ISSN: 0973-7510
E-ISSN: 2581-690X
The escalation of environmental pathogenic microorganisms and disregard of public hygiene practices have resulted in the emergence of various skin infections within communities. Recent investigations have proposed that diverse snail mucus compositions may possess antimicrobial properties. Therefore, it is imperative to conduct further research to elucidate specific antibacterial characteristics inherent in the mucus of White Garden snail (Theba pisana). This study aimed to evaluate antibacterial activity of Theba pisana mucus extract against selected ATCC bacterial strains being Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Enterococcus faecalis, and MRSA. The assessment involved comparing antimicrobial activity of the mucus extract to various broad-spectrum antibiotics. The susceptibility of bacterial isolates to snail mucus secretions was assessed using agar well diffusion method on Muller Hilton Agar plates. After incubation at 37°C for 24 hours, various concentrations of snail slime including 100%, 75%, 50%, and 25% were applied. The findings reported that E. faecalis exhibited highest activity, with zone of inhibition measuring 24 mm, followed by K. pneumonia and S. aureus (16 mm), MRSA (14 mm), and E. coli (12 mm). However, at concentration of 25%, mucus extract exhibited effect only against E. faecalis. Moreover, the antimicrobial activity of several tested antibiotics demonstrated similarity to that of the mucus extract. Therefore, it revealed that secretions of T. pisana mucus may possess the potential to act as a source of antibacterial agents. This may become as an alternative agent to costly synthetic antibacterial compounds. However, further studies are required to exploit the mucus secretion in addressing the issue of antibiotic resistance.
White Garden Snail, Theba pisana, Bacteria, Antibacterial Activity, Bacterial Resistance
A skin wound is an injury or harm to the outer layer of the body’s integumentary system, which is the skin.1-3 These injuries can disrupt the skin’s protective layer, potentially exposing underlying tissues and blood vessels to outer environment.1-4 Consequently, when bacteria successfully breach the skin’s defenses and proliferate, they can give rise to bacterial skin infections, constituting a significant public health concern.4,5 Among these infections, the most prevalent cases are attributed to Staphylococcus aureus and Streptococcus pyogenes.5,6 These infections can result in symptoms such as pain, warmth, redness, and swelling.7,8 Proper wound care, antibiotics, and keeping the affected area clean and dry are all common treatments.7,8
The advent of antibiotics during the mid-20th century has had a profound impact on mitigating the morbidity and mortality associated with infectious diseases.5,9,10 Nevertheless, bacteria have survived for millions of years by resistance development against some viruses, mobile genetic elements, heat, cold, acid osmotic stressors and different antibiotics.9-11 This adaptive resistance often involves simple enzymatic modifications, effectively disrupting the interactions between target proteins and antibiotics.9,11 The emergence of antibiotic-resistant bacterial infections on a daily basis contributes to the ineffectiveness of treatments.11–14 Land snails represent a diverse assemblage of mollusks exhibiting a global distribution across a multitude of ecosystems, encompassing forests, deserts, and grasslands.15,16 This phylum comprises an extensive repertoire, exceeding 40,000 documented species, manifesting considerable variations in morphological attributes, dimensions, and pigmentation.15,17 Among the prevalent land snail varieties are the ubiquitous garden snail, frequently encountered within horticultural spaces and recreational parks, and the notable Roman snail, characterized by its distinctive spiral-shaped shell, frequently employed in culinary practices.15,16,18-20 Furthermore, additional noteworthy land snail classifications encompass the channeled apple snail, indigenous to South America and discernible by its distinct yellow or brown shell, and the remarkable giant African land snail, renowned for its imposing size and visually striking characteristics, even gaining recent applications in the domain of beauty products.20-22
Snails have survived extreme environmental conditions for over 600 million years, positioning them among the oldest extant species on Earth.21,22 These special features include the shell power, estivation and hibernation, water conservation, tolerance to temperature extremes, and reproductive strategies. This longstanding survival implicates the development of unique adaptive mechanisms enabling their persistence in diverse habitats.5,23 A prominent survival strategy employed by snails entails the production and utilization of a specialized viscous-elastic secretion termed “mucus slime”.5,23 However, there are several key components that are commonly found in snail’s slime.11 These include water, which makes up around 90% of the slime, and mucin, a glycoprotein that gives snail slime its slimy texture and contributes to its healing properties.11,24,25 Snail’s slime also contains hyaluronic acid, a polysaccharide that helps to hydrate and plump the skin, as well as glycolic acid, an alpha-hydroxy acid that can help to exfoliate the skin and improve its texture.11,24 Another important component of snail slime is allantoin, a natural compound known for its soothing, healing and antimicrobial properties.11,24 Together, these components give snail’s slime its unique properties, which can help to hydrate, soothe, and heal the skin.11,24 This remarkable slime, exhibits adhesive and lubricative properties.22,24 Moreover, the mucus slime potentially serves multifaceted functions such as moisture retention, friction reduction, and a protective shield against pathogenic microorganisms.23 Remarkably, the manifold nutritional, medicinal, and therapeutic attributes inherent in snails have rendered them a significant component of human sustenance, medical practice, and dermatological well-being.5,24 Notably, scientific investigations have unveiled the antimicrobial properties inherent in the natural constituents of snail mucus slime, thus endorsing its utility as a natural supplement across diverse domains.5,24 Consequently, concerted research endeavors have been directed towards identifying alternative solutions, involving the exploration of natural compounds or extracts derived from animals and plants.9 Among these alternative avenues, a notable focus has been directed towards investigating the mucus slime produced by various snail species. The aim of this research is to comprehensively elucidate its potential applications and to propose its use as a natural antimicrobial agent, paving the way for future studies and therapeutic prospects.
Experimental Area
The study was conducted fundamentally at the Department of Medical Laboratories, College of Applied Medical Sciences, Qassim University, Buraydah, Saudi Arabia, during March-May 2023.The climate is sunny with a mean temperature of 38.8°C and mean relative humidity of 14 %.
Materials and samples collection
Twenty (20) white garden snails (T. pisana) were used. The snails were kept in one plastic box (45x37x30 cm, length/width/height) at room temperature. The snails were handled in accordance with the principles of animal welfare in scientific experiments. The plastic box was sprinkled with water daily in order to maintain humidity. The snail slime specimens were extracted according to the method of Alessia et al.26 from the snail samples by using citric acid solution (4% w/v) as it acts as a stimulant to encourage snails to produce more slime. The extracted slime secretion considered as 100% concentration was stored in the refrigerator at 4°C.5 The stored extracted slime was prepared into various concentrations (25%, 50%, 75% and 100% v/v) with distilled water as diluents. This was done by dissolving the respective volumes of slime into corresponding volumes of sterile distilled water.
Assessment of antibacterial activity
The antibacterial activity of the slime was determined in vitro by the agar well diffusion method as described by Ali et al.,12 against some pathogenic bacterial strains brought from the Department of Medical Laboratories stock at Qassim University and isolate them on blood agar media. One milliliter of different standardized organisms (0.5 McFarland) was introduced separately on Mueller Hilton Agar after culturing the bacteria using Lawn culture method to provide a uniform surface growth for bacteria, and allowed to set and labeled. A sterile cork borer 6 mm was used to punch hole (i.e., 4 wells) in the inoculated agar. The formed four wells were filled with 100 µl of snail slime extract with different concentration that is: 25%, 50%, 75% and 100% v/v, respectively. The plates were left for 1 hour for proper diffusion of the slime and incubated at 37°C for 24 hours. After incubation, the plates were observed for the zone of inhibition by the rulers. Five antibiotics were also tested on the same bacteria in order to compare antibacterial activity between the snail slime and the antibiotics.
Data analysis
The data for zone of inhibition from the agar well diffusion was expressed as mm to indicate the most significant concentration of the slime on each bacteria tested.
Physical properties of the snail slime
Small (between 1.90 mL and 2.40 mL), sufficient quantity of snail slime was successfully collected from the T. pisana snail during experiment time. The physical properties of the snail slime secretions from this species were observed. Snail slime was observed as a colorless fluid with medium sliminess and stretchy low texture thickness. The volume released at stimulation from each snail was 2.15±0.25 mL (Table 1).
Table (1):
The physical properties of the T. pisana slime.
Parameters |
T. pisana |
|---|---|
Color |
Colorless and clear |
Texture/Thickness |
Low thickness and stretchy |
Sliminess |
Medium |
Volume |
2.15±0.25 mL |
pH |
7.3 |
Antibacterial potential of the snail slime
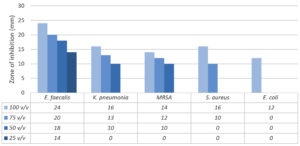
The results of testing the slime mucus of the T. pisana snail in four dilutions (100% v/v, 75% v/v, 50% v/v and 25% v/v) with distilled water against five ATCC strains of pathogenic bacteria determined by agar well diffusion assay, are shown in Figure. The pathogenic bacterial strains included three gram-positive bacteria, being Staphylococcus aureus ATCC 29213, Enterococcus faecalis ATCC 29212 and methicillin-resistant Staphylococcus (MRSA) ATCC 43300. Two gram-negative bacteria, being Escherichia coli ATCC 25922 and Klebsiella pneumonia ATCC 700603 were also included.
Figure. Antibacterial properties of the snail slime from T. pisana against five ATCC strains of pathogenic bacteria, as determined by agar well diffusion assay
Clear, small measurable zones of inhibition were obtained. The result showed that antibacterial activity of the slime increased with increase in concentration of the slime. When the snail slime was tested at 100% v/v concentration during 2 hours after collection, all the tested bacteria showed inhibition with varied zones unlike the remaining concentrations (75%, 50% and 25% v/v).
From the five pathogenic bacterial strains we tested, E. faecalis showed the maximum inhibition in all snail slime concentrations (24 mm, 20 mm, 18 mm and 14 mm), followed by K. pneumonia, MRSA, S. aureus then E. coli. E. coli showed the least susceptibility to 100% v/v snail slime concentration, while no activity was observed with the other concentrations. However, no inhibitory activity was observed against most bacteria at 25% v/v concentration.
The same five ATCC strains of pathogenic bacteria used in the snail slime extraction were also tested against five standard antibiotics, and the inhibitory zones were determined by disc diffusion assay shown in Table 2. Chloramphenicol antibiotic demonstrated a larger inhibition zone against all bacteria at a concentration of 100% v/v, except for E. faecalis and K. pneumonia, which were more effectively inhibited by the T. pisana snail slime extraction. Bacitracin antibiotic was particularly effective against S. aureus and MRSA compared to the other bacteria, but snail slime was found to be much more effective to others. The inhibitory activity of most of the remaining antibiotics tested was found to be similar to that of the snail slime.
Table (2):
Standard antibiotic discs used as control
| Antibiotic | Conc. | Bacteria | ||||
|---|---|---|---|---|---|---|
| E. faecalis | K. pneumonia | MRSA | S. aureus | E. coli | ||
| C | 30 µg | 22 | 13 | 24 | 23 | 27 |
| BA | 10 units | 17 | 0 | 20 | 20 | 0 |
| GM | 10 µg | 18 | 14 | 9 | 8 | 21 |
| NE | 30 µg | 13 | 13 | 9 | 8 | 17 |
| PB | 300 units | 0 | 16 | 10 | 9 | 16 |
Legend: C – Chloramphenicol, BA – Bacitracin, GM – Gentamicin, NE – Neomycin, PB – Polymyxin B.
Land snails encompass a diverse array of species, notably including the giant African snail, golden apple snail, garden snail, and Roman snail, alongside numerous subspecies.15-17,27 Investigations have revealed that the natural constituents found within the slime typically comprises water (about 90% of its composition), mucin (a glycoprotein responsible for its slimy texture and healing properties), hyaluronic acid (which hydrates and plumps the skin), glycolic acid (promoting skin exfoliation and texture improvement), and allantoin (known for its soothing and antimicrobial properties).5,11,22,24,25 Collectively, these components grant snail slime its unique ability to hydrate, soothe, and heal the skin.11,24,25
The present research constitutes the first investigation conducted on this organism regarding the properties under consideration. The antimicrobial efficacy against several pathogenic bacteria responsible for wound and skin infections, namely E. faecalis, K. pneumonia, MRSA, S. aureus, and E. coli, was assessed through inhibitory assay. Remarkably, the organism displayed activity against all five strains in the agar well diffusion assay. Furthermore, the observed variations in the assay results might be attributed to diverse factors associated with the snail slime, including its pH, temperature, and potential protein denaturation.
The outcomes of this investigation align with the findings reported by Muhammad Ali et al.,12 who similarly documented the antibacterial potential of the African snail (A. marginata) against K. pneumonia, S. aureus, and E. coli. Nevertheless, it is worth noting that their study did not provide evidence of anti-MRSA properties in the snail’s inhibitory activity results. In contrast to our own findings, which demonstrated a moderate inhibitory effect on MRSA bacteria, it is important to highlight the findings of Periyasamy et al.,28 who observed significant activity of crude snail skin methanol extract against S. aureus, and Lawrence et al.,5 who reported the susceptibility of Staphylococcus sp. to mucus derived from A. marginata. Compared to our findings, it was observed that the snail slime from the brown garden species H. aspersa exhibited a comparatively weaker effect against S. aureus when contrasted with the mucus derived from T. pisana.10 Indeed, another study conducted by Ajiboye on the species A. marginata corroborated the absence of any evidence of antibacterial activity in the snail slime.29 Numerous researchers have reported compelling evidence of antibacterial activity in the snail slime derived from snail secretions.5,30 Notably, the antibacterial effects of mucin present in the mucus of land snails have been linked to specific antibacterial factors within the protein component, rather than acting directly on the bacterial cell surface.24
These discrepancies in outcomes could be attributed to dissimilar experimental approaches, variations in the snail species examined, or other factors pertaining to the experimental conditions adopted. However, to establish the precise antimicrobial ingredient and comprehensively understand the role of snail slime in providing antimicrobial protection, further investigations are imperative to illuminate its potential as a natural defense against pathogenic bacteria.
In summary, this study focuses on investigating the antimicrobial activity of white garden snail (T. pisana) slime against cultured pathogenic bacteria. The research revealed that the presence of active antibacterial components within the snail slime effectively inhibits the growth of various pathogenic bacteria, including E. faecalis, K. pneumonia, MRSA, S. aureus, and E. coli, to an extent. Notably, the slime demonstrated noticeable efficacy against E. faecalis and K. pneumonia, the two bacterial strains commonly associated with wound infections. These findings hold promising implications, indicating that snail slime could potentially serve as a valuable source of antibacterial agents, offering a viable alternative to costly synthetic agents for wound treatment. This is particularly significant in the context of MRSA, a bacterium known for its substantial resistance challenges on a global scale. Additionally, this study represents the first investigation into the antimicrobial properties of slime derived from the white garden snail (T. pisana). The isolation of the active ingredients from the slime is essential for further research and potential use in developing pharmacological and therapeutic products.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Nichols RL. Surgical wound infection. Am J Med. 1991;91(3B):S54-S64.
Crossref - Leaper DJ, Harding KG. Wounds: biology and management. Oxford Academic. 1998.
Crossref - Hutchinson J. The wound programme. Centre for Medical Education: Dundee. 1992.
- EL-Zawawy NA, Mona MM. Antimicrobial efficacy of Egyptian Eremina desertorum and Helix aspersa snail mucus with a novel approach to their anti-inflammatory and wound healing potencies. Sci Rep. 2021;11(1):24317.
Crossref - Etim L, Aleruchi C, Obande G. Antibacterial properties of snail mucus on bacteria isolated from patients with wound infection. Br Microbiol Res J. 2016;11(2):1-9.
Crossref - Nantarat N, Tragoolpua Y, Gunama P. Antibacterial Activity of the Mucus Extract from the Giant African Snail (Lissachatina Fulica) and Golden Apple Snail (Pomacea Canaliculata) against Pathogenic Bacteria Causing Skin Diseases. Tropical Natural History. 2019;19(2):103–112.
- Wiya C, Nantarat N, Saenphet K. Anti-Inflammatory Activity of Slime Extract from Giant African Snail (Lissachatina Fulica). Indian J Pharm Sci. 2020;82(3):508-514.
Crossref - Chinaka NC, Chuku LC, George G, Oraezu C, Umahi G, Orinya OF. Snail slime: Evaluation of anti-inflammatory, phytochemical and antioxidant properties. J Complement Alternat Med Res. 2021;13(1):8-13.
Crossref - Abiona J, Akinduti A, Osinowo O, Onagbesan O. Comparative evaluation of inhibitory activity of Epiphgram from albino and normal skinned giant African land snail (<i>Archachatina marginata</i>) against selected bacteria isolates. Ethiopian Journal of Environmental Studies and Management. 2013;6(2).
Crossref - Pitt SJ, Graham MA,Dedi CJ, Taylor-Harris PN, Gunn A. Antimicrobial Properties of Mucus from the Brown Garden Snail Helix Aspersa. 2015;72(4):174-181.
Crossref - Okeniyi FA, Oghenochuko OM, Olawoye SO, Animashahun RA, Adeyonu AG, Akpor OB. Antimicrobial potentials of mucus mucin from different species of giant African land snails on some typed culture pathogenic bacteria. Asian J Agric Biol. 2022;(4).
Crossref - AM Ahmad, M Ali, LM Azu, MS Abdallah, IU Zungum. Assessment of Antibacterial Activity of Snail (Archachatina Marginata) Slime on some Clinical Wound Isolates. Innovat International Journal Of Medical & Pharmaceutical Sciences, 2021;6(2):8-11.
- Taiwo SS, Okesina AB, Onile BA. In vitro antimicrobial susceptibility pattern of bacterial isolates from wound infections in University of Ilorin Teaching Hospital. Afr J Clin Experiment Microbiol. 2002;3(1):6-10.
Crossref - Andhoga J, Macharia AG, Maikuma IR, Awnyonyi ZS, Ayumba BR, Kakai R. Aerobic pathogenic bacteria in post-operative wounds at Moi Teaching and Referral Hospital. East Afr Med J. 2002;79(12):640-644.
Crossref - Ershler RH, Ponder WF. A Review of Morphological Characters of Hydrobioid Snails Ifc Smithsonian Contributions to Zoology Number 600.
- Speiser B. Food and feeding behaviour. In: The Biology of Terrestrial Molluscs. CABI Wallingford UK. 2001:259-288.
Crossref - Elgar MA, BJ Crespi. Ecology and evolution of cannibalism. (eds), 1992;1-12.
Crossref - Dimitriadis VK. Structure and function of the digestive system in Stylommatophora. In: The Biology of Terrestrial Molluscs. CABI Wallingford UK. 2001:237-257.
Crossref - Ademolu KO, Idowu AB, Mafiana CF, Osinowo OA. Performance, proximate and mineral analyses of African giant land snail (Archachatina marginata) fed different nitrogen sources. Afr J Biotechnol. 2004;3(8):412-417.
Crossref - Okonkwo TO, Anyaene LU. Meat yield and the effects of curing on the characteristics of snail meat. Agro-Science. 2009;8(1).
Crossref - Etengeneng AE, Moh LG, Landry SKA. The effects of using chemicals to remove slime from African giant land snails flesh during processing on some nutritional and biochemical parameters. Int J Food Sci. 2021;6691609.
Crossref - Adikwu MU, Nnamani PO. Some physiological and toxicological properties of snail mucin extracted from Archachatina marginata. Bio-Research. 2005;3(2):1-6.
Crossref - Kenawy ER, Ali SS, Al-Etewy M, Sun J, Wu J, El-Zawawy N. Synthesis, characterization and biomedical applications of a novel Schiff base on methyl acrylate-functionalized chitosan bearing p-nitrobenzaldehyde groups. Int J BiolMacromol. 2019;122:833-843.
Crossref - Cilia G, Fratini F. Antimicrobial properties of terrestrial snail and slug mucus. J Complement Integr Med. 2018;15(3).
Crossref - Kim YS, Jo YY, Chang IM, Toida T, Park Y, Linhardt RJ. A New Glycosaminoglycan from the Giant African Snail Achatina fulica. J Biol Chem. 1996;271(20):11750-11755.
Crossref - Ricci A, Gallorini M, Feghali N, Sampo S, Cataldi A, Zara S. Snail slime extracted by a cruelty free method preserves viability and controls inflammation occurrence: A focus on fibroblasts. Molecules. 2023;28(3):1222.
Crossref - Ogunyemi O. Growth Performance of African Giant Land Snail Archachatina Marginata Fed Varying Dietary Protein Levels of Plant Source. JOJ Wildl Biodivers. 2021: 3(3): 555616.
Crossref - Periyasamy N, Srinivasan M, Balakrishnan S. Antimicrobial activities of the tissue extracts of Babylonia spirata Linnaeus, 1758 (Mollusca: Gastropoda) from Thazhanguda, southeast coast of India. Asian Pac J Trop Biomed. 2012;2(1):36-40.
Crossref - Sodipe OG, Osinowo OA, Onagbesan OM, Bankole MO. Evaluation of the Haemolymph of the Giant African Land Snails Achatina achatina and Archachatina marginata for Bacteria Sterility and Inhibitory Properties. J. Agric. Sci. Environ. 2013:13(1);10-14. https://journal.funaab.edu.ng/index.php/JAgSE/article/view/1205
- Khaheshi I, Keshavarz S, Fooladi AAI, et al. Loss of expression of TGF-βs and their receptors in chronic skin lesions induced by sulfur mustard as compared with chronic contact dermatitis patients. BMC Dermatol. 2011;11(1):2.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.