ISSN: 0973-7510
E-ISSN: 2581-690X
Urinary tract infections (UTIs) are most common infections encountered in both community and hospital settings and are frequently treated with antibiotics. Escherichia coli and Klebsiella pneumoniae, members of the Enterobacteriaceae family, are the predominant uropathogens. The rise of resistant uropathogens, that exhibit significant rates of antibiotic resistance, is making therapy more demanding. Increasing usage of carbapenem in complicated urinary infections is resulting in the Carbapenem-resistant escalation property in the gram-negative bacilli. This research was done to assess in vitro susceptibility of fosfomycin against both uropathogenic E. coli and K. pneumoniae. Over an interval of three months, 1070 urine samples were processed (January to March 2024). After an exclusion criteria, 1000 Specimens were inoculated on blood and Macconkey agar and incubated at 37 °C. Microbial growth and colony counts were noted. Speciation and antibiotic sensitivity pattern were analysed using Automated compact system of VITEK 2 (BioMerieux Inc., France). Fosfomycin discs were used to determine CRE and ESBL producers. Overall, 99.43% of E. coli isolates were susceptible to fosfomycin. E. coli that produce carbapenemase and ESBL had a fosfomycin susceptibility of 99.17% and 98.78%, respectively. The overall fosfomycin susceptibility of K. pneumoniae is 91.30%. Fosfomycin is effective against 91.6% and 87.5% of the carbapenem-resistant and ESBL-producing strains of K. pneumoniae, respectively. Although fosfomycin resistance is currently low, it may be a therapeutic alternative relative to other medications used for the management of UTIS caused by ESBL and carbapenem-resistant E. coli and K. pneumoniae. To preserve the efficacy of fosfomycin it must be used carefully following antimicrobial stewardship principles and guidelines.
E. coli, Klebsiella spp., Fosfomycin, ESBL, CRE
Urinary tract infections (UTIs) are among the most common infections encountered in both community and healthcare settings and frequently require antibiotic treatment.1 The most often reported agents are gram-negative bacteria belonging to the Enterobacteriaceae family, specifically Escherichia coli and Klebsiella pneumoniae.2 The uropathogens that produce Extended-spectrum beta-lactamases (ESBLs), shows significant antibiotic resistance, which makes therapy more complicated.3 Increased utilization of carbapenem drugs in complicated urinary tract infections leads to the development of carbapenem drug-resistance in gram-negative bacilli. Organisms that exhibit resistance for at least three antibiotic classes are referred to as multidrug-resistant organism (MDRO).4 Treating infections caused by ESBL-producing strains often necessitates alternative agents, including aminoglycosides, newer beta-lactam/beta-lactamase inhibitor combinations, carbapenems, fosfomycin, and agents like piperacillin/tazobactam or cefoperazone/tazobactam.
Fosfomycin, a broad-spectrum antibiotic is administered in the form of fosfomycin tromethamine, inhibits bacterial cell wall formation by interferes peptidoglycan synthesis for the treating of mild to severe infections of urinary tract caused by the organisms that are susceptible.5-8 Fosfomycin is a broad-spectrum antibiotic that is administered orally and excreted in the urine with high concentrations up to 24 hours, making it highly efficient against uropathogenic organisms.9,10 Due to its unique chemical structure, mechanism of action and low chromosomal mutations which do not spread rapidly, maintain a low fosfomycin resistance rate. All the systematic reviews and Meta analysis showed that single dosage of fosfomycin was effective as longer courses of alternate antibiotics agents for treating uncomplicated UTI.11
New antibiotics, antimicrobial resistance profiles must be updated on a regular basis, to guide appropriate antibiotic stewardship measures. This study set out to examine of fosfomycin in vitro susceptibility against both uropathogenic MDR E. coli and K. pneumoniae, as there haven’t been many studies done in this area. The objective was to evaluate the potential choice of drug for treating UTIs caused due to Uropathogenic E. coli (UPEC) and Klebsiella species, evaluating its clinical efficacy and suitability.
A retrospective research was performed within the Microbiology Department, Saveetha Medical College, Thandalam, Chennai, Tamil Nadu, India, over a period of 3 months from January 2024 to March 2024.
Inclusion criteria
Urine samples were collected from symptomatic persons. Single sample per subject was included in the study.
Exclusion criteria
Samples were excluded if the individuals were on antibiotic treatment, as well as those who underwent recurrent sampling.
Processing of urine samples
In the course of three months, 1070 urine samples were processed from January to March 2024. After applying the exclusion criteria, 70 samples were excluded-45 due to recent antibiotic use and 25 due to duplicate submissions-resulting in 1,000 samples included in the final analysis. These specimens were cultured onto routine culture media and held at 37 °C for a duration of 18 to 24 hours. After incubation, growth was evaluated. A pure bacterial growth culture with a colony count greater than 105 CFU/ml is considered significant bacteriuria. Standard microbiological techniques were employed to determine the growth of each sample.12
Detection and Characterization of urinary isolates
After acquiring the pure growth of bacterial culture, antibiotic susceptibility was further identified and determined by automated VITEK 2 compact system (BioMerieux SA). For Identification of isolates GN ID and AST cards were used, especially the AST405 card for fermenter bacteria and the AST 407 card for critical care patients. A quantitative measurement of growth was carried out using an optical reader.13 Simultaneously antimicrobial susceptibility test of these culture-positive isolates were determined by using the Kirby Bauer disc diffusion method by inoculating on MHA plate after adjusting the turbidity to 0.5 McFarlands.14 Fosfomycin disks (HiMedia Laboratories Pvt. Limited, India), 200 µg comprising 50 µg of glucose-6 phosphate are inserted. Incubated the culture Plates at 37 °C for 24 hrs. The zone of inhibition and its diameter were measured. E. coli ATCC 25922, ATCC 35218 and K. pneumoniae ATCC 13883 were served as a reference strains.
Out of the 1000 specimens, 222 (22.2%) non-duplicated uropathogenic Enterobacteriaceae were isolated. The uropathogenic multidrug-resistant organisms isolated were confirmed by VITEK 2 Advanced expert system (AES) as Phenotypes flagged ESBL, CRE producing phenotypes.15,16
Statistics
The fosfomycin susceptible uropathogenic organisms were calculated by using descriptive statistics.
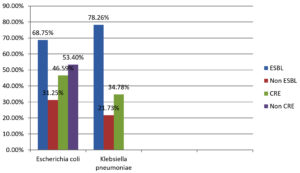
The study analyzed 1000 urine samples. Among these, 222 (20.74%) non-duplicated uropathogenic Enterobacteriaceae were isolated (Table 1). Out of these isolates, 176 (79.27%) were E. coli and 46 (20.72%) were Klebsiella species. Among the 176 E. coli isolates, 121 (68.75%) are ESBL producers, 55 (31.25%) are non-ESBL producers. Additionally, 82 (46.59%) were carbapenem-resistant E. coli (CRE), while 94 (53.4%) were non-carbapenem-resistant (non-CRE). Among the 46 isolates of Klebsiella species, 36 (78.26%) were found to produce ESBL, and 10 (21.73%) were non-ESBL producers. Furthermore, 16 (34.78%) were carbapenem-resistant strains (CRS), while 30 (65.21%) were non-carbapenem-resistant Klebsiella species, as illustrated in Figure 1.
Table (1):
Total number of Enterobacteriaceae isolates showing ESBL, Non-ESBL and CRE, Non-CRE producers
Organisms |
Total (n%) |
ESBL (n%) |
Non-ESBL (n%) |
CRE (n%) |
Non-CRE (n%) |
|---|---|---|---|---|---|
E. coli |
176 (79.2%) |
121 (68.75%) |
55 (31.25%) |
82 (46.59%) |
94 (53.40%) |
K. pneumoniae |
46 (20.7%) |
36 (78.26%) |
10 (21.73%) |
16 (34.78%) |
30 (65.21%) |
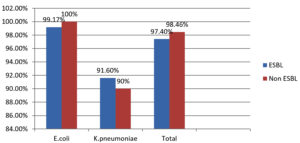
E. coli’s overall susceptibility to fosfomycin is 175 (99.4%). While 98.78% of bacteria are resistant to carbapenems. 99.17% of ESBL-producing E. coli are susceptible to fosfomycin (Table 2 and 3).
Table (2):
Fosfomycin susceptibility comparison in ESBL and Non-ESBL producers
Organism Fosfomycin susceptibility (n%) |
ESBL (n%) |
Non-ESBL producers (n%) |
|---|---|---|
E. coli (99.4%) |
120/121 (99.17%) |
55/55 (100%) |
Klebsiella pneumoniae (91.3%) |
33/36 (91.6%) |
9/10 (90%) |
Total |
153/157 (97.4%) |
64/65 (98.46%) |
Table (3):
Fosfomycin susceptibility among Carbapenem-resistant and Non-carbapenem-resistant Enterobacteriaceae
Organisms (n%) |
CRE (n%) |
Non-CRE (n%) |
|---|---|---|
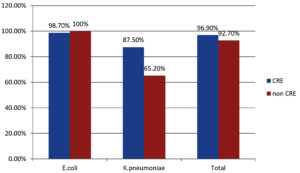
E. coli (99.4%). |
81/82 (98.7%) |
94/94 (100%) |
Klebsiella pneumoniae (93.4%) |
14/16 (87.5%) |
29/30 (65.2%) |
Total |
95/98 (96.9%) |
115/124 (92.7%) |
The overall susceptibility of fosfomycin among K. pneumoniae is 42 (91.30%) in ESBL-producing strains and 43 (93.4%) in carbapenem-resistant strains (CRE) of Klebsiella spp.
The ESBL-producing K. pneumoniae susceptible to fosfomycin was seen in 33 (91.6%), while among carbapenem-resistant strains, it was found in 14 (87.5%).
Significant differences were not found between susceptibility patterns of fosfomycin determined by the automated VITEK 2 compact system and manually used disc diffusion method (Figure 2 and 3).
The rise in drug-resistance in the uropathogenic organisms including K. pneumoniae and E. coli constitute a increasing risk to the effective management of UTIs. The production of extended-spectrum β-lactamases (ESBLs) and carbapenemases limit the therapeutic options available and contribute to treatment failures and disease recurrence.17
Drug fosfomycin originally known as phosphonomycin, this compound has wide range of antibacterial activity that was accidentally discovered in the fermentation broth Streptomyces fradiae in 1969.18 Fosfomycin inhibits the pyruvyl transferase enzyme, which is essential for producing the precursors of peptidoglycan. Because fosfomycin interferes with the formation of peptidoglycan, an important component of bacterial cell walls, the bacteria cannot maintain the integrity of their cell walls, ultimately resulting in their death.19
In our study, we identified 176 isolates of E. coli out of 222 isolates and 46 isolates of K. pneumoniae. Among the 176 samples in which E. coli was isolated, 121 were found to produce ESBL, demonstrating sensitivity to fosfomycin at a rate of 99.1%, while 55 were non-ESBL producers, showing susceptibility to fosfomycin at 100%. Among the 82 carbapenem-resistant E. coli, 81 showed sensitivity to fosfomycin, while the remaining 94 noncarbapenem-resistant E. coli exhibited sensitivity. Similarly, 36 ESBL-producing Klebsiella spp. showed susceptibility to fosfomycin, while 16 carbapenem-resistant Klebsiella spp. showed susceptibility to fosfomycin.
In the AREC (Antimicrobial Resistance Epidemiological Survey on Cystitis) research group, 98.1% of E. coli isolated from urine samples were susceptible to fosfomycin,20,21 which is quite similar to our data of 99.4%. Falgas et al. found that fosfomycin sensitivity was present in 97% of the ESBL-producing E. coli isolates.11 Similarly, in research by Sardar et al., all isolates showed uniform sensitivity to fosfomycin (100%). which mirrored our findings.21
A study from the Netherlands has shown that the susceptibility rates for fosfomycin were 95.9% and 87.6% for E. coli and K. pneumoniae.22 This includes overall vulnerability to ESBL and carbapenem-resistant strains of E. coli and K. pneumoniae.
Similar findings to ours were reported in India, Tulara et al.,23 where it was reported that 99.6% of 384 ESBL-producing E. coli strains and 87.7% of 80 ESBL-producing K. pneumoniae strains were fosfomycin-sensitive.
In a study by Dalai and Modak et al.,3 96.8% of E. coli and 91.4% of K. pneumoniae were found to be susceptible to fosfomycin, indicating our data exhibit higher fosfomycin susceptibility against ESBL and carbapenemase producing uropathogenic E. coli and K. pneumoniae.
Fosfomycin has significant effectiveness against ESBL- and carbapenem-resistant E. coli and K. pneumoniae, making it a good choice for empirical therapy of urinary tract infections. Its oral bioavailability and broad-spectrum efficacy support its use in outpatient settings. Integrating fosfomycin into local therapy protocols may reduce dependency on carbapenems. This helps preserve last-line antibiotics and slow resistance development. Overall, fosfomycin supports both effective therapy and antimicrobial stewardship goals.
Limitations
The importance of the study was done to find the in vitro susceptibility of fosfomycin to E. coli and K. pneumoniae only. The present study included limited number of isolates, and the results obtained may not be generalizable to other areas with different epidemiological antibiotic susceptibility patterns. Additionally, there was no MIC testing that might have yielded more accurate quantitative data about fosfomycin’s activity. The study is also not designed to address clinical outcome, the follow-up of which was not included, and it was not possible to determine any association between in vitro susceptibility and patient response. In addition, genotyping of the mechanisms of resistance including ESBL or carbapenemase genes was not performed and this precludes an interpretation of the molecular mechanisms which underpin resistance.
In conclusion, the study emphasizes the effectiveness of fosfomycin against uropathogenic ESBL-producers and carbapenem-resistant E. coli and K. pneumoniae. In comparison with other drugs, fosfomycin is a better therapeutic option for managing UTIs caused by ESBL and carbapenem-resistant K. pneumoniae, while current resistance to fosfomycin remains low. To preserve the efficacy of fosfomycin, it is crucial to ensure its prudent and appropriate use following antimicrobial stewardship principles and guidelines. Incorporating fosfomycin susceptibility tests into standard panels for multidrug-resistant (MDR) urinary tract infections (UTIs) may enhance the more precise and effective treatment recommendations.
ACKNOWLEDGMENTS
The authors thank the Department of Microbiology at Saveetha Medical College for providing the resources and facilities required for this study. We extend our gratitude to all the technical personnel who contributed to the collection and processing of the samples.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
The study protocol was reviewed and approved by the Institutional Ethics Committee, Saveetha Medical College, vide approval no.: SMC/IEC/2024/156.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Simsek M. Sensitivity of extended spectrum of b-lactamase producing E. coli and Klebsiella species to Fosfomycin. J Pak Med Assoc. 2020;70(7):1187-1192.
Crossref - Kang CI, Kim J, Park DW, et al. Clinical Practice Guidelines for the Antibiotic Treatment of Community-Acquired Urinary Tract Infections. Infect Chemother. 2018;50(1):67-100.
Crossref - Dalai S, Modak M, Lahiri K. Fosfomycin susceptibility among uropathogenic E. coli and K. pneumoniae. Int J Sci Res. 2019;8(1):282-284.
- Magiorakos AP, Srinivasan A, Carey RB, Wonacott A, Hutchinson SE, Kelly VA, Duncan K. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268-281.
Crossref - Skarzynski T, Mistry A, et al. Structure of UDP-N-acetylglucosamine enolpyruvyl transferase, an enzyme essential for the synthesis of bacterial peptidoglycan, complexed with substrate UDP-N-acetylglucosamine and the drug fosfomycin. Structure. 1996;4(12):1465-1474.
Crossref - Lu CL, Liu CY, Huang YT, et al. Antimicrobial susceptibilities of commonly encountered bacterial isolates to fosfomycin determined by agar dilution and disk diffusion methods. Antimicrob Agents Chemother. 2011;55(9):4295-4301.
Crossref - Barry AL, Brown SD. Antibacterial spectrum of fosfomycin trometamol. J Antimicrob Chemother. 1995;35(1):228-230.
Crossref - de Cueto M, Lopez L, Hernandez JR, Morillo C, Pascual A. In vitro activity of fosfomycin against extended-spectrum-b-lactamase-producing Escherichia coli and Klebsiella pneumoniae: comparison of susceptibility testing procedures. Antimicrob Agents Chemother. 2006;50(1):368-370.
Crossref - Marchese A, Gualco L, Debbia EA, Schito GC, Schito AM. In vitro activity of fosfomycin against gram-negative urinary pathogens and the biological cost of fosfomycin resistance. Int J Antimicrob Agents. 2003;22(Suppl 2):853-859.
Crossref - Nabin KS, Walton TJ. Fosfomycin: A review. Infect Dis Clin Pract. 2001;10(5):255-260.
Crossref - Falagas ME, Kastoris AC, Kapaskelis AM, Karageorgopoulos DE. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum b-lactamase producing, Enterobacteriaceae infections: A systematic review. Lancet Infect Dis. 2010;10(1):43-50.
Crossref - Bergey DH, Buchanan RE, Gibbons NE. Bergey’s manual of determinative bacteriology. Baltimore: Williams & Wilkins Co.; 1975;71-348.
Crossref - BioMerieux. VITEK 2 Compact Reference Manual. Ref. 414532.Published 2018. https://www.biomerieuxusa.com/sites/subsidiary_us/files/18-vitek2-systembrochure_v2.pdf
- Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. 32nd informational supplement. CLSI document M100-S32. Wayne, PA: CLSI; 2023.
- Young AL, Nicol MP, Moodley C, Bamford CM. The accuracy of extended-spectrum beta-lactamase detection in Escherichia coli and Klebsiella pneumoniae in South African laboratories using the Vitek 2 Gram-negative susceptibility card AST-N255. S Afr J Infect Dis. 2019;34(1):114.
Crossref - Govindaswamy A, Bajpai V, Khurana S, et al. Prevalence and characterization of beta-lactamase-producing Escherichia coli Isolates from a tertiary care Hospital. J Lab Physicians. 2019;11(2):123-127.
Crossref - Nicolle LE. Antimicrobial stewardship in long term care facilities: what is effective? Antimicrob Resist Infect Control. 2014;3(1):6.
Crossref - Hendlin D, Stapley EO, Jackson M, et al. Phosphonomycin. A new antibiotic produced by strains of Streptomyces. Science. 1969;166(3901):122-123.
Crossref - Gardiner BJ, Stewardson AJ, Abbott IJ, Peleg AY. Nitrofurantoin and fosfomycin for resistant urinary tract infections: old drugs for emerging problems. Aust Prescr. 2019;42(1):14-19.
Crossref - Schito GC, Naber KG, Botto H, et al. The ARESC study: An international survey on the antimicrobial resistance of pathogens involved in uncomplicated urinary tract infections. Int J Antimicrob Agents. 2009;34(5):407.
Crossref - Sardar A, Basireddy SR, Navaz A, Singh M, Kabra V. Comparative Evaluation of Fosfomycin Activity with other Antimicrobial Agents against E.coli Isolates from Urinary Tract Infections. J Clin Diagn Res. 2017;11(2):DC26-DC29.
Crossref - van den Bijllaardt W, Schijffelen MJ, Bosboom RW, et al. Susceptibility of ESBL Escherichia coli and Klebsiella pneumoniae to fosfomycin in the Netherlands and comparison of several testing methods including Etest, MIC test strip, Vitek2, Phoenix and disc diffusion. J Antimicrob Chemother. 2018;73(9):2380-2387.
Crossref - Tulara NK. Nitrofurantoin and Fosfomycin for Extended Spectrum Beta-lactamases Producing Escherichia coli and Klebsiella pneumoniae. J Glob Infect Dis. 2018;10(1):19-21.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.