ISSN: 0973-7510
E-ISSN: 2581-690X
Among group of bacteria screened for exopolymer production, Bacillus species DAS10-1 producing exopolysaccharide was detected. The bacterial strain was isolated from indoor air of Dammam slaughterhouse, in KSA and identified based on 16S rRNA gene sequencing. Phylogenetic analysis revealed its closeness to Bacillus mojavensis. In comparison with static culture, shake culture recorded 27.9-fold increase. FT-IR Spectroscopy showed sharp bands at 3415.6, 2942.1, 1646.5, 1183.1 and 1111.8 cm-1 that are typical for carbohydrate. Furthermore, strong absorbance in 1200-950 cm”1 indicated polysaccharide nature of the polymer. Result of GPC indicated that weight average (Mw), number average (Mn) and size average molecular weight (Mz) for EPS polymer were 1151Da, 987Da and 1302Da, respectively. Maximum EPS yield (5.62 mg/mg) was reported during growth on M3 medium supplemented with C:N ratio of 4:1 for sucrose and ammonium sulfate, respectively. Supplementation of medium with trace element solution-I resulted in remarkable decrease in EPS yield (1.62 mg/mg). Maximum EPS production (63.02 mg/mg) was recorded during growth on synthetic medium M3 in presence of 20 g/L sucrose. Successful use of the agro-industrial carbon source date syrup or DEPS rather than sucrose might significantly lower process economy and increase promises for production of EPS on industrial scale.
Exopolysaccharide; Bacillus mojavensis; FT-IR Spectroscopy; GPC Molecular Weight; EPS Production
Exopolysaccharides are naturally synthesized macromolecules containing repeated units of sugar moieties, secreted into the medium during growth of many organisms including bacteria1, 2. In many bacterial species, EPSs are involved in bacterial biofilm formation3. Bacterial exopolysaccharides (EPS) gains tremendous imputes in numerous health and industrial applications namely as; antitumor, antioxidants, anti-inflammatory, bioabsorbants, bioflocculants, encapsulation materials, drug carriers, ion exchange resins and emulsifying agents due to their unique physical and chemical properties1, 4-8. Production of EPS by bacteria belong to genus Bacillus, was reported by many scientists9-12. Although many exopolysaccharides are currently available in market including; alginate, xanthan, carrageenan, galactan, leuvan, cellulose and hyaluronic acid, the interest in exploitation of valuable EPS for industrial applications has been increased13-16.
In this study an attempt was made to isolate, characterize and identify an EPS producing bacilli from soil, sewage and air samples from Dammam city, Eastern province, Saudi Arabia. Potent strain was identified by 16S rDNA analysis revealing its closeness to B. mojavensis. Chemical characterization of the produced polymer was analyzed by FT-IR spectroscopy and GPC techniques to detect the distinctive peaks of the polymer. Emphasis was given to the impact of different media composition on EPS production as well as the use of the raw agro-industrial waste (DEPS) as a sole C source during EPS production from B. mojavensis DAS10-1.
Microorganism; isolation and identification
Screening was carried out among a group of bacilli isolated from different sources in Dammam city, Eastern Province, Saudi Arabia as previously reported (17-18). Bacterial candidates were tested for exopolymer production by cultivation on complex medium of the following composition (g/L): Beef; 3, peptone, 5; K2HPO4; 3, NaCl; 5 with agar; 15 as a solidifying agent and supplemented with 10 g/L glucose (M1) or sucrose (M2) as a carbon source. Visible viscous colonies were selected and the most potent EPSs producing were chosen for further investigation. Isolates maintained on nutrient agar slant composed of (g/L): peptone; 5, beef extract; 3, NaCl; 2 and agar; 20. Stock cultures were subcultured at regular intervals of one month and stored under refrigeration.
Molecular identification by 16S RNA gene analysis
Potent EPS producing strain Bacillus sp DAS10-1 was identified by amplification of 16S RNA gene sequencing using set of primers previously descirbed in Berekaa et al. (18). After PCR amplification and subsequent sequencing 16S rDNA sequence was aligned with published sequences through BLAST sequence tool from NCBI database. Subsequently, sequence deposited in the GenBank under the accession number KU199819. The other six bacilli strains were molecularly identified and accession numbers were given as previously reported18.
Growth and production conditions
The bacterium grew in 50 ml aliquot of nutrient broth dispensed in 250 ml Erlenmeyer flask and incubated at 37°C for 24 h at 120 rpm as a seed culture. For quantitative estimation of EPS 1.5% inoculums of overnight culture was used to inoculate complex media M1 and M2 with glucose or sucrose as a sole carbon source, respectively. For optimization of EPS production another 5 modified basal salt production media (M3-M7) were tested. M3 basal production medium with the following composition (g/L): Sucrose, 20; K2HPO4; 8, KH2PO4; 2, MgSO4.7H2O; 0.5; (NH4)2SO4; 5 and traces of yeast extract. M4 was similar to M3 medium plus 1.5 mL trace element solution-I with the following composition; FeSO4.4H2O, CaCl2.2H2O, MnSO4.4H2O, ZnCl2 1 mM each). However, M5 was modified M4 medium with equal amount of sucrose and ammonium sulfate (10 g/L). Furthermore, M6 was similar to M3 medium plus 1.5 mL trace element solution-II with the following composition (g/L): (FeSO4.7H2O, 10; CaCl2 2; MnSO4.4H2O, 0.5; ZnSO4.7H2O, 2.2; CuSO4.5H2O, 1.9; (NH4)Mo7O24 0.1; Na2B4O7.10H2O, 0.02). Finally, M7 medium was similar to M6 plus equal concentration of sucrose and ammonium sulfate (10 g/L). The most potent exopolymer-producing bacteria were cultivated in 50 mL aliquot of the tested medium and dispensed in 250 mL Erlenmeyer flask and incubated at 37°C for 48 h under shake condition.
Extraction and quantification of EPS
For recovery of exopolysaccharide biopolymer, modified method of Kumar et al19 was applied. Cultivation medium was first centrifuged to remove cells (30 min, 5000 rpm at 4°C) and the EPS was precipitated using ice-cold ethanol (1:2 volume ratio) and kept at 4°C overnight. Crude precipitated EPS was separated by centrifugation and purified by repeated precipitation for 2 times. Finally, precipitate was dried overnight at 60°C and weighed. For estimation of cell dry weight, harvested cells washed once with distilled water, dried and weighed till constant weight.
Characterization of EPS polymer by FT-IR spectroscopy
Chemical characterization of exopolysaccharide biopolymer was carried out by Fourier transform infrared spectroscopy (FT-IR). Extracted dry EPS polymer from B. mojavensis DAS10-1 was used in FT-IR analysis. Spectra were recorded between 400 and 4000 cm-1 using Nicolet 6700 FITR spectrometer from the Nicolet Instrument Corporation, USA.
Determination of EPS molecular weight by GPC
Gel Permeation Chromatography (GPC) instrument from Waters Corporation (USA) equipped with refractive index detector (RI) model 2410 and HPLC pump 515 was applied to identify each of weight average molecular weight (Mw), number average molecular weight (Mn) and size average molecular weight (Mz) for the EPS polymer. Practically, 200 µL of the extracted EPS polymer dissolved in THF was injected to Waters Styragel column and then isocratically eluted with THF as a mobile phase. The Flow rate of the mobile phase was adapted to be 1 mL/min, while, the temperature of column was 40oC. The calibration was done using polystyrene standards and the data managed by GPC data processing software.
Isolation, Identification of EPS producing bacteria
In a screening program for exploring microbial EPS production, seven bacterial strains belong to genus Bacillus sp. were detected. The strains showed highly viscous growth on solid basal medium supplemented with glucose or sucrose as a carbon source. Results of polymer productivity (Table 1) indicated that both complex media M1 and M2 support EPS production by Bacillus strains, in different degrees. Among tested strains, Bacillus sp. DAS10-1 and Bacillus megaterium DG8 showed the optimal EPS productivity especially on M2 medium supplemented with sucrose (8.54 and 6.89 mg/mg, respectively). Morphologically, bacterial strain Bacillus sp DAS10-1 was rod in shape, Gram positive and spore forming. It is capable of growing on different sugars such as: sucrose, glucose, fructose, mannose, xylose and arabinose. It showed catalase, amylase and protease enzymatic activities. It is also sensitive to wide number of antibiotics including; ampicillin (10 µg), tetracycline (30 µg), erythromycin (5 µg), kanamycin (30 µµg), amoxicillin (30 µg) and neomycin (30 µg) however, less sensitive to streptomycin (10 µg). Additionally, it showed high sensitivity to toxic metals such as Cr (2 mg/mL); Ag, Zn (4 mg/mL) and Hg (1 mg/mL) ions while, showed high tolerance to Pb (4 mg/mL). It showed alpha-hemolytic activity during growth on blood agar medium. For phylogenetic analysis, complete 16S rRNA gene of the most potent EPS producing strains was amplified and partially sequenced. Analysis of Bacillus sp. DAS10-1 16S rDNA gene sequence showed high similarity to the corresponding sequences of Bacillus mojavensis strains deposited in GenBank, NCBI, NIH, USA.
Table (1):
Screening of different bacteria belong to genus Bacillus sp for exopolysaccharide production
| Bacterial Strain | EPS Yield (mg/mg) | |
|---|---|---|
| M1 (GLUCOSE) | M2 (SUCROSE) | |
| B. megaterium DT7 (KU199811) | 4.23 | 3.33 |
| B. megaterium DPS6 (KU199807) | 4.51 | 3.75 |
| B. megaterium DPS8 (KU199804) | 3.74 | 2.56 |
| B. megaterium DG8 (KU199809) | 5.45 | 6.89 |
| B. megaterium DP7 (KU199812) | 4.63 | 1.94 |
| B. axarquiensis DSG5 (KU199808) | 3.87 | 4.24 |
| B. mojavensis DAS10-1 (KU199819) | 6.69 | 8.54 |
FT-IR spectroscopy analysis
For chemical characterization, B. mojavensis DAS10-1 cells were cultivated on liquid production medium M3 and EPS polymer was extracted. FT-IR Spectroscopic analysis of purified EPS polymer (Figure 1) showed clear bands at 3415.62, 2942.13, 1646.51, 1183.01 and 1111.81 cm-1. Additionally, strong absorbance in the region 1200-950 cm•1 was observed.
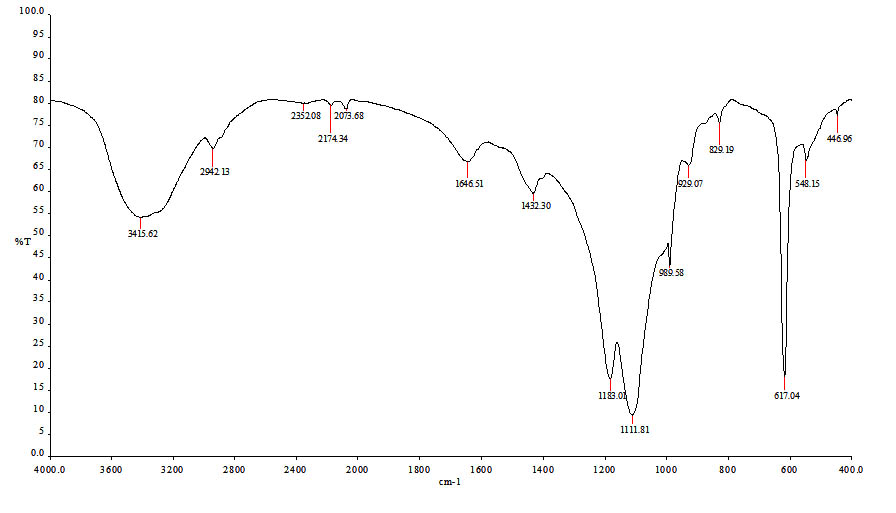
Fig. 1. FT-IR spectroscopy of exopolysaccharide (EPS) polymer produced from B. mojavensis DAS10-1
Molecular weight of EPS produced by B. mojavensis DAS10-1
Under the above mentioned separation conditions (in the experiment part) only one peak was noticed and identified at retention time of 34.2 min. in the GPC chromatogram shown in figure 2. The identified polymer was of weight average molecular weight (Mw), number average molecular weight (Mn) and size average molecular weight (Mz) 1151Da, 987Da and 1302Da, respectively.
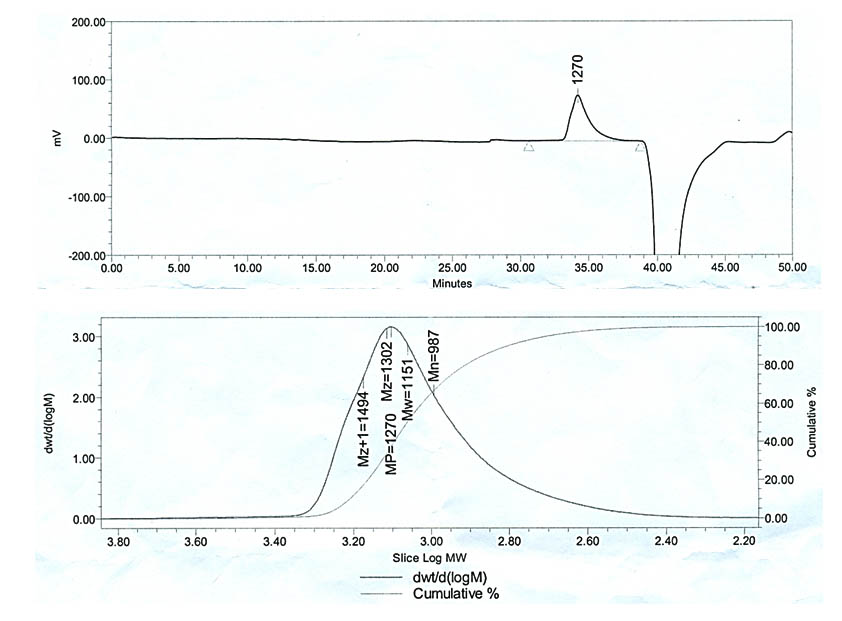
Fig. 2. Gel permeation chromatogram and Molar Mass Distribution pattern of exopolysaccha-ride (EPS) polymer produced by B. mojavensis DAS10-1
Production of EPS biopolymer by B. mojavensis DAS10-1
For further analysis, B. mojavensis DAS10-1 cells were cultivated on complex M2 medium and incubated under different aeration conditions. Results indicated that polymer production is dependent on aeration condition. Maximum EPS yield (16.2 mg/mg) was recorded when cells were incubated at 120 rpm (approximately 27.9-fold increase polymer yield in comparison with the static culture).
Exopolysaccharide (EPS) production on different media
Level of two main critical parameters involved in EPS production were simultaneously tested namely; C:N ratio and the use of trace element solution (-I & -II). Results in figure 3 revealed that maximum EPS yield (5.62 mg/mg) was recorded during growth on M3 medium with 4:1 C:N ratio, without trace element solution. Furthermore, addition of any of these trace-element solutions (-I or -II) to modified basal production media resulted in dramatic decreased in EPS yield, even in presence of equal or 4:1 C:N ratios (M4-M7). Interestingly, the EPS produced durig cultivation on complex medium (M2) recorded approximately 2.89-fold increase in yield. Results also indicated that EPS production enhanced by increasing sucrose concentration as well as trace amount of yeast extract.
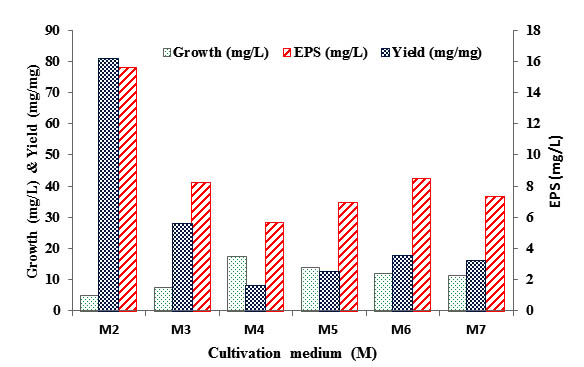
Fig. 3. EPS production by B. mojavensis DAS10-1 cells cultivated on different pro-duction media
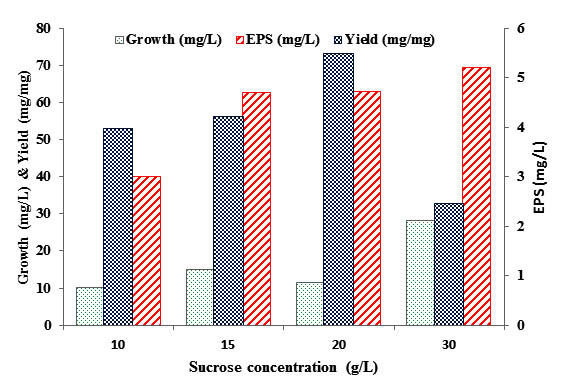
Fig. 4. EPS production by B. mojavensis DAS10-1 cells cultivated in presence of different sucrose concentration
Production of EPS by Bacillus mojavensis DAS10-1 using different sucrose levels
Results in figure 4 indicated that EPS gradually increased with sucrose concentration increase while, the maximum EPS production (63.02 mg) and yield (5.48 mg/mg) were noticed in presence of sucrose (20 g/L). Further increase in sucrose concentration led to remarkable increase in cell biomass while, lowering EPS yield.
Production of EPS by Bacillus mojavensis DAS10-1 using DEPS
Production of EPS by B. mojavensis DAS10-1 during growth of bacterial cells on date syrup “DEPS”; most dominant raw feedstock material in Saudi Arabia, was tested. Bacterial cells were cultivated on M3 medium in presence of DEPS as a sole carbon source. Results revealed successful production of EPS polymer in presence of DEPS as a carbon source. Surprisingly, increasing DEPS concentration from 0.5 to 1 mg/L led to simultaneous increase in polymer yield from 5.6 to 6.93 (mg/mg), respectively.
Bacterial exopolysaccharides (EPS) gains tremendous imputes in numerous health and industrial applications due to their unique physical and chemical properties6-8. In this study, group of bacilli explored for potential EPS production (Table 1). Among tested strains, Bacillus sp. DAS10-1 showed the optimal EPS productivity. 16S rDNA gene was partially sequenced. This sequence deposited in the GenBank with the accession number KU199819. On the other hand, the other 6 bacilli were previously identified by 16S rDNA gene analysis and accession numbers were given as reported in Berekaa et al18. Analysis of Bacillus sp. DAS10-1 16S rDNA gene sequence showed high similarity to the corresponding sequences of Bacillus mojavensis strains. Sequence recorded close similarity to xylanase producing bacterium B. mojavensis (accession No. KC297104), endophytic bacteria B. mojavensis (accession No. KY127359 and KY127359), and B. subtilis strains involved in phosphate solubilization (accession No. KX710213) and mannanase production (accession No. FJ485822).
FT-IR Spectroscopic analysis of purified EPS polymer showed clear band at 3415.62 cm-1 due to -OH stretching or hydroxyl stretching vibration of the polysaccharide20-22. Bands due to C-H stretching vibration (C-H asymmetric) in the sugar ring and C-O and carboxyl group stretching vibration were identified at 2942.13 cm-1 and 1646.51 cm-1 peaks, respectively20, 22. The bands at 1183.01 cm-1 and 1111.81 cm-1 due to valent vibration of C-O-C bond and C-O bond, respectively are typical for carbohydrate20, 21, 23. In concordance with Kavitake et al14, the presence of strong absorbance in the region 1200-950 cm”1 indicated the polysaccharide nature of the EPS. The FT-IR pattern collectively showed very close similarity with the results obtained by Singh et al24 during profiling of EPS produced by B. licheniforms associated with seaweeds. GPC is known to be one of the powerful tools to separate the different type of polymers according to their molecular size25. Interestingly, only one polymer species was recorded. The molecular values of the identified polymer Mw, Mn and Mz 1151Da, 987Da and 1302Da, respectively were characteristics for polymer. However, the polydispersity value (PDI) was 1.16 (very close to 1) indicating only one length of polymer is present which usually belongs to biological polymers26.
In the current study, production of the EPS polymer was enhanced by increase in aeration condition. Likewise, the increase of aeration and rate of agitation resulted in enriched exopolysaccharide production by Aeribacillus palidus. This is due to the influence of aeration on availability of nutrient, dissolved oxygen as well as the rate of metabolite release from cells27. Also, it was evident that 4:1 C:N ratio is suitable for optimal EPS yield. Ratio of carbon to nitrogen plays a crucial role in microbial biomass as well as exopolymer production with favor to higher carbon to decreased nitrogen content as reported by Abdul Razack et al28. Moreover, EPS production was enhanced by increasing sucrose concentration and with traces of yeast extract. It is assumed that the disaccharide sugar, sucrose could act as a precursor of EPS in many bacteria28. Production of EPS in presence of yeast extract was previously reported by many authors29, 30. Mostly, yeast extract was used as vitamin and nitrogen source30,31.
Interestingly, B. mojavensis DAS10-1 showed great capability of EPS production during growth on date syrup or “DEPS”; most dominant raw feedstock material in Saudi Arabia. Production of EPS from B. subtilis using agro-industrial wastes such as rice bran and sugar can molasses was reported by Abdul Razack et al28. Likewise, date syrup was successfully used as a raw material for bacterial production of levan32. It is assumed that sugars components in date syrup “DEPs” (mainly sucrose) can be used as a carbon source by many microbes during biopolymers production32, 33.
In conculsion, B. mojavensis DAS10-1 is recognized as a potent EPS producer. FT-IR spectroscopy and GPC analysis revealed characteristics typically for polysaccharides. Maximum EPS yield was recorded with C: N ratio of 4:1 for sucrose and ammonium sulfate. Raw agro-industrial waste (DEPS) can be used as alternative substrates for commercial production of EPS.
- Freitas, F., Alves, V.D., Reis, M.A.M. Advances in bacterial exopolysaccharides from production to biotechnological applications. Trends Biotechnol., 2011; 29(8): 388-398.
- Donot, F., Fontana, A., Baccou, J.C., Schorr-Galindo, S. Microbial exopolysaccharides: main examples of synthesis, excretion, genetics and extraction. Carbohydr Polym., 2012; 87(2): 951-962.
- Vu, B., Chen, M., Crawford, R.J., Ivanova, E.P. Bacterial extracellular polysaccharides involved in biofilm formation. Molecules,2009; 14(7): 2535-2554.
- Rehm, B.H.A., Bacterial polymers: biosynthesis, modifications and applications. Nat Rev Microbiol., 2010; 8(8): 578-592.
- Ismail, B., Nampoothiri, K. M. Exposition of antitumour activity of a chemically characterized exopolysaccharide from a probiotic Lactobacillus plantarum MTCC 9510. Biologia, 2013; 68: 1041-1047.
- Sathiyanarayanan, G., Vignesh, V., Saibaba, G., Vinothkanna, A., Dineshkumar, K. P., Viswanathana, M. and Selvin, J. Synthesis of carbohydrate polymer encrusted gold nanoparticles using bacterial exopolysaccharide: a novel and greener approach. RSC Adv., 2014; 4: 22817-22827.
- Lin, H., Zhang, M., Wang, F., Meng, F., Liao, B. Q., Hong, H., Chen, J. and Gao, W. A Critical review of extracellular polymeric substances (EPSs) in membrane bioreactors: characteristics, roles in membrane fouling and control strategies. J Membr Sci., 2014; 460: 110-125.
- Nouha, K., Kumar, R. S., Balasubramanian, S. and Tyagi, R. D. Critical review of EPS production, synthesis and composition for sludge flocculation. J Environ Sci., 2017; 225-245.
- Li, Z., Zhong, S., Lei, H. Y., Chen, R. W., Yu Q. and Li, H. L. Production of a novel bioflocculant by Bacillus licheniformis X14 and its application to low temperature drinking water treatment. Bioresour Technol., 2009; 100: 3650-3656.
- Elkady, M. F., Farag, S., Zaki, S., Abu-Elreesh, G.A. and Abd-El-aleem, D. Bacillus mojavensis strain 32A, a bioflocculant-producing bacterium isolated from an Egyptian salt production pond. Bioresour Technol., 2011; 102: 8143-8151.
- Orsod, M., Mugambwa, J. and Huyopf, M. Characterization of exopolysaccharides produced by Bacillus cereus and Brachybacterium sp. isolated from asian sea bass (Lates calcarifer). Malaysian J Microbiol., 2012; 8: 170-174 .
- Malick, A., Khodaei, N., Benkerroum, N. and Karboune, S. Production of exopolysaccharides by selected Bacillus strains: Optimization of media composition to maximize the yield and structural characterization. Int J Biol Macromol., 2017;102: 539-549 .
- Torino, M. I., Font de Valdez, G. and Mozzi, F. Biopolymers from lactic acid bacteria. Novel applications in foods and beverages. Front Microbiol., 2015; 6: Article no. 834.
- Kavitake, D. P., Devi1, B., S. Singh, P. and Shetty, P. H. Characterization of a novel galactan produced by Weissella confusa KR780676 from an acidic fermented food. Int J Biol Macromol., 2016; 86, 681-689 .
- Kielak, A. M., Castellane, T. L., Campanharo, J. C., Colnago, L. A., Costa, O.Y.A., da Silva, M. L. C., van Veen, J. A., E. Lemos, G. M. and Kuramae, E. E. Characterization of novel Acidobacteria exopolysaccharides with potential industrial and ecological applications. Sci Rep., 2017; 7: 41193-41204 .
- Zhao, M., Cui, N., Qu, F., Huang, X., Yang, H., Nie, S., Zha, X., Cuif, S.W., Nishinari, K., Phillips G.O. and Fang, Y. Novel nano-particulated exopolysaccharide produced by Klebsiella sp. PHRC1. Carbohydr Polym., 2017;171: 252-258 .
- Berekaa, M. and Salama, K. Comprehensive Assessment of Microbiological and Bioaerosol Contaminants in Dammam Slaughterhouse, Saudi Arabia. J Pure Appl Microbiol., 2015; 9: 69-78.
- Berekaa, M., Salama, K. and Alkharsah, K. Antibiotics sensitivity and heavy metals resistance in PHB-producing bacilli isolated from eastern province, Saudi Arabia. Int J Agric Biol., 2016; 18: 1032- 1037.
- Kumar, C. G., Joo, H. S., Choi, J. W., Koo, Y. M. and Chang, C. S. Purification and characterization of an extracellular polysaccharide from haloalkalophilic Bacillus sp. I-450. Enzyme Microb Technol., 2004; 34: 673-681.
- Purama, R. K., Goswami, P., Khan, A. T. and Goyal, A. Structural analysis and properties of dextran produced by Leuconostoc mesentroides NRRL B-640. Carbohydr Polym., 2009; 76: 30-35 .
- Ahmed, R. Z., Siddiquia, K., Arman, M. and Ahmed, N. Characterization of high molecular weight dextran produced by Weissella cibaria CMGDEX3. Carbohydr Polym, 2012; 90: 441-446 .
- Li, W., Ji1, J., Chen, Z., Jiang, M., Rui X. and Dong, M. Structural elucidation and antioxidant activities ofexopolysaccharides from Lactobacillus helveticus MB2-1. Carbohydr Polym., 2014; 102: 351-359.
- Ortega-Morales, B. O., Santiago-García, J. L., ChanBaca, M. J., Moppert, X., Miranda-Tello, E., Fardeau, M. L., Carrero, J. C., Bartolo-Pérez, P., ValadézGonzález A. and Guezennec, J. Characterization of extracellular polymers synthesized by tropical intertidal biofilm bacteria. J Appl Microbiol., 2007; 102: 254-264 .
- Singh, R. P., Shukla, M. K., Mishra, A., Kumari, P., Reddy C. R. K. and Jha, B. Isolation and characterization of exopolysaccharides from seaweed associated bacteria Bacillus licheniformis. Carbohydr Polym., 2011; 84: 1019-1026 .
- Ismail, B., and Nampoothiri, K. M. Molecular characterization of an exopolysaccharide from a probiotic Lactobacillus plantarum MTCC 9510 and its efficacy to improve the texture of starchy food. J Food Sci Technol., 2014; 51(12): 4012-4018.
- Kanamarlapudi, S. L. R. and Muddada, S. Characterization of Exopolysaccharide Produced by Streptococcus thermophilus CC30. Biomed Res Int., Article ID 4201809.
- Radchenkova, N., Vassilev, S., Martinov, M., Kuncheva, M., Panchev, I., Vlaev, S. Kambourova, M. Optimization of the aeration and agitation speed of Aeribacillus palidus 418 exopolysaccharide production and the emulsifying properties of the product. Process Biochem., 2014; 49: 576- 582.
- Abdul Razack, S., Velayutham, V. and Thangavelu, V. Medium optimization for the production of exopolysaccharide by Bacillus subtilis using synthetic sources and agro-wastes. Turk J Biol., 2013; 37: 280-288.
- Mukherjee, S., Ghosh, S., Sadhu, S., Ghosh, P. and Maiti, T. K. Extracellular polysaccharide production by a Rhizobium sp. isolated from legume herb Crotalaria saltiana Andr. Indian J Biotechnol., 2011; 10: 340-345.
- Van Dyk, J. S., Kee, N. L. A., Frost, C. L. and Pletschke, B. I. Extracellular polysaccharide production in Bacillus licheniformis SVD1 and its immunomodulatory effect. Bioresour., 2012; 7: 4976- 4982.
- Bhatia, S.K., Kumar N., and Bhatia, R. K. Stepwise bioprocess for exopolysaccharide production using potato starch as carbon source. Biotech., 2015; 5(5): 735-739 .
- Moosavi-Nasab, M., Layegh, B., Aminlari L. and Hashemi, B. Microbial Production of Levan using Date Syrup and Investigation of Its Properties. Int J Nut Food Eng., 2010; 44: 603-609.
- Tang, Z., Shi, L. and Aleid, S. M. Date and Their Processing Byproducts as Substrates for Bioactive Compounds Production. Braz arch biol technol., 2014; 57: 706-713.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


