ISSN: 0973-7510
E-ISSN: 2581-690X
Echinococcus granulosus recombinant proteins including two antigen B sub-units EgAgB8/1 and EgAgB8/2 and Echinococcus protoscolex calcium binding protein (EPC1) were expressed in prokaryotic expression vectors. The diagnostic potential of these three recombinant proteins was evaluated in the detection of cystic echinococcosis in goats in IgG-ELISA. The EgAgB8/1 and EgAgB8/2 recombinant proteins reacted fairly with the hydatid infected goats with sensitivity of 66.7% and 80.0% and specificity of 71.3% and 73.3%, respectively while EPC1 recombinant protein showed lower sensitivity (60%) but comparable specificity (72.3%). Cross-reactivity of these three antigens with goat gastro-intestinal strongyle nematodes and Taenia hydatigena under field conditions was studied. Results showed that EgAgB8/1, EgAgB8/2 and EPC1 antigens cross-reacted with most of the parasites in the goat host.
Echinococcus granulosus; goat; Antigen B8 sub-units; EPC1; Diagnosis; IgG-ELISA; Cross-reactivity.
Cystic echinococcosis (CE) is a cyclozoonosis caused by the larval stage of the taeniid cestode Echinococcus granulosus. It is a serious public health concern and in India the economic losses of ~ US $ 212 million associated with cystic echinococcosis in livestock are high.1 Infection of the intermediate host occurs by incidental ingestion of the oncosphere that develops in the liver and lungs to hydatid fluid filled cyst with protoscolices and daughter cysts.2 Early detection of the infection significantly reduces morbidity and mortality associated with CE. However, early detection of hydatidosis in animals and man has been a challenge. The diagnosis of CE largely depends on imaging scans and serological tests. Diagnosis of CE in animals by imaging methods is not feasible in the developing countries due to cost factors and in the absence of accurate serological tests hydatid infected animals remain in the morbid state. Hydatid cyst fluid has been used most frequently as a source of E. granulosus antigens and its components have been comprehensively investigated for their applicability in serological tests. Among several proteins of hydatid cyst fluid, antigen 5 (EgAg5) and antigen B (EgAgB) are known to be potent diagnostic antigens but the native and recombinant EgAgB antigens allow for better diagnostic performance.2-4 EgAgB is a thermostable, lipoprotein encoded by a multigene family.5 The literature describing the immunological diagnosis of hydatid disease in humans is extensive2,6-9 but research efforts towards the development of immunodiagnostic tests for E. granulosus infection in domestic ruminants are limited and the results have not been generally consistent due to poor sensitivity and specificity of the immuno-assays in animals.10 The only reliable method of diagnosing hydatidosis in animals is by detection of cysts at necropsy. Therefore, present studies were undertaken to express three recombinant antigens EgAgB8/1, EgAgB8/2 and EPC1 known to be potent diagnostic antigens in human cystic echinococcosis and to assess their immunodiagnostic potential for cystic echinococcosis in goats.
Three recombinant proteins of E. granulosus including two EgAgB sub-units EgAgB8/1 and EgAgB8/2 and Echinococcus protoscolex calcium binding protein (EPC1) were expressed in the prokaryotic expression vectors. Total RNA was isolated from the protoscolices retrieved from a fertile hydatid cyst from buffalo liver at a local abattoir using Trizol reagent (Invitrogen, USA). Briefly, ~0.2 x104 protoscolices were treated with Trizol reagent (1ml) and manually homogenized with a micropestle in a sterile 2.0 ml microcentrifuge tube. The lysed protoscolices were freeze-thawed at -80°C for multiple cycles together with manual homogenization to completely lyse the parasites. Total RNA was isolated from the lysed protoscolices following standard RNA isolation protocol (Invitrogen, USA). The RNA was converted to single stranded cDNA using oligo-dT primer and reverse transcriptase enzyme (MBI Fermentas, USA) following standard protocols of cDNA synthesis. The cDNA coding for each of the above three target proteins was PCR amplified with gene specific primers (Table1). The PCR products of three target cDNAs were cloned in pDRIVE cloning vector (Qiagen, Germany) and sequence confirmed for each cDNA.
Expression of the recombinant EgAgB8 sub-units and EPC1 protein
The cDNAs coding for EgAgB8/1, EgAgB8/2 and EPC1 proteins were PCR amplified with primers designed with suitable restriction enzyme sites and expressed in three different prokaryotic expression vectors (Table 1).
EgAgB8/1 protein was expressed in pRham vector in frame with solubility enhancing sumo-fusion protein (Expresso™ Rhamnose cloning and expression kit; Lucigen, USA). E. cloni® 10G chemically competent cells were transformed with the recombinant pRham vector and a moderate level of the recombinant fusion protein was generated on induction of the culture with 0.2% L-rhamnose for 8 h at 37oC. The bacterial cells were disrupted with 6 M guanidine hydrochloride in the lysis buffer (pH 8.0) supplemented with 18 mM imidazole and 10 mM â-mercaptoethanol, followed by sonication of the cell lysate with 3 bursts of 30 sec each at 10 micron amplitude. The protein was purified to complete homogeneity using Ni-NTA affinity chromatography. The recombinant protein was bound to Ni-NTA resin (Qiagen, Germany) at room temperature for 2 h with constant shaking. Affinity column was washed with wash buffer (pH 6.0) supplemented with 18 mM imidazole and protein eluted with elution buffer (pH 4.2).
Expression of the EgAgB8/2 recombinant protein was carried out in pET32a (+) vector in Escherichia coli BL21 (DE3).The cDNA was PCR amplified and cloned in the expression vector in frame with the vector fusion tag. An optimum level of expression of the EgAgB8/2 recombinant protein was achieved at 6 h post-1mM IPTG induction at 37 oC. The recombinant protein was purified by lysis of the bacterial cells in lysis buffer (pH 8.0) containing 8 M urea and supplemented with 12 mM imidazole and 10 mM â-mercaptoethanol for 2 h at RT. The cell lysate was then sonicated on ice for 5 cycles of 30 sec each at 5 micron amplitude. The recombinant protein was bound to Ni-NTA resin at room temperature for 2 h with constant shaking. The affinity column was washed with wash buffer (pH 6.5) supplemented with 12 mM imidazole and recombinant protein eluted with elution buffer at pH 4.2.
The EPC1 target protein was expressed in pPROEXHT-b vector, with a higher level of expression of the recombinant protein achieved at 8 h post-IPTG induction at 37oC. E. coli BL21 (DE3) cells induced with 1mM IPTG were disrupted in lysis buffer (pH 8.0) containing 6 M guanidine hydrochloride and supplemented with 16 mM imidazole at room temperature for 2 h and the recombinant protein was purified by Ni-NTA affinity chromatography. The wash buffer (pH 5.9) was supplemented with 16 mM imidazole and recombinant protein was eluted at pH 4.2. The composition of the lysis, wash and elution buffers used in the purification steps of each recombinant protein was 10 mM tris and 100 mM potassium dihydrogen phosphate containing 6M guanidine hydrochloride or 8M urea as protein denaturant.
The purified recombinant fusion proteins EgAgB8/1, EgAgB8/2 and EPC1 resolved at 25, 29 and 10.5 kDa, respectively in the SDS-PAGE.
Collection of goat sera
Goats (n=116) were screened for hydatid infection at necropsy at the local abattoir. Out of the 116 animals examined at necropsy, 15 were positive for hydatid cysts in the liver or lungs and remaining 101 were negative for hydatid cysts. Sera were retrieved from these animals and screened for anti-hydatid antibodies by IgG-ELISA with the above three recombinant antigens. Sera were also collected from goats infected with other parasites for their cross-reactivity studies. Sera collected from healthy goats, maintained at Indian Veterinary Research Institute, Izatnagar, were used as negative control in the subsequent immuno-assays. All experiments on goats were conducted as per the guidelines of the Institute Animal Ethics Committee.
Enzyme Linked Immunosorbent Assay
Checker board titrations were done to optimize the concentration of each antigen. The amount of recombinant EgAgB8/1, EgAgB8/2 and EPC1 antigens coated on each well of the 96-well microtitre plate was optimized to 1.0 µg /ml, 2.0 µg /ml and 2.0 µg/ml of coating buffer, respectively. The optimal dilutions of serum samples and anti-goat IgG-HRP conjugate (Sigma Chemicals, USA) used in the assay were 1:100-1:200 and 1:6000-1:12000, respectively for different antigens. Receiver Operating Characteristic (ROC) curve analysis was performed to determine the cut-off value for each recombinant antigen. Levels of sensitivity were plotted against the levels of one minus specificity at each cut-off point on a ROC curve. Cut-off values were selected that gave the highest sum of sensitivity (%) and specificity (%), as described by Amagai et al11. The area under the ROC curve (AUC) was the parameter used to define the antigen’s discriminatory values between ELISA positive and negative animals.
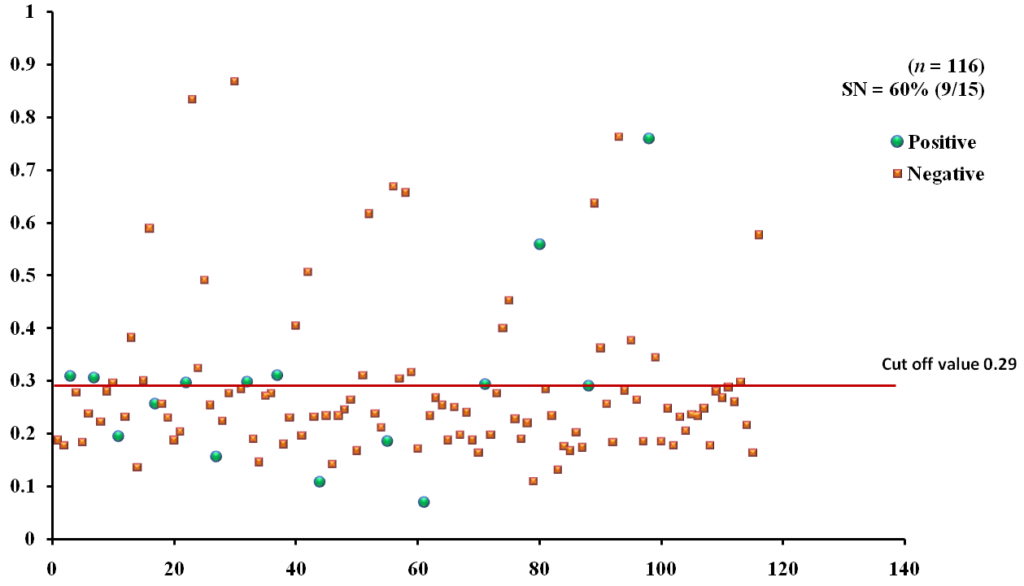
The EgAgB8/1 antigen showed positive reactivity with 10/15 necropsy positive animals with OD492 ranging between 0.22-1.18 above the cut-off value 0.22. However, 5/15 necropsy confirmed positive animals were negative with EgAgB8/1 ELISA leading to the sensitivity of 66.7%. The IgG-ELISA with this antigen showed 29/101 (28.7%) necropsy negative animals for hydatid cyst as sero-positive (Fig 1a). With antigen EgAgB8/2, 12/15 necropsy confirmed positive animals were ELISA positive with OD492 values above the cut-off (0.29), thereby depicting the sensitivity of 80.0%. The IgG-ELISA with this antigen showed 27/101 (26.7%) necropsy negative animals as ELISA positive (Fig 2a). The EPC1 based ELISA carried out on the same number of sera had a lower sensitivity of 60% with 9/15 necropsy confirmed positive animals showing A492 above the cut-off value (0.29) and 6 were sero-negative with A492 below cut-off. A number of necropsy negative animals (26/101) were sero-positive (25.7%) with this antigen with A492 above the cut-off (Fig 3a). The comparative sensitivity, specificity, positive and negative predictive values of the three assays showed that EgAgB8/2 antigen has higher sensitivity and specificity than the other two antigens (Table 2).
Table (1):
Primer sequences designed for PCR amplification (A) and cloning of three cDNAs coding for the recombinant antigens in expression vectors (B). *Sequences with restriction enzyme sites are indicated in bold and sequences for homologous recombination with SUMO / pRham vector sequence are indicated in bold with italics (B).
| Gene | Primer name | Primer length | Primer Sequence (5ʹ à 3ʹ) | Amplicon size | |
|---|---|---|---|---|---|
|
A |
EgAgB8/1 | B8/1-FOR | 24 bp | ATG CTT CTC GCT CTG GCT CTC GTC | 247 bp |
| B8/1-REV | 24 bp | CTA TTC ACC TTC AGC AAT CAA CCC | |||
| EgAgB8/2 | B8/2-FOR | 21 bp | AAA GAT GAG CCA AAA GCA CAC | 250 bp | |
| B8/2-REV | 24 bp | TTA CTT TGA ATC ATC ATC TTT TTC | |||
| EPC1 | EPC-FOR | 21 bp | TGC GTT TGT CGT TCC TGC CGT | 231 bp | |
| EPC-REV | 22 bp | TTA GAA GAG AGC CAT TAA CTC A | |||
|
B |
EgAgB8/1 | B8/1-FOR-SUM | 39 bp | CGC GAA CAG ATT GGA GGTATG CTT CTC GCT CTG GCT CTC | 281 bp |
| B8/1-REV-SUM | 40 bp | GTG GCG GCC GCT CTA TTATTC ACC TTC AGC AAT CAA CCC T | |||
| EgAgB8/2 | B8/2-FOR-EX | 33 bp | GGA TCC ATG GGC AAA GAT GAG CCA AAA GCA CAC | 270 bp | |
| B8/2-REV-EX | 32bp | CTC GAA GCT TAC TTT GAA TCA TCA TCT TTT TC | |||
| EPC1 | EPC-FOR-EX | 31 bp | CCA TGG ATC CTG CGT TTG TCG TTC CTG CCG T | 251 bp | |
| EPC-REV-EX | 32 bp | CGA GTC TAG ATT AGA AGA GAG CCA TTA ACT CA |
Table (2):
Showing sensitivity, specificity and accuracy of IgG-ELISA with three recombinant antigens based on ROC analysis.
Antigen |
AUC |
Cut-off |
Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
Accuracy (%) |
|---|---|---|---|---|---|---|---|
EgAgB8/1 |
0.690 |
0.22 |
66.7 |
71.3 |
25.6 |
93.5 |
70.6 |
EgAgB8/2 |
0.740 |
0.29 |
80 |
73.3 |
30.7 |
96.1 |
74.1 |
EPC1 |
0.558 |
0.29 |
60 |
72.3 |
25.7 |
92.5 |
72.4 |
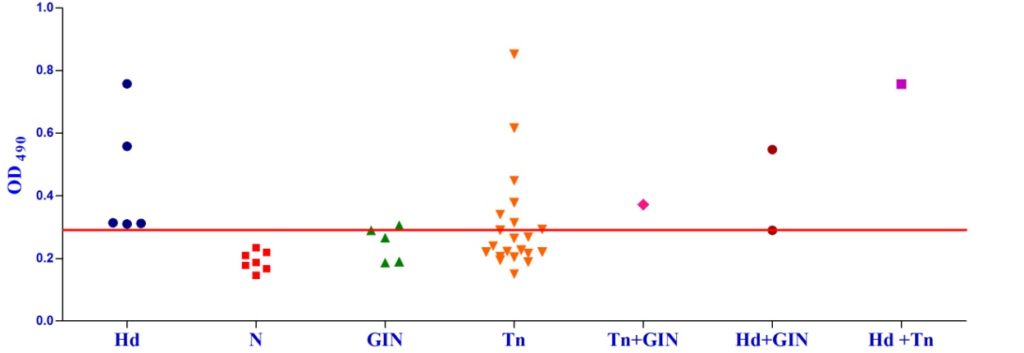
Studies on the cross-reactivity of each recombinant antigen were conducted with goat sera positive for gastrointestinal (GI) strongyle nematodes and Taenia hydatigenia. The EgAgB8/1 antigen showed immuno-reactivity with sera of 3/5 GI nematode infected goats and 14/21 T. hydatigena positive goats with OD492 above cut off (0.22). However, serum of single goat with a mixed infection of T. hydatigena and GI nematodes showed OD492 below negative cut-off. All the goats with a mixed infection of hydatid, T. hydatigena and GI nematodes reacted with the antigen EgAgB8/1(Fig 1b).
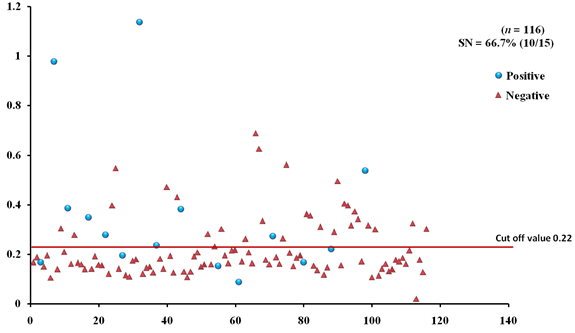
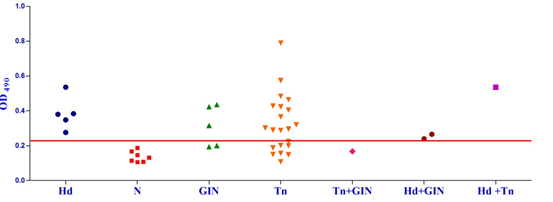 Fig. 1a & b. IgG-ELISA showing immuno-reactivity (a) and cross reactivity (b) of the goat sera with recombinant antigen EgAgB8/1 (Hd -Hydatid positive sera, N- Healthy goat sera (Negative control), GIN – Gastro intestinal nematodes, Tn-Taenia hydatigena positive sera)
Fig. 1a & b. IgG-ELISA showing immuno-reactivity (a) and cross reactivity (b) of the goat sera with recombinant antigen EgAgB8/1 (Hd -Hydatid positive sera, N- Healthy goat sera (Negative control), GIN – Gastro intestinal nematodes, Tn-Taenia hydatigena positive sera) The EgAgB8/2 antigen showed reactivity with 4/5 sera positive for GI nematodes and 16/21 T. hydatigena positive sera with OD492 above cut off (0.29). The serum of a single goat with mixed infection of T. hydatigena and GI nematodes showed OD492 above negative cut-off. Goats with a mixed infection of hydatid, T. hydatigena and GI nematodes also reacted with the antigen EgAgB8/2 (Fig 2b).
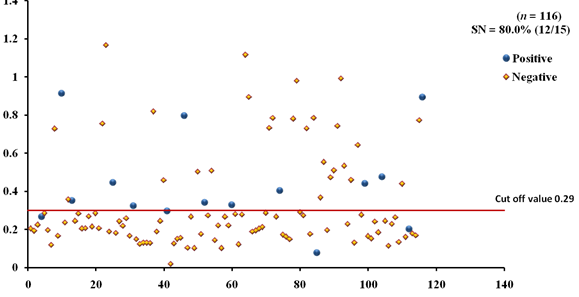
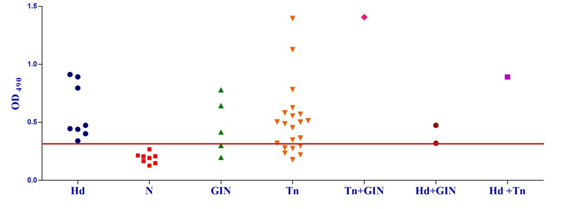 Fig. 2a & b. IgG-ELISA showing immuno-reactivity (a) and cross reactivity (b) of the goat sera with recombinant antigen EgAgB8/2 (Hd -Hydatid positive sera, N- Healthy goat sera (Negative control), GIN – Gastro intestinal nematodes, Tn-Taenia hydatigena positive sera)
Fig. 2a & b. IgG-ELISA showing immuno-reactivity (a) and cross reactivity (b) of the goat sera with recombinant antigen EgAgB8/2 (Hd -Hydatid positive sera, N- Healthy goat sera (Negative control), GIN – Gastro intestinal nematodes, Tn-Taenia hydatigena positive sera) The EPC1 antigen showed sero-reactivity with 2/5 GI nematodes, 8/21 T. hydatigena infected goats with OD492 above cut-off (0.29). The serum of the goat with a mixed infection of T. hydatigena and GI nematodes reacted with this antigen with OD492 above negative cut-off. Goats with a mixed infection of hydatid, T. hydatigena and GI nematodes also reacted with the antigen EPC1 in IgG ELISA (Fig 3b).
Two sub-units of E. granulosus AgB viz. EgAgB8/1 and EgAgB8/2 along with EPC1 were expressed as recombinant proteins and evaluated in the present investigation for the detection of CE in goats. The two antigen B sub-units EgAgB8/1 and EgAgB8/2 showed a sensitivity of 66.7% and 80% and specificity of 71.3% and 73.3%, respectively. However, EPC1 antigen showed a lower sensitivity (60%) but comparable specificity (72.3%). The three recombinant antigens were found to cross-react with the sera of goats infected with gastrointestinal strongyle nematodes and Taenia hydatigenia equally. Likewise, EPC1 that has shown a potential in the immunodiagnosis of human hydatidosis12-14 also cross-reacted with the above parasites in goat host. The results indicate that these proteins share cross-reacting epitopes between the taeniid cestodes and strongyle nematodes. IgG4 sub-class antibody has been shown to reduce the cross-reactivity of EgAgB8/2 in human patients15,16 but no such study has been carried out in goats. Therefore, immuno-assays using IgG4 isotype of the antibody could be evaluated for better sensitivity and specificity of these antigens in goats. Peptide based ELISA for improving the sensitivity and specificity of these antigens may also be tested.
Immunodiagnosis of CE in different hosts like pig, cattle, goat, buffalo and camel has been investigated but specific and sensitive diagnosis has been compromised due to false positive reactions and weak serological response generated in infected animals.17-19 Serological cross-reactivity of the hydatid cyst antigens with taeniid cestodes including Taenia hydatigena and T. ovis has hindered the accurate serological diagnosis of hydatid infection in sheep.17 Furthermore, natural intermediate host animals produce very poor antibody responses to infection compared with the relatively high levels of specific antibody evident in human infection.17 Different groups have reported different findings on using the same antigenic component in diagnostic assays, with some authors reporting low antibody response in sheep to antigen B20, 21 while others showing reasonably high and specific antibody reactivity against antigen B in livestock.18,22-26 Cross-reactivity of antigen B (similar to antigen 5) with other helminth parasites has been a recurrent challenge to the immunodiagnostic potential of these antigens10.
The cross-reactivity of the three recombinant antigens with other helminths in goats detected in the present study compromises the specificity of the test. However, these antigens can be used in the sero-surveillance of the goat flocks for cystic echinococcosis in the endemic regions of the country. The present results provide first information on the cross-reactivity of the three potent human CE diagnostic molecules EgAgB8/1, EgAgB8/2 and EPC1 of E. granulosus with the helminth parasites of goat. This investigation was carried out on a smaller number of goats but screening of larger sample size and determining the cross-reactivity with more species of helminths parasitizing goats is needed for validating the utility of these three antigens in the sero-diagnosis of cystic echinococcosis in goats.
ACKNOWLEDGMENTS
The authors are highly thankful to the Director, Indian Council of Agricultural Research-Indian Veterinary Research Institute, Izatnagar, India for providing necessary facilities for completing the present research work. The first author is also thankful to Department of Science and Technology, Government of India, New Delhi for providing INSPIRE fellowship for her Ph D programme.
- Singh, B.B., Dhand, N.K., Ghatak, S., Gill, J.P. Economic losses due to cystic echinococcosis in India: Need for urgent action to control the disease. Prev. Vet. Med., 2014; 113: 1-12.
- Brunetti, E., Kern, P., Vuitton, D.A. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop., 2010; 114: 1–16.
- Lorenzo, C., Last, J.A., Gonzalez-Sapienza, G.G. The immunogenicity of Echinococcus granulosus antigen 5 is determined by its post-translational modifications. Parasitology, 2005; 131: 669–677.
- Ortona, E., Rigano, R., Margutti, P., Notargiacomo, S., Ioppolo, S., Vaccari, S., Barca, S., Buttari, B., Profumo, E., Teggi, A., Siracusano, A. Native and recombinant antigens in the immunodiagnosis of human cystic echinococcosis. Parasite Immunol., 2000; 22: 553–559.
- Haag, K.L, Alves-Junior, L., Zaha, A., Ayala, F.J. Contingent, non-neutral evolution in a multicellular parasite: natural selection and gene conversion in the Echinococcus granulosus antigen B gene family. Gene, 2004; 333: 157–167.
- Zhang, W., McManus, D.P. Recent advances in the immunology and diagnosis of echinococcosis. FEMS Immunol. Med. Microbiol., 2006; 47: 24–41.
- Craig, P.S., McManus, D.P., Lightowlers, M.W., Chabalgoity, J.A., Garcia, H.H., Gavidia, C.M., Gilman, R.H., Gonzalez, A.E., Lorca, M., Naquira, C., Nieto, A. Prevention and control of cystic echinococcosis. Lancet Infect. Dis., 2007; 7(6): 385-394.
- McManus, D.P., Gray, D., Zhang, W., Yang, Y.R. Diagnosis, treatment and management of echinococcosis. Brit. Med. J., 2012; 344: e3866.
- Zhang, W., Wen, H., Li, J., Lin, R., McManus, D.P. Immunology and immunodiagnosis of cystic echinococcosis – an update. Clin. Dev. Immunol., 2012; 101895.
- McManus, D.P. Immunodiagnosis of sheep infections with Echinococcus granulosus: in 35 years where have we come? Parasite Immunol., 2014; 36: 125–130.
- Amagai, M., Komai, A., Hashimoto, T., Shirakata, Y., Hashimoto, K., Yamada, T., Kitajima, Y., Ohya, K., Iwanami, H., Nishikawa, T. Usefulness of enzyme-linked immunosorbent assay using recombinant desmogleins 1 and 3 for serodiagnosis of pemphigus. Br. J. Dermatol., 1999; 140: 351–357.
- Li, J., Zhang, W.B., Wilson, M., Ito, A., McManus, D.P. A novel recombinant antigen for immunodiagnosis of human cystic echinococcosis. J. Infect. Dis., 2003; 188: 1951-1960.
- Cai, H.X., Shen, Y.J., Han, X.M., Yuan, Z.Y., Wang, H., Xu, Y.X., Hu, Y., Lu, W.Y., Guan, Y.Y., Cao, J.P. Cloning, expression and immunodiagnostic evaluation of antigen EPC1 from Echinococcus granulosus. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi., 2011; 29: 167-171.
- Etebar, F., Jalousian, F., Hosseini, S.H., Kordafshari, S., Najafi, A. Immunoproteomics approach for EPC1 antigenic epitope prediction of G1 and G6 strains of Echinococcus granulosus. Parasitol. Res., 2013; 112: 3129-3135.
- McVie, A., Ersfeld, K., Rogan, M.T., Craig, P.S. Expression and immunological characterization of Echinococcus granulosus recombinant antigen B for IgG4 subclass detection in human echinococcosis. Acta Trop., 1997; 67: 19–35.
- Khalilpour, A., Sadjjadi, S.M., Moghadam, Z.K., Yunus, M.H., Zakaria, N.D., Osman, S., Noordi. Lateral flow test using Echinococcus granulosus native antigen B and comparison of IgG and IgG4 dipsticks for detection of human cystic echinococcosis. Am. J. Trop. Med. Hyg., 2014; 91: 994-999.
- Lightowlers, M.W., Gottstein, B. Echinococcosis/hydatidosis: antigens, immunological and molecular diagnosis. In Thomson RCA & Lymbery AJ (eds): Echinococcus and Hydatid Disease. Wallingford, Oxon, UK, CAB International, 1995; pp 355–419.
- Ibrahem, M.M., Rafiei, A., Dar, F.K., Azwai, S.M., Carter, S.D., Craig, P.S. Serodiagnosis of cystic echinococcosis in naturally infected camels. Parasitology, 2002; 125: 245–251.
- Golassa, L., Abebe, T., Hailu, A. Evaluation of crude hydatid cyst fluid antigens for the serological diagnosis of hydatidosis in cattle. J. Helminthol., 2011; 85(01): 100-108.
- Lightowlers, M.W., Rickard, M.D., Honey, R.D., Obendorf, D.L., Mitchell, G.F. Serological diagnosis of Echinococcus granulosus infection in sheep using cyst fluid antigen processed by antibody affinity chromatography. Aust. Vet. J., 1984; 61: 101–108.
- Kittelberger, R., Reichel, M.P., Jenner, J., Heath, D.D., Lightowlers, M.W., Moro, P., Ibrahem, M.M., Craig, P.S., O’Keefe, J.S. Evaluation of three enzyme-linked immunosorbent assays (ELISAs) for the detection of serum antibodies in sheep infected with Echinococcus granulosus. Vet. Parasitol., 2002; 110(1): 57-76.
- Ibrahem, M.M., Craig, P.S., McVie, A., Ersfeld, K., Rogan, M.T. Echinococcus granulosus antigen B and seroreactivity in natural ovine hydatidosis. Res. Vet. Sci., 1996; 61: 102–106.
- Kanwar, J.R., Kanwar, R. Purification and partial immunochemical characterization of a low molecular mass, diagnostic Echinococcus granulosus immunogen for sheep hydatidosis. FEMS Immunol. Med. Microbiol., 1994; 9: 101–107.
- Moro, P., Verastegui, M., Gilman, R.H., Falcon, N., Bernal, T., Gavidia, C., Gonzalez, A., Malqui, V., Moro, M.H., Dueger, E. Enzyme-linked immunoelectrotransfer blot assay for diagnosis of hydatidosis (Echinococcus granulosus) in sheep. Vet. Record, 1997; 140: 605-606.
- Dueger, E.L., Verastegui, M., Gilman, R.H. Evaluation of the enzyme-linked immunoelectrotransfer blot (EITB) for ovine hydatidosis relative to age and cyst characteristics in naturally infected sheep. Vet. Parasitol., 2003; 114: 285–293.
- Pan, D., Bera, A.K., Bandyopadhyay, S., Das, S., Rana, T., Das, S.K., Bandyopadhyay, S., Manna, B., Bhattacharya, D. Molecular characterization of antigen B2 subunit in two genotypes of Echinococcus granulosus from Indian bubaline isolates, its stage specific expression and serological evaluation. Mol. Biol. Rep., 2011; 38: 2067-2073.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.