that target ergosterol biosynthetic pathway i.e. terbinafine, caspofungin73. At high temperatures, it is involved in maintaining homeostasis of glycerol, glycogen, and trehalose73. Susceptibility to various antifungal drugs was seen in the deletion mutant of hsp21of C. albicans74. Hsp21 can be an effective target to develop a treatment strategy for C. albicans infection.
Hsp10
Hsp10p is present in association with Hsp60p and assisting Hsp60 function75. In-vivo Hsp60p and Hsp10p did not always act as a single functional unit in-vitro. Hsp10p is crucial for cell survival and acting as a co-chaperon to assist the folding of the proteins in the mitochondrial matrix76. Functionally defective protein aggregation was seen in the mutant of Hsp10. It imparts in the sorting of the RieskeFeS protein during the transport from the matrix to the intermembrane space77.
Hsp30/31
In C. albicans, oxidative stress upregulates Hsp30p besides other heat shock proteins61. A gene of A. nidulans, Hsp30 is homologous to the Hsp26 gene of Saccharomyces cerevisiae and found upregulated in numerous stress conditions including low pH18,78. In another study, Iron deprivation is sensed as the nutritional deficiency in Candida cells, leading to the upregulation of Hsp30p79.
Targeting small heat shock proteins (sHSPs) can be an effective combat strategy to overcome drug resistance in pathogenic fungi. Sequence alignment analysis of sHSPs showed no similarity in the nucleotides or protein sequences with that of humans (data not shown). Understanding sHSPs and its interactive partners not only allow us to study drug resistance but also can be the promising lead therapeutic molecules. It will be an interesting study to see their expression pattern during the progression of drug resistance in pathogenic fungi.
Pathogenic fungi are a major cause of secondary infection and hospital-acquired infections. Drug resistance in fungi is the major setback in the treatment of these infections. Several studies have shown the promising role of sHSPs as novel targets to develop an effective treatment against these drug resistant fungal infections. A study showed that potential antifungal treatment of C. albicans is achieved by over-expressing Hsp1265. Role of Hsp10 and Hsp30/Hsp31 are still not very clear, while on the other hand it will be fascinating to find the crucial function of Hsp21 in the understanding of antifungal drug resistance development. Hsps are not only playing vital roles in many major cellular pathways, such as calcium-calcineurin, MAPK, Ras1-cAMP-PKA, and cell cycle control signaling but several client proteins of Hsps are signaling molecules. Moreover, different groups have reported the involvement of Hsps to confer antifungal drug resistance by modulating these signaling pathways in C. albicans. Thus, developing new effective antifungal targets are required to investigate HSPs and other signaling molecules of HSPs-associated pathways in C. albicans. Therefore, the study of HSPs expression and the functional role will help us better in exploring not only their roles as chaperones but also their indulgent in disease development and progression caused by fungal parasites.
This review summarizes some of the explored functions of sHSPs in direction of drug resistance in fungal biology and indicating that available information is insufficient to understand the in-depth role of these sHSPs in drug resistance. Hence sHSPs provide a new horizon to explore the unexplored heat shock molecules to understand their regulation and function in the interactome of cell molecules and could be promising targets to develop new therapeutics to combat drug resistance in pathogenic fungi. Moreover sHsps have not been given enough attention for a long time to understand their biological roles and targeting them for therapeutic use for overcoming fungal drug resistance.
ACKNOWLEDGMENTS
The author is thankful to Department of Zoology, Acharya Narendra Dev College, University of Delhi.
FUNDING
None.
ETHICS STATEMENT
Not applicable.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
- Dimopoulos G, Karabinis A, Samonis G, Falagas ME. Candidemia in immunocompromised and immunocompetent critically ill patients: a prospective comparative study. Eur J Clin Microbiol Infect Dis.: The official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2007;26:377-384.
Crossref - Odds FC, Brown AJ, Gow NA. Antifungal agents: mechanisms of action. Trends in Microbiology. 2003;11:272-279.
Crossref - Monge RA, Roman E, Nombela C, Pla J. The MAP kinase signal transduction network in Candida albicans. Microbiology. 2006;152:905-912.
Crossref - Steinbach WJ, Reedy JL, Cramer RAJr, Perfect JR, Heitman J. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat Rev Microbiol. 2007;5:418-430.
Crossref - Sanglard D, Ischer F, Marchetti O, Entenza J, Bille J. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Molecular Microbiology. 2003;48:959-976.
Crossref - Perepnikhatka V, Fischer FJ, Niimi M, et al. Specific chromosome alterations in fluconazole-resistant mutants of Candida albicans. Journal of Bacteriology. 1999;181:4041-4049.
Crossref - Selmecki A, Forche A, Berman J. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science. 2006;313:367-370.
Crossref - Cowen LE, Sanglard D, Howard SJ, Rogers PD, Perlin DS. Mechanisms of Antifungal Drug Resistance. Cold Spring Harb Perspect Med. 2014;5:a019752.
Crossref - Kriengkauykiat J, Ito JI, Dadwal SS. Epidemiology and treatment approaches in management of invasive fungal infections. Clinical epidemiology. 2011;3:175-191.
Crossref - Wirk B, Wingard JR. Assessing responses to treatment of opportunistic mycoses and salvage strategies.Current Infectious Disease Reports. 2011;13:492-503.
Crossref - Cuenca-Estrella M. Antifungal drug resistance mechanisms in pathogenic fungi: from bench to bedside. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2014;20(Suppl 6):54-59.
Crossref - Veri AO, Robbins N, Cowen LE. Regulation of the heat shock transcription factor Hsf1 in fungi: implications for temperature-dependent virulence traits. FEMS Yeast Research. 2018;18.
Crossref - Leach MD, Tyc KM, Brown AJ, Klipp E. Modelling the regulation of thermal adaptation in Candida albicans, a major fungal pathogen of humans. PloS one. 2012;7:e32467.
Crossref - Becherelli M, Tao J, Ryder NS. Involvement of heat shock proteins in Candida albicans biofilm formation. Journal of Molecular Microbiology and Biotechnology. 2013;23:396-400.
Crossref - Shapiro RS, Zaas AK, Betancourt-Quiroz M, Perfect JR, Cowen LE. The Hsp90 co-chaperone Sgt1 governs Candida albicans morphogenesis and drug resistance. PloS one. 2012;7:e44734.
Crossref - Leach MD, Klipp E, Cowen LE, Brown AJ. Fungal Hsp90: a biological transistor that tunes cellular outputs to thermal inputs. Nat Rev Microbiol. 2012;10:693-704.
Crossref - O’Meara TR, Cowen LE. Hsp90-dependent regulatory circuitry controlling temperature-dependent fungal development and virulence, Cell Microbiol. 2014;16:473-481.
Crossref - Burnie JP, Carter TL, Hodgetts SJ, Matthews RC. Fungal heat-shock proteins in human disease. FEMS Microbiology Reviews. 2006;30:53-88.
Crossref - Wirk B. Heat shock protein inhibitors for the treatment of fungal infections. Recent Patents on Anti-infective Drug Discovery. 2011;6:38-44.
Crossref - Basha E, O’Neill H, Vierling E. Small heat shock proteins and alpha-crystallins: dynamic proteins with flexible functions. Trends in Biochemical Sciences. 2012;37:106-117.
Crossref - Lopes-Caitar VS, de Carvalho MC, Darben LM, et al. Genome-wide analysis of the Hsp20 gene family in soybean: comprehensive sequence, genomic organization and expression profile analysis under abiotic and biotic stresses. BMC Genomics. 2013;14:577.
Crossref - Lindquist S. The heat-shock response. Annual Review of Biochemistry. 1986;55:1151-1191.
Crossref - Jaya N, Garcia V, Vierling E. Substrate binding site flexibility of the small heat shock protein molecular chaperones. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15604-15609.
Crossref - Narberhaus F. Alpha-crystallin-type heat shock proteins: socializing minichaperones in the context of a multichaperone network. Microbiol Mol Biol Rev.: MMBR. 2002;66:64-93.
Crossref - Cannon RD, Lamping E, Holmes AR, Niimi K, Tanabe K, Niimi M, Monk BC. Candida albicans drug resistance another way to cope with stress. Microbiology. 2007;153:3211-3217.
Crossref - Sanchez Y, Lindquist SL. HSP104 required for induced thermotolerance. Science. 1990;248:1112-1115.
Crossref - Glover JR, Lindquist S. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell. 1998;94:73-82.
Crossref - Fiori A, Kucharikova S, Govaert G, Cammue BP, Thevissen K, Van Dijck P. The heat-induced molecular disaggregase Hsp104 of Candida albicans plays a role in biofilm formation and pathogenicity in a worm infection model. Eukaryotic cell. 2012;11:1012-1020.
Crossref - Chatterjee S, Tatu U. Heat shock protein 90 localizes to the surface and augments virulence factors of Cryptococcus neoformans. PLoS Neglected Tropical Diseases. 2017;11:e0005836.
Crossref - Cordeiro Rde A, Evangelista AJ, Serpa R, et al. Inhibition of heat-shock protein 90 enhances the susceptibility to antifungals and reduces the virulence of Cryptococcus neoformans/Cryptococcus gattii species complex. Microbiology. 2016;162:309-317.
Crossref - Lamoth F, Juvvadi PR, Steinbach WJ. Heat shock protein 90 (Hsp90): A novel antifungal target against Aspergillus fumigatus. Critical Reviews in Microbiology. 2016;42:310-21.
- Weissman Z, Pinsky M, Wolfgeher DJ, Kron SJ, Truman AW, Kornitzer D. Genetic analysis of Hsp70 phosphorylation sites reveals a role in Candida albicans cell and colony morphogenesis. Biochimica et Biophysica Acta Proteins and Proteomics. 2020;1868:140135.
Crossref - Cowen LE, Singh SD, Kohler JR, et al. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2818-2823.
Crossref - Li L, An M, Shen H, et al. The non-Geldanamycin Hsp90 inhibitors enhanced the antifungal activity of fluconazole. American Journal of Translational Research. 2015;7:2589-2602.
- Soroka J, Wandinger SK, Mausbacher N, et al. Conformational switching of the molecular chaperone Hsp90 via regulated phosphorylation. Molecular Cell. 2012;45:517-528.
Crossref - Martinez-Ruiz A, Villanueva L, Gonzalez de Orduna C, et al. S-nitrosylation of Hsp90 promotes the inhibition of its ATPase and endothelial nitric oxide synthase regulatory activities. Proc Natl Acad Sci U S A. 2005;102:8525-8530.
Crossref - Retzlaff M, Stahl M, Eberl HC, et al. Hsp90 is regulated by a switch point in the C-terminal domain. EMBO Reports. 2009;10:1147-1153.
Crossref - Lopez-Ribot JL, Alloush HM, Masten BJ, Chaffin WL. Evidence for presence in the cell wall of Candida albicans of a protein related to the hsp70 family. Infection and Immunity. 1996;64:3333-3340.
Crossref - Eroles P, Sentandreu M, Elorza MV, Sentandreu R. The highly immunogenic enolase and Hsp70p are adventitious Candida albicans cell wall proteins. Microbiology. 1997;143(Pt 2):313-320.
Crossref - Krakowiak J, Zheng X, Patel N, et al. Hsf1 and Hsp70 constitute a two-component feedback loop that regulates the yeast heat shock response. eLife. 2018;7.
Crossref - Leach MD, Stead DA, Argo E, Brown AJ. Identification of sumoylation targets, combined with inactivation of SMT3, reveals the impact of sumoylation upon growth, morphology, and stress resistance in the pathogen Candida albicans. Molecular Biology of the Cell. 2011;22:687-702.
Crossref - Cabiscol E, Belli G, Tamarit J, Echave P, Herrero E, Ros J. Mitochondrial Hsp60, resistance to oxidative stress, and the labile iron pool are closely connected in Saccharomyces cerevisiae. The Journal of Biological Chemistry. 2002;277:44531-44538.
Crossref - Cunha DA, Zancope-Oliveira RM, Sueli M, et al. Heterologous expression, purification, and immunological reactivity of a recombinant HSP60 from Paracoccidioides brasiliensis. Clin Diagn Lab Immunol. 2002;9:374-377.
Crossref - Raggam RB, Salzer HJ, Marth E, Heiling B, Paulitsch AH, Buzina W. Molecular detection and characterisation of fungal heat shock protein 60. Mycoses. 2011;54:e394-e399.
Crossref - Sonna LA, Hawkins L, Lissauer ME, et al. Core temperature correlates with expression of selected stress and immunomodulatory genes in febrile patients with sepsis and noninfectious SIRS. Cell Stress & Chaperones. 2010;15:55-66.
Crossref - de Jong WW, Leunissen JA, Voorter CE. Evolution of the alpha-crystallin/small heat-shock protein family. Mol Biol Evol. 1993;10:103-126.
- Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. The Journal of Biological Chemistry. 1993;268:1517-1520.
- McHaourab HS, Dodson EK, Koteiche HA. Mechanism of chaperone function in small heat shock proteins. Two-mode binding of the excited states of T4 lysozyme mutants by alphaA-crystallin. The Journal of Biological Chemistry. 2002;277:40557-40566.
Crossref - Haslbeck M, Walke S, Stromer T, et al. Hsp26: a temperature-regulated chaperone. The EMBO Journal. 1999;18:6744-6751.
Crossref - Bepperling A, Alte F, Kriehuber T, et al. Alternative bacterial two-component small heat shock protein systems. Proc Natl Acad Sci USA. 2012;109:20407-20412.
Crossref - Haslbeck M, Braun N, Stromer T, et al. Hsp42 is the general small heat shock protein in the cytosol of Saccharomyces cerevisiae. The EMBO Journal. 2004;23:638-649.
Crossref - McHaourab HS, Godar JA, Stewart PL. Structure and mechanism of protein stability sensors: chaperone activity of small heat shock proteins. Biochemistry. 2009;48:3828-3837.
Crossref - Kaufmann SH. Heat-shock proteins and pathogenesis of bacterial infections. Springer Seminars in Immunopathology. 1991;13:25-36.
Crossref - Yamaguchi H, Osaki T, Taguchi H, Hanawa T, Yamamoto T, Kamiya S. Flow cytometric analysis of the heat shock protein 60 expressed on the cell surface of Helicobacter pylori. J Med Microbiol. 1996;45:270-277.
Crossref - Hubel A, Krobitsch S, Horauf A, Clos J. Leishmania major Hsp100 is required chiefly in the mammalian stage of the parasite. Mol Cell Biol. 1997;17:5987-5995.
Crossref - Meibom KL, Dubail I, Dupuis M, et al. The heat-shock protein ClpB of Francisella tularensis is involved in stress tolerance and is required for multiplication in target organs of infected mice. Molecular Microbiology. 2008;67:1384-1401.
Crossref - de Koning-Ward TF, Gilson PR, Boddey JA, et al. A newly discovered protein export machine in malaria parasites. Nature. 2009;459:945-949.
Crossref - Shonhai A. Plasmodial heat shock proteins: targets for chemotherapy. FEMS Immunology and Medical Microbiology. 2010;58:61-74.
Crossref - Sun JN, Solis NV, Phan QT, et al. Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathogens. 2010;6:e1001181.
Crossref - Pacheco A, Pereira C, Almeida MJ, Sousa MJ. Small heat-shock protein Hsp12 contributes to yeast tolerance to freezing stress. Microbiology. 2009;155:2021-2028.
Crossref - Enjalbert B, Nantel A, Whiteway M. Stress-induced gene expression in Candida albicans: absence of a general stress response. Molecular Biology of the Cell. 2003;14:1460-1467.
Crossref - Smith DA, Nicholls S, Morgan BA, Brown AJ, Quinn J. A conserved stress-activated protein kinase regulates a core stress response in the human pathogen Candida albicans. Molecular Biology of the Cell. 2004;15:4179-4190.
Crossref - Sheth CC, Mogensen EG, Fu MS, Blomfield IC, Muhlschlegel FA. Candida albicans HSP12 is co-regulated by physiological CO2 and pH. Fungal Genetics and Biology : FG & B. 2008;45:1075-1080.
Crossref - Davis-Hanna A, Piispanen AE, Stateva LI, Hogan DA. Farnesol and dodecanol effects on the Candida albicans Ras1-cAMP signalling pathway and the regulation of morphogenesis. Molecular Microbiology. 2008;67:47-62.
Crossref - Fu MS, De Sordi L, Muhlschlegel FA. Functional characterization of the small heat shock protein Hsp12p from Candida albicans, PloS one. 2012;7:e42894.
Crossref - Vianna RN, Souza Filho JP, Deschenes J, Burnier MNJr. Bilateral Candida chorioretinitis: involvement of the second eye after 3 years. Journal Canadien d’ophtalmologie. 2005;40:75-78.
Crossref - Nicholls S, Leach MD, Priest CL, Brown AJ. Role of the heat shock transcription factor, Hsf1, in a major fungal pathogen that is obligately associated with warm-blooded animals. Molecular Microbiology. 2009;74:844-861.
Crossref - Enjalbert B, Moran GP, Vaughan C, et al. Genome-wide gene expression profiling and a forward genetic screen show that differential expression of the sodium ion transporter Ena21 contributes to the differential tolerance of Candida albicans and Candida dubliniensis to osmotic stress. Molecular Microbiology. 2009;72:216-228.
Crossref - Ramsdale M, Selway L, Stead D, et al. MNL1 regulates weak acid-induced stress responses of the fungal pathogen Candida albicans. Molecular Biology of the Cell. 2008;19:4393-4403.
Crossref - Lorenz MC, Bender JA, Fink GR. Transcriptional response of Candida albicans upon internalization by macrophages, Eukaryotic Cell. 2004;3:1076-1087.
Crossref - Thewes S, Kretschmar M, Park H, Schaller M, Filler SG, Hube B. In vivo and ex vivo comparative transcriptional profiling of invasive and non-invasive Candida albicans isolates identifies genes associated with tissue invasion. Molecular Microbiology. 2007;63:1606-1628.
Crossref - Fradin C, De Groot P, MacCallum D, et al. Granulocytes govern the transcriptional response, morphology and proliferation of Candida albicans in human blood. Molecular Microbiology. 2005;56:397-415.
Crossref - Mayer FL, Wilson D, Jacobsen ID, et al. Small but crucial: the novel small heat shock protein Hsp21 mediates stress adaptation and virulence in Candida albicans, PloS one. 2012;7:e38584.
Crossref - Mayer FL, Wilson D, Hube B. Hsp21 potentiates antifungal drug tolerance in Candida albicans. PloS one. 2013;8:e60417.
Crossref - Dubaquie Y, Looser R, Rospert S. Significance of chaperonin 10-mediated inhibition of ATP hydrolysis by chaperonin 60. Proc Natl Acad Sci USA. 1997;94:9011-9016.
Crossref - Miura T, Minegishi H, Usami R, Abe F. Systematic analysis of HSP gene expression and effects on cell growth and survival at high hydrostatic pressure in Saccharomyces cerevisiae. Extremophiles: Life Under Extreme Conditions. 2006;10:279-284.
Crossref - Hohfeld J, Hartl FU. Role of the chaperonin cofactor Hsp10 in protein folding and sorting in yeast mitochondria. The Journal of Cell Biology. 1994;126:305-315.
Crossref - Amoros M, Estruch F. Hsf1p and Msn2/4p cooperate in the expression of Saccharomyces cerevisiae genes HSP26 and HSP104 in a gene- and stress type-dependent manner. Molecular Microbiology. 2001;39:1523-1532.
Crossref - Hameed S, Prasad T, Banerjee D, et al. Iron deprivation induces EFG1-mediated hyphal development in Candida albicans without affecting biofilm formation.FEMS Yeast Research. 2008;8:744-755.
Crossref
© The Author(s) 2021. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.