ISSN: 0973-7510
E-ISSN: 2581-690X
Millions of people die every year as a result of infections’ multidrug resistance (MDR) pathogens. Therefore, the creation of novel antibiotics is urgently required for the treatment of bacterial illnesses that are resistant to a variety of drugs. The current study concentrated on the development of copper oxide nanoparticles conjugated with Vitamin-E (CuO NPs-Vit E complex), which was evaluated using various approaches like FTIR (Fourier transform infrared spectroscopy), UV-Vis (ultraviolet-visible spectroscopy), SEM (scanning electron microscopy), XRD (X-ray diffraction). The antibacterial and anti-biofilm activity was investigated against MDR Escherichia coli and Staphylococcus aureus. The antioxidant properties of CuO NPs-Vit E complex were determined using DPPH and FRAP assay. The CuO NPs-Vit E complex showed excellent anti-bacterial, anti-biofilm, and antioxidant activities. Moreover, it was found to be biocompatible and non-toxic to normal cells. Therefore, the synthesized CuO NPs-Vit E complex can be used for the creation of new drugs for the treatment of multidrug-resistant bacterial infections.
Anti-bacterial, Anti-biofilm, Antioxidant, Copper Nanoparticles, Multidrug Resistance, Vitamin-E
Multidrug resistance (MDR) among pathogens is a global threat. Millions of deaths occur every year and there is an urgent need for the creation of innovative antibiotics for the treatment of multidrug-resistant bacterial infections. Successful treatment is still significantly hampered by multi-drug resistance (MDR). Pathogenic bacteria are now posing a serious threat to worldwide public health due to their development of resistance to various antimicrobial drugs. Drug-resistant bacterial infections are a significant cause of patient mortality and morbidity, and developing antibiotic resistance is significantly harming the tremendous medical advances made feasible by antibiotics over the last 70 years.1 Antimicrobial resistance has a considerable impact on the efficacy of antimicrobial drugs and is linked with elevated rates of mortality and expensive medical care. By increasing the likelihood that resistant infections will spread, MDR causes blockage in the control of the disease. This results in decreased treatment effectiveness, which prolongs the patient’s infection period.2
Nanoparticles are of particular importance due to their incredibly small size and high surface-to-volume ratio, which results in chemical and physical changes in their characteristics. Metal nanoparticles have been garnering attention among researchers due to their applications in various fields. They can be combined with aqueous suspension and act as colloids because they are made of metals and metal oxides, such as titanium dioxide, metal oxide nanomaterial, cupric oxide, etc. Metal nanoparticles’ ability to interact closely with microbial membranes due to their small size and the high surface-to-volume ratio has been assigned as the cause of their bactericidal activity, rather than just the release of metal ions in solution.3 It is possible to immobilize and coat surfaces with metal nanoparticles that have bactericidal action, which may find use in a variety of industries. In order to prepare them for possible use in medicine and other biomedical applications, nanosized organic and inorganic nanoparticles are created.4 Nanoparticle biosynthesis, which uses non-toxic reactants and materials, has been hailed as a technology that is cost-effective, ecologically safe, energy-efficient, and economically sound.5
Copper is a naturally occurring element that permeates the entire environment. In the Earth’s crust, it is concentrated at 146, or 60 g per tonne. Copper-based nanoparticles are useful for a variety of applications, including water treatment, the textile industry, food preservation, and agricultural techniques. Copper nanoparticles have been shown in studies to exhibit antibacterial characteristics, and they hold great promise as a bactericidal agent.6 Different techniques have been used to create and characterize copper nanoparticles. The two main barriers to the use and advancement of the metal cluster in a new type of nanoelectronic device are stability and reactivity. Copper compounds (Cu), which can replace silver and composites made of other precious metals, have a significant potential for use as antibacterial agents. Copper oxide nanoparticles (CuO NPs) have gained interest primarily due to their antibacterial and biocidal capabilities and are potentially useful in a variety of biological applications.7
Vitamin E refers to eight fat-soluble substances that are produced by plant organisms as well as exhibited in varying amounts in foods high in fat, such as seeds and edible oils, or are mostly found in fortified foods such as -tocopherol. Vitamin E normally guards against oxidative damage brought on by copper. It is a lipid-soluble vitamin, and its most active form, -tocopherol, is a potent biological antioxidant.8 In biological systems, vitamin E may successfully reduce oxidative stress, lipid peroxidation, and the harmful effects of reactive oxygen species. Additionally, it has been demonstrated that vitamin E is useful in minimizing the genotoxic effects of a variety of genotoxic substances.9 The immunostimulatory properties of vitamin E have led to improved resistance to a number of diseases.10 Supplementation with vitamin E has been shown to improve humoral reactions. Both animals and people have shown increased antibody responses.11 The vitamin E increases the capacity of naive T cells to divide and produce IL-2, boosts the proportion of T cells that can successfully form immunological synapses, and corrects the age-related deficit.12 Numerous research on people has established the benefits of vitamin E on the occurrence of infectious illnesses naturally and immunostimulatory properties of vitamin E boost resistance to infections.13
Hence, the current study focused on the production of the copper oxide nanoparticles conjugated with Vitamin-E (CuO NPs-Vit E complex) and evaluated its anti-bacterial, anti-biofilm, and antioxidant capabilities. Additionally, the biocompatibility and cytotoxicity of synthesized CuO NPs-Vit E complex were also investigated.
Synthesis of Copper Oxide Nanoparticles
Two prepared solutions were used separately to create copper oxide nanoparticles. 14
Solution – 1
In 100 ml of distilled water, 0.27M copper sulphate pentahydrate was dissolved. To completely dissolve, the mixture was agitated in a magnetic stirrer at continuous temperatures and speeds (37°C and 200 rpm).
Solution – 2
About 1.2M sodium potassium tartrate and 3M sodium hydroxide was dissolved in 100ml of distilled water and stirring conditions were carried out using a magnetic stirrer at constant conditions (37°C and 200rpm).
5g of glucose was added after vigorously mixing 50 ml each of Solutions 1 and 2, respectively. The liquid was then agitated for 600rpm for 30 minutes and then heated to 60°C in a water bath. The resulting mixture is centrifuged, twice washed in distilled water, then twice washed in ethanol. The powdered material was air-dried and used for additional examination.
Preparation of CuO NPs-Vit E complex
The copper oxide nanoparticles were gradually added to a 20 ml magnetically agitated ethanol solution. Once the reaction reached equilibrium, Vitamin-E oil was carefully added to the first reaction mixture and agitated at a temperature of between 60 and 80°C. The reaction mixture was then placed in a fume closet to allow the solvent to evaporate, and the nano-metallic vitamin E complex was formed.
Synthesized Cuo NPs Characterization
UV-Vis (ultraviolet-visible) spectroscopy
In a spectrometer (Beckman-Model No. DU-50, Fullerton) with a resolution of 1 nm, UV-vis spectra between the wavelengths 190 and 800 nm were used to analyze the produced CuO NPs.
FTIR evaluation
Fourier-transform infrared (FTIR) spectroscopy was used to conduct the FTIR study for the created CuO NPs (SHIMADZU, IRSpirit). With a resolution of 16 cm-1, the transmittance spectra were captured in the wave number range of 4000 – 600 cm-1.
XRD studies
XRD is used to examine the structure and crystallinity of the synthesized CuO NPs using XPERT-PRO diffractometer instrument operating at 45kV. Using the Debye-Scherrer formula, the average crystallite size of CuONPs is calculated. Cu-NP size (in nm), λ =X-ray wavelength, β =complete width at half maximum of the diffraction peak, and θ = observed Bragg angle are all included in the formula D = 0.9l/bcosq.
Scanning Electron Microscopy (SEM)
CuO NPs and CuO NPs-Vit E complex are morphologically examined by Scanning electron microscopy (SEM) (Carl Zeiss, Germany). The scanning data were collected from an average of 47 scans with a resolution of 4 cm-1 and a scanning range of 4000-400 cm-1. It is constrained in certain morphological research since it yields scant data on the true population and dispersion of average size.
Anti-Biotic Sensitivity Testing (Abst) For The MDR Pathogens
Using the dehydrated medium, prepare MHA as directed by the manufacturer. Distilled or deionized water was used to prepare the media. The medium was boiled and heated while being stirred frequently and sterilized at 121°C for 15 minutes. The agar medium was allowed to cool and poured into the sterile petri dish.
A sterile cotton swab should be dipped into the predetermined bacterial suspension of MDR Staphylococcus aureus and MDR Escherichia coli and streaked across the agar plates with the inoculum-containing swab. The streaking process was repeated after rotating the plate by 60 degrees. The antibiotic disc such as Amoxicillin, Ampicillin, Streptomycin, Ciprofloxacin, and Tetracycline was placed on the inoculated and dried plate’s surface using the disc dispenser and lightly press down with the tool to make sure the disc is completely in touch with the agar surface. The zone of inhibition that resulted from overnight incubation of plates at 37°C was measured and recorded.15
Antibacterial Activity
The antibacterial activity of CuO NPs and the CuO NPs-Vit E combination against MDR Pathogens (Escherichia coli and Staphylococcus aureus) was evaluated using the well diffusion method. Bacterial inoculums from cultures that have been growing for 1-3 days were seeded onto petri plates with 20 ml of Muller-Hinton agar medium. CuO NPs and CuO NPs-Vit E complex were introduced at various concentrations (0.125 g, 0.25 g, 0.5 g, and 1 g) and incubated for 24-48 hours at 37°C in wells that were 6 mm in diameter. The experiment was carried out in duplicate, and the diameter of the resulting zone of inhibition surrounding the well was determined in millimeters.15
Minimum Inhibitory Concentration (MIC)
The procedure outlined in the CLSI (2012)16 guideline was used to determine the MIC of the synthesized CuO NPs-Vit E complex. Using the Resazurin method, the MIC test was carried out in a 96-well round bottom microtiter plate.
The concentration of the bacterial inoculums was maintained at 106 CFU/mL. In order to perform the MIC test, 100 µL of the test sample solution (500 µg/mL) was added and diluted twice with 100 µL of MHB containing bacterial inoculums from columns 1 to 12. The test samples were concentrated mostly in column 1 of the microtiter plate and least in column 12 of the plate. Row 2 functioned as the positive control, while Row 1 was the negative control (only medium). 30 µL of the resazurin solution (0.02% (wt/vol)) was added to each well of the microtiter plate, which was then incubated for 24 hours at 37°C. Any colour variations were noticed and the colour shift from purple to pink indicates the existence of living cells. The lowest concentration at which a color change could be seen was identified as the minimum inhibitory concentration (MIC) value.
Evaluation of Formation and Inhibition of Bacterial Biofilm
Mueller-Hinton broth was used to grow Staphylococcus aureus and Escherichia coli, and the adherence assay on 96-well tissue culture was used to examine the bacteria’s capacity to produce biofilm. Each well of the microtiter plate was filled with 100µl overnight bacterial solution, then the 1 MIC and 2 MIC concentrations of CuO NPs-Vit E complex were added. Each strain was examined in triplicate. Escherichia coli and Staphylococcus aureus served as the positive control, while wells with sterile Mueller-Hinton broth served as the negative controls. At 37°C, the plates were incubated for 24 hours.
Following the incubation, the culture was removed, the plates were thoroughly washed three times using phosphate-buffered saline (200ml), and they were dried inverted to get rid of non-adherent cells. The adhering biofilm was fixed with ethanol (95%) and stained for five minutes with crystal violet (1%). The unbound crystal violet was then removed from the wells using sterile distilled water, and the plates were air-dried for 30 minutes. For each well, the optical density was calculated at a wavelength of 570 nm.17
Antioxidant Activity
DPPH Method
The DPPH assay was performed by the method suggested by Formagio et al.18 3ml of the methanolic DPPH reagent (0.002% w/v) was combined with different concentrations of the CuO NPs-Vit E complex. A spectrophotometer was used to measure the absorbance at 517 nm following the incubation at room temperature in the dark for 30 min. Ascorbic acid served as the standard.
Inhibition (%) = (Absorbance of Control – Absorbance of Sample)/ (Absorbance of Control) × 100
FRAP ASSAY
The ferric-reducing antioxidant power (FRAP) was assessed using the Benzie and Strain, (1996) method.19 A 300 mM sodium acetate buffer, 10 mM 2,4,6-tri-(2-pyridyl)-5-triazine (TPTZ), and a 20 mM FeCl3•6H2O solution make up the FRAP solution. Various aliquots of CuO NPs-Vit E complex were added to 900 µl of FRAP solution, which was then incubated for 30 minutes at room temperature and in the dark. The absorbance was then spectrophotometrically determined at 593 nm.
RBC Hemolysis Assay
Healthy volunteers’ blood was drawn and centrifuged for five minutes at a speed of 5000 rpm. The blood pellet was subsequently washed three times in a sterile pH 7.2 phosphate buffer saline solution.20
The RBC suspension was combined with 1X and 2X MIC of CuO NPs-Vit E complex and the reaction mixture was then incubated for 60 min at 37°C, followed by centrifugation for 10 minutes at 1500 rpm. A UV-Vis spectrophotometer was used to determine the optical density of the resulting supernatant at a wavelength of 540 nm. 0.1% (v/v) Triton X-100 was used as a positive control, and PBS was utilized as a negative control.21
Cytotoxicity (MTT Assay)
The L929 (normal fibroblast) cell line, which was bought from the National Centre for Cell Sciences (NCCS), situated in Pune, India, was utilized in the MTT experiment to examine the cytotoxicity of CuO NPs-Vit E complex. Fetal bovine serum was added to MEM medium in which the cell line was grown and incubated with 5% CO2 at 37°C. A freshly made growth medium containing various aliquots of CuO NPs-Vit E complex was used after the initial 24-hour incubation period. Following that, 10 µl of MTT (5 mg/mL in PBS) was added to each well, and the plates were left incubating for an additional 4 hours. The intractable formazan crystals were dissolved by thoroughly combining 200 µl of DMSO with the supernatant and the optical density was measured at 550nm.22
The percentages of viability (%V) and inhibition (%I) were calculated using the following formula:
Percentage of viability (%V) =100 (At /Ac)
Percentage of inhibition (%I) =100 [1-(At /Ac)]
Where, At – absorbance of treated cells; Ac – absorbance of control cells
LDH ASSAY
Using a cytotoxicity detection kit, the activity of the lactate dehydrogenase (LDH) produced from the wounded cells was used to gauge the cytotoxicity of the CuO NPs-Vit E complex on the L929 mouse fibroblast cell line (Sigma Aldrich Inc., USA). The procedure recommended by the manufacturer was followed during the trial. Briefly, 100 µl of the reaction mixture was added to the same volume of untreated and treated cells’ MEM. Supplemented with fetal bovine serum media. The plates were kept for 30 minutes at 25°C in the dark. The release of LDH activity was measured by using the optical density value read at 490 nm.23
Then, the L929 cells treated with different concentrations of CuO NPs-Vit E complex were evaluated by acridine orange staining.24
SYNTHESIS OF CuO NPs
The formation of CuO NPs were visually confirmed by the colour shift occurred in the solution mixture (Figure 1). The pH of the synthesized nanoparticles was found to be 8.32. The total yield of nanoparticles were 1.5g/ml (21.73%). The Figure 2 shows the formation of CuO NPs-Vit E complex.
Characterization Of Synthesized CuO NPs
Ultraviolet-Visible (UV-VIS) Spectroscopy
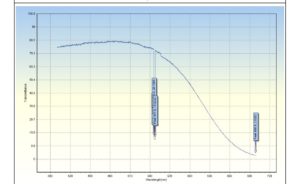
UV-Vis spectroscopy was used to analyze the CuO nanoparticles. The UV–vis absorption peak at 550 nm is attributed to the copper oxide nanoparticles (Figure 3). This shows the successful synthesis of the nanoparticles. The copper and oxygen peaks in the spectrum were the greatest, indicating that the copper oxide nanoparticles created were extremely pure.
FTIR Analysis
The synthesized copper oxide NPs’ FTIR measurements are shown in Figure 4, which aids in finding potential biomolecules that function as reducing and capping agents. The FTIR band at wavelength 416 cm-1 corresponds to the metal ligand, which indicated the presence of copper. The peaks at 679 cm-1 correspond to Cu (I)–O vibration of copper-oxide nanoparticles. The absorption peak at 555.50 cm-1 corresponds to the halogenated compounds, and it might be stretching of Cu-O. The existence of the C-O stretching group was indicated by the absorption band at 1203.58 cm-1. The bands at 1481.53, 1643.35, and 3309.85cm-1 correspond to the CH2 bend, mono-substituted alkene (C=C stretching), and aliphatic primary amine (N-H stretching), respectively.
Pos. [°2Th.] Height [cts] FWHM Left [°2Th.] d-spacing [Å] Rel. Int. [%]
36.8613 101.37 0.2040 2.43645 100.00
Figure 5. XRD analysis
XRD Analysis
The XRD results of CuO NPs showed the peak positions (Figure 5) with 2ט values of, 35.6°, 38.7°, 66.4°, 68.1°, 72.5°, and 75.1° can be assigned to the planes (002), (111), (202), (113), (311) and (400) which corresponds to the copper-oxide (JCPDS card no 45-0937). The peak at 2θ value of 36.86° represented the crystallinity of the synthesized CuO NPs. The presence of significant line stretching of the diffraction peaks demonstrates that the synthesised materials are in the nanometre range. The average particle size of the synthesized nanoparticles was calculated using Scherrer’s equation from the FWHM (full width at half maximum) of the diffraction peaks. The crystalline size of the CuO NPs was found to be 42.89nm.
SEM Analysis
The surface morphology of the CuO NP and CuO NPs-Vit E complex was observed using scanning electron microscopy. The micrograph of CuO NPs (Figure 6) showed the presence of nanoparticles with more or less uniform circular or spherical shaped but varying in sizes due to the complex formation and also no aggregation is seen. The SEM images (Figure 7) of the CuO NPs-Vit E complex revealed the agglomeration of particles, which evidenced the binding of Vitamin E to the CuO NPs. The CuO NPs-Vit E complex was also found to be in spherical shaped.
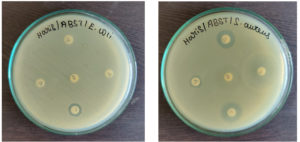
Antibiotic Sensitivity Testing
This test was done by using standard antibiotics like amoxicillin, ampicillin, tetracycline, streptomycin, and ciprofloxacin against both the MDR pathogens such as Staphylococcus aureus and Escherichia coli (Figure 8). The diameter of the inhibition zone reflects the magnitude of the susceptibility of microbes. No zone of inhibition was observed around the amoxicillin, ampicillin, ciprofloxacin, and tetracycline in the MDR Escherichia coli seeded plate. But, streptomycin inhibited the growth of Escherichia coli slightly with an 8mm inhibitory zone. For Staphylococcus aureus, the zones for Amoxicillin were 13 mm, Ampicillin was 7 mm, Streptomycin was 12 mm, and Ciproflaxin was 14 mm, whereas, the Tetracycline had no zones (Table 1). Hence, considering the size of the zone of inhibition and comparing it with the standard antibiotic chart both the isolated pathogens were observed to be multi-drug resistant.
Table (1):
ABST against MDR pathogens (Escherichia coli and Staphylococcus aureus).
Organism |
Amoxicillin |
Ampicillin |
Streptomycin |
Ciprofloxacin |
Tetracycline |
|---|---|---|---|---|---|
Escherichia coli |
– Resistant |
– Resistant |
8 Resistant |
– Resistant |
– Resistant |
Staphylococcus aureus |
13 Resistant |
7 Resistant |
12 Resistant |
14 Resistant |
– Resistant |
Anti-bacterial Activity
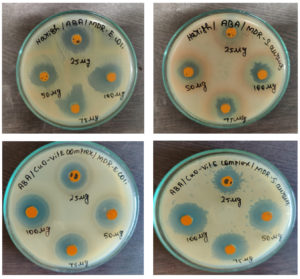
Both the MDR pathogens Escherichia coli and Staphylococcus aureus were effectively inhibited by all concentrations of synthesized CuO NPs and the CuO NPs-Vit E complex
(Figure 9). Table 2 displays the findings from the study of the antibacterial activity using the well diffusion method. By measuring the zone of inhibition against each of the tested bacterial strains, the antibacterial activity was ascertained. Four different concentrations underwent this testing. For Escherichia coli, the zone range for the CuO NPs is 16 mm for 25 mg, 19 mm for 50 mg, and 23 mm for 75 mg, but Staphylococcus aureus has no zone for 25 mg, 12 mm for 50 mg, 15 mm for 75 mg, and 18 mm for 100 mg. The zone range of the Cu NPs + Vit E complex is 18 mm for 25 mg, 22 mm for 50 mg, 27 mm for 75 mg, and 31 mm for 100 mg for Escherichia coli, but 15 mm for 25 mg, 18 mm for 50 mg, 24 mm for 75 mg, and 29 mm for 100 mg for Staphylococcus aureus. CuO NPs had a maximum zone of inhibition of 28 mm for Escherichia coli at a concentration of 100 mg, whereas the CuO NPs-Vit E complex had a maximum zone of inhibition of 31 mm for Escherichia coli at the same concentration. CuO NPs were shown to have a maximum zone of inhibition for Staphylococcus aureus of 18 mm for 100 mg, while the CuO NPs- Vitamin E complex had a maximum zone of inhibition of 29 mm for the 100g. The maximum zone of inhibition was recorded for the CuO NPs + Vit E complex than the CuO Nps. Hence, the anti-bacterial activity of the CuO NPs was enhanced by the combination of Vitamin E.
Table (2):
Antibacterial activity of CuO NPs-Vit E complex against MDR pathogens – Escherichia coli and Staphylococcus aureus
| Microorganisms | Zone of Inhibition (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| CuO NPs | CuO NPs-Vit E complex | |||||||
| 25µg | 50µg | 70µg | 100µg | 25µg | 50µg | 75µg | 100µg | |
| Escherichia coli | 16 | 19 | 23 | 18 | 18 | 22 | 27 | 31 |
| Staphylococcus aureus | – | 12 | 15 | 15 | 15 | 18 | 24 | 29 |
Figure 9. Antibacterial activity of CuO NPs-Vit E complex against MDR pathogens – Escherichia coli and Staphylococcus aureus
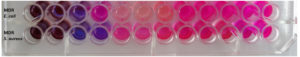
Minimum Inhibitory Concentration (MIC)
The Minimum Inhibitory Concentration (MIC) of the synthesized CuO NPs-Vit E complex was found to be 25 and 12.5 µg/ml for Escherichia coli, and Staphylococcus aureus respectively (Table 3). Hence, the Staphylococcus aureus was more susceptible to the CuO NPs – Vit E complex than the Escherichia coli. Figure 10 represents MIC of the CuO NPs-Vit E complex against the test organisms.
Table (3):
MIC
Microorganisms |
MIC (µg/ml) |
|---|---|
MDR Escherichia coli |
25 |
MDR Staphylococcus aureus |
12.5 |
Evaluation of Formation and Inhibition of Bacterial Biofilm
Biofilm Formation
The optical Density Value (Biofilm growth) of the treatment CuO NPs-Vit E complex against MDR Escherichia coli and MDR Staphylococcus aureus was provided in Table 4. Both tested bacterial strains such as MDR Staphylococcus aureus and MDR Escherichia coli showed the considerable formation of biofilm with OD values of 0.28±0.024 and 0.28±0.07, respectively.
Table (4):
Bio-film formation of MDR Staphylococcus aureus, and MDR Escherichia coli (OD values)
| Organism | OD value | |||
|---|---|---|---|---|
| 1st | 2nd | 3rd | Average | |
| MDR Staphylococcus aureus | 0.26 | 0.32 | 0.28 | 0.28±0.024 |
| MDR Escherichia coli | 0.39 | 0.23 | 0.24 | 0.28±0.07 |
Biofilm Inhibition
All the tested concentrations such as 1X MIC, and 2X MIC of CuO NPs-Vit E complex inhibited the biofilm formation of MDR Staphylococcus aureus and MDR Escherichia coli (Table 5). The absorbance of 1X concentration of CuO NPs-Vit E complex was found to be 0.14 for MDR Staphylococcus aureus and 0.19 for MDR Escherichia coli. The 2X MIC of CuO NPs – Vit E complex effectively inhibited the biofilm formation with OD values of 0.05 and 0.07 for MDR Staphylococcus aureus and MDR Escherichia coli, respectively.
Table (5):
Anti-biofilm activity (OD values)
| Microorganisms | OD value | |
|---|---|---|
| 1*mic | 2*mic | |
| MDR Staphylococcus aureus | 0.14 | 0.05 |
| MDR Escherichia coli | 0.19 | 0.07 |
Antioxidant Activity
DPPH Method
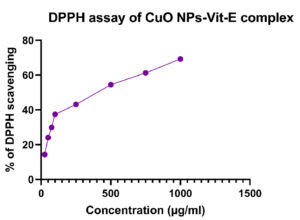
The synthesized CuO NPs-Vit E complex demonstrated concentration-dependent DPPH radical scavenging ability (Figure 11). The DPPH scavenging activity is shown to be 14.31% at 25 µg, 24.06% at 50 µg, 29.89% at 75 µg, 37.41% at 100 µg, 43.07% at 250 µg, 54.46% at 500 µg, 61.21% at 750 µg, and 69.22% at 1000 µg (Table 6). The highest amount of scavenging activity was achieved at 1000 µg/ml of the synthesized complex, while the lowest amount was 14.31% at 25 µg/ml. The IC50 value was determined as 514.68 µg/ml.
Table (6):
DPPH assay of CuO NPs-Vit E complex
Concentration (µg/ml) |
DPPH (%) |
|---|---|
25 |
14.31 |
50 |
24.06 |
75 |
29.89 |
100 |
37.41 |
250 |
43.07 |
500 |
54.46 |
750 |
61.21 |
1000 |
69.22 |
IC50 |
514.68 µg/ml |
FRAP ASSAY
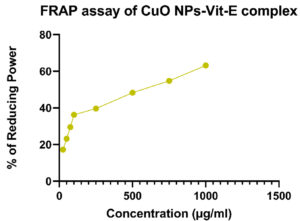
Ferric Reducing Antioxidant Power (FRAP) values of synthesized CuO NPs-Vit E complex were depicted in Table 7. The data showed the maximum FRAP value of synthesized CuO NPs-Vit E complex was 63.18% for 1000µg/ml. The scavenging activity of FRAP against various concentrations, such as 25 µg, 50 µg, 75 µg, 100 µg, and 250 µg, 750 µg revealed the FRAP value of 17.22, 23.14, 29.51, 36.20, 39.66, 48.29, and 54.79% respectively. The IC50 value was found to be 614.72 µg/ml. The Figure 12 shows the FRAP assay carried out for CuO NPs-Vit E complex.
Table (7):
FRAP assay of CuO NPs-Vit E complex
Concentration (µg/ml) |
FRAP (%) |
|---|---|
25 |
17.22 |
50 |
23.14 |
75 |
29.51 |
100 |
36.20 |
250 |
39.66 |
500 |
48.29 |
750 |
54.79 |
1000 |
63.18 |
IC50 |
614.72 µg/ml |
RBC Hemolysis Assay
The result for 1X MIC shows 13.86% and for 2X MIC reveals 28.73% (Table 8). Hence, all of the evaluated concentrations of CuO NPs-Vit E complex are regarded as safe and non-toxic for humans because the maximum concentration (2X MIC) was less than 30% hemolysis activity.
Table (8):
RBC hemolysis of CuO NPs-Vit E complex
Concentration |
Percentage of hemolysis inhibition |
|---|---|
1X MIC |
13.86% |
2X MIC |
28.73% |
Cytotoxicity Activity (MTT Assay)
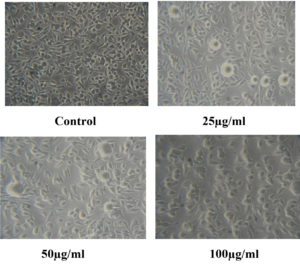
The cytotoxic activity of CuO NPs-Vit E complex against the L929 cell line was investigated using the MTT assay (Figure 13). The treatment of different concentrations of Cuo NPs-Vit E complex does not affect the cell viability of the L929 cells. The maximum cytotoxicity obtained was 19% at the concentration of 100 µg/ml (Table 9). Hence, the CuO NPs-Vit E complex had no significant cytotoxic impacts on L929 cells.
Table (9):
MTT assay
Concentration (µg) |
Cytotoxicity (%) |
Cell Viability (%) |
Cytotoxic Reactivity |
|---|---|---|---|
Control |
0 |
100 |
Good |
25 |
8 |
92 |
Mild |
50 |
13 |
87 |
Mild |
100 |
19 |
81 |
Mild |
LDH Assay
LDH assay is used to evaluate the release of lactate dehydrogenase released upon the death of the cells. Increasing concentrations increased the LDH and apoptosis of the cells (Figure 14). 100µg showed 64% LDH release, 58% at 75µg, 45% at 50µg, and 26% at 25µg (Table 10). Similarly, the fluorescent dye stained viable cells were found to be higher at the lower concentrations and only mild reduction in cells was observed at higher concentrations.
Table (10):
LDH ASSAY
Concentration (µg/ml) |
Standard (%) |
Sample (%) |
|---|---|---|
25 |
8 |
26 |
50 |
21 |
45 |
75 |
41 |
58 |
100 |
56 |
64 |
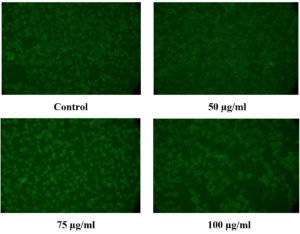
The existence of live cells could be seen in Figure 15, cells stained with acridine orange after being exposed to various concentrations of the CuO NPs-Vit E complex. The green cells are alive cells stained with acridine orange (Fluorescent dye). This result correlated with the MTT and the fluorescent staining (apoptosis). This shows that the developed CuO NPs-Vit E complex is safe and biocompatible.
Particles with a size between 1 and 100 nm are referred to as nanoparticles (NPs). They can be combined with an aqueous suspension and act as colloids because they are made of metals and metal oxides, such as titanium dioxide, metal oxide nanomaterials, cupric oxide, etc. It is possible to immobilize and coat surfaces with metal nanoparticles that have bactericidal action, which may find use in a variety of industries. As a result, nanosized organic and inorganic nanoparticles are created in order to be used in biomedical applications and maybe in medicine. Everywhere in the environment, copper is a naturally occurring element. Studies on copper nanoparticles’ antibacterial characteristics demonstrate that these particles hold great promise as bactericidal agents. Metal oxide nanoparticles, including copper oxide (CuO), have attracted attention for their antibacterial and biocidal properties, and they could be used in a variety of biological applications. Vitamin E normally guards against oxidative damage caused by copper.7 It is a lipid-soluble vitamin, and its most active form, tocopherol, is a potent biological antioxidant. MDR is an inevitable natural occurrence that poses a severe global threat to human health. To tackle the MDR, a global cooperation effort is necessary. Pathogens frequently use a variety of resistance mechanisms to endure harsh circumstances.
The current study evaluated the ability of synthesized CuO NPs-Vit E complex to suppress the growth of the MDR pathogens Escherichia coli and Staphylococcus aureus.
The surface vibration of valence electrons in the metal nanoparticles causes them to absorb visible electromagnetic radiation. This phenomenon is termed as surface plasmon resonance effect. The idea of exploiting this phenomenon as a tracer for the presence of metal nanoparticles is possible with a basic UV-visible spectrophotometer.25 The production of copper oxide NPS is indicated by a peak at 550nm in the UV-visible spectrum. Likewise, the Cu-nanoparticles exhibited an absorption maxima at 562 nm, which corresponded to the typical isoionic spectral peak.26
Rajeshkumar and Rinitha reported27 that the diffraction peaks for copper were found to be at 2θ = 24°, 30°, and 42°, which correlate to (111), (111), and (200), respectively, which indicated the formation of Copper nanoparticles. Likewise, Peaks at 2 values of 43.39°, 50.49°, and 74.18° are associated with the (111), (200), and (220) metallic Cu planes, respectively. Other diffraction peaks, in addition to the metallic Cu peaks, were observed at 29.63°, 36.54°, 42.44°, 61.57°, 73.58°, and 77.49°, respectively, corresponding to the (110), (111), (200), (220), (311) and (222) planes of cuprite. These peaks represent the emergence of cubic copper (I) oxide nanocrystals. 28
The work of Shiny et al.29 confirmed the existence of CuO nanoparticles and it was found to be spherically shaped. Likewise, the cubic-shaped CuO nanoparticles were examined using SEM,28 which showed similarity to our findings. It is further validated by FTIR, which reveals a band that is consistent with the Cu-O bond. The primary peak was observed to be 576 cm-1, which indicates that Cu-O should be stretched.30 In the current study, the Cu-O stretching band was observed at 555.50 cm-1. The FTIR peak appearances at many wavenumber bands, including the 3400 cm-1, 1630–1761, 1142–1345, and 724–928 cm-1 were reported. N-CH3 stretching vibration was shown to have peaks at a bandwidth of 900–1000 cm-1. The absorption bands at 471 cm-1 in the spectra indicate the occurrence of metal-oxygen. 31 The FTIR spectra of synthesized CuO NPs showed peaks in the 400–4000 cm-1 range that can be related to the vibrations of metal-oxygen (M=Cu), further demonstrating that the CuO NPs formed in this manner. 2
The copper oxide nanoparticles exhibit bactericidal action against gram-positive and gram-negative bacterial species.32 The bactericidal action of synthesized CuO NPs was consistent and showed potential for usage in biomedical applications.33 Escherichia coli and Staphylococcus aureus were two bacterial strains against which the antimicrobial activity of CuO NPs was examined. The antibacterial efficacy of CuO NPs against Staphylococcus aureus and Escherichia coli was demonstrated in a well-diffusion assay. The Copper nanoparticles have a distinctively high surface-to-volume ratio allowing them to engage with the surface of a bacterium’s cell membrane, killing the bacterium as a result.34 Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, and Proteus vulgaris were employed as the four different bacterium species to test the antibacterial efficacy of CuO NPs. The findings showed that Escherichia coli had a larger inhibition zone (26.0±1.00 mm), making it more sensitive to CuO NPs than other bacterial species at the highest dose tested (1000 µg mL).35 The contact-killing process of copper oxides (CuO and Cu2O) in which bacteria initially experience significant cell membrane destruction. Then additional damage occurs via a separate channel used by each copper oxide nanoparticle. The main factors of the toxicity for Escherichia coli were found to be the production of free radicals from CuO (cupric oxide) and the formation of copper (I)-peptide combination from Cu2O (cuprous oxide).36
Cuo NPs-Vit E complex was studied for its effects on bacterial biofilm growth of Escherichia coli, and Staphylococcus aureus. The fact that Cuo NPs-Vit E complex has an impact on bio lm formation is mostly due to the size of their nanoparticles, as smaller particles have a bigger surface area for contact with pathogens than the bacterial control. Copper ions have the power to restrict bacterial formation and growth by rupturing the bacterial cell wall or cell membrane. They will react with them once inside the cell due to their propensity for phosphorus and sulfur-rich compounds like DNA.37 The ability of Cu2+ ions released by CuO NPs, which completely submerge the bacterial cell surface and harm cells by modifying the arrangement of proteins and enzyme properties, has been linked to anti-biofilm actions.38
DPPH has been widely utilized as a stable free radical to examine the reducing compounds and as a suitable solution for studying the free radical scavenging capability.39 The current study revealed the maximum DPPH radical scavenging of Cuo NPs-Vit E complex was 69.22% at 1000 µg. The present study employed the Ferric-reducing antioxidant power test to evaluate the antioxidant effects of the synthesized copper nanoparticles complexed with vitamin E. This assay relies on the existence of antioxidants in the sample to convert the ferricyanide complex into the ferrous form 19. The FRAP value was identified as 63.18% for 1000µg/ml.
The rupture or bursting of RBC during hemolysis indicates that it possesses cytotoxic effects. If there is more than 30% hemolysis, the test samples are thought to be toxic to erythrocytes. 40 The current study revealed 28.73% at the 2X MIC concentration, which was considered to be safe.
The cytotoxic activity of the Cuo NPs-Vit E complex was tested using a healthy normal mouse fibroblasts cell line (L929) to assess the biocompatibility of the synthesized Cuo NPs-Vit E complex. The cell viability was observed to be 81% at the concentration of 100µg/ml, as the absorbance value did not reduce even at the increased concentration of CuO NPs- Vit E complex, which showed similarities to the finding of Jahan et al.41 However, the CuO NPs – Vit E complex demonstrated their significant bactericidal efficacy against two MDR bacterial strains such as Escherichia coli and Staphylococcus aureus at the same concentration (100 µg/mL). The release of LDH was used to assess the extent of damage in the L929 fibroblasts cell line, which is taken as an indicator of the disruption of the cell membrane. The results of the LDH assay showed 64% in CuO NPs – Vit E complex, which is comparably higher than the standard used (56%).
Hence, the CuO NPs-Vit E complex had potent anti-bacterial, anti-biofilm, and anti-oxidant activity and also it was found to be safe, biocompatible, and non-toxic.
From the present study, the synthesized Cuo NPs-Vit E complex was characterized by UV Vis, FTIR, XRD, and SEM analysis. The significant antibacterial and anti-biofilm activity against MDR Staphylococcus aureus and Escherichia coli. Moreover, the antioxidant effects of Cuo NPs-Vit E complex were proved in terms of Ferric ion-reducing antioxidant power and DPPH radical scavenging activity. This study demonstrated that the pharmacological properties were enhanced and the toxicity was reduced for the Cuo NPs upon the conjugation with Vitamin E. Therefore, the developed Cuo NPs-Vit E complex can be used for the creation of new drugs for the treatment of multidrug-resistant bacterial infections. Additionally, the non-toxicity of the synthesized Cuo NPs-Vit E complex on healthy mouse cells indicates their potential for usage in a wide range of sectors, including agriculture, medicine, and biomedical disciplines.
ACKNOWLEDGMENTS
The authors would like to thank the Department of Microbiology, Vivekanandha College of Arts and Sciences for Women (Autonomous), Elayampalayam, Namakkal (DT), Tamilnadu, India, for their guidance and support in completing this article.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Payne DJ. Desperately seeking new antibiotics. Science. 2008;321(5896):1644-165.
Crossref - Tanwar J, Das S, Fatima Z, Hameed S. Multidrug resistance: an emerging crisis. Interdiscip Perspect Infect Dis. 2014;541340.
Crossref - Azam A, Ahmed AS, Oves M, Khan MS, Habib SS, Memic A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int J Nanomedicine. 2012;5:6003-6009.
Crossref - Mahapatra O, Bhagat M, Gopalakrishnan C, Arunachalam KD. Ultrafine dispersed CuO nanoparticles and their antibacterial activity. J Exp Nanosci. 2008;3(3):185-193.
Crossref - Tran N, Mir A, Mallik D, Sinha A, Nayar S, Webster TJ. Bactericidal effect of iron oxide nanoparticles on Staphylococcus aureus. Int J Nanomed. 2010;15:277-283.
Crossref - Bibi I, Nazar N, Iqbal M, et al. Green and eco-friendly synthesis of cobalt-oxide nanoparticle: Characterization and photo-catalytic activity. Advanced Powder Technology. 2017;28(9):2035-2043.
Crossref - Akhter SM, Mohammad F, Ahmad S. Terminalia belerica mediated green synthesis of nanoparticles of copper, iron and zinc metal oxides as the alternate antibacterial agents against some common pathogens. BioNanoScience. 2019; 15(9):365-372.
Crossref - Gaetke LM, Chow CK. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. 2003;189(1-2):147-63.
Crossref - Sundl I, Murkovic M, Bandoniene D, Winklhofer-Roob BM. Vitamin E content of foods: comparison of results obtained from food composition tables and HPLC analysis. Clin Nutr. 2007;26(1):145-153.
Crossref - Radhakrishnan AK, Lee AL, Wong PF, Kaur J, Aung H, Nesaretnam K. Daily supplementation of tocotrienol-rich fraction or a-tocopherol did not induce immunomodulatory changes in healthy human volunteers. British J Nutr. 2009;101(6):810-815.
Crossref - Meydani SN, Meydani M, Blumberg JB, et al. Vitamin E supplementation and in vivo immune response in healthy elderly subjects: a randomized controlled trial. JAMA. 1997;277(17):1380-1386.
Crossref - Adolfsson O, Huber BT, Meydani SN. Vitamin E-enhanced IL-2 production in old mice: naive but not memory T cells show increased cell division cycling and IL-2-producing capacity. J Immunol. 2001;167(7):3809-3817.
Crossref - Dalia AM, Loh TC, Sazili AQ, Jahromi MF, Samsudin AA. Effects of vitamin E, inorganic selenium, bacterial organic selenium, and their combinations on immunity response in broiler chickens. BMC Vet Res. 2018;14(1):1-10.
Crossref - Singh M, Kaur P, Sandhir R, Kiran R. Protective effects of vitamin E against atrazine-induced genotoxicity in rats. Mutat Res. 2008;654(2):145-149.
Crossref - Jayasree KV, Neelakanndeswari K, Elayarajah B, Rajesh R. Synthesis and Characterization Of Copper Oxide Nanoparticles Against Dental Implant-Associated Infections. Int J Appl Eng Res. 2015;10(2):2895-2908.
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement. Wayne, PA: The Clinical and Laboratory Standards Institute. 2012.
- Noumi E, Snoussi M, Merghni A, et ak. Phytochemical composition, anti-biofilm and anti-quorum sensing potential of fruit, stem, and leaves of Salvadora persica L. methanolic extracts. Microb Pathog. 2017;109:169-176.
Crossref - Formagio AS, Volobuff CR, Santiago M, Cardoso CA, Vieira MD, Pereira ZV. Evaluation of antioxidant activity, total flavonoids, tannins and phenolic compounds in Psychotria leaf extracts. Antioxidants. 2014;3(4):745-757.
Crossref - Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70-76.
Crossref - Shabbir M, Khan MR, Saeed N. Assessment of phytochemicals, antioxidant, anti-lipid peroxidation and anti-hemolytic activity of extract and various fractions of Maytenus royleanus leaves. BMC Complement Altern Med. 2013;13:1-3.
Crossref - Tabassum S, Ahmad S, ur Rehman Khan K, et al. Phytochemical profiling, antioxidant, anti-inflammatory, thrombolytic, hemolytic activity in vitro and silico potential of Portulacaria afra. Molecules. 2022;27(8):2377.
Crossref - Elsayed EA, Sharaf-Eldin MA, Wadaan M. In vitro evaluation of cytotoxic activities of essential oil from Moringa oleifera seeds on HeLa, HepG2, MCF-7, CACO-2, and L929 cell lines. Asian Pac J Cancer Prev. 2015;16(11):4671-4675.
Crossref - Vijayakumar S, Ganesan S. In vitro cytotoxicity assay on gold nanoparticles with different stabilizing agents. J Nanomater. 2012;14.
Crossref - Shafagh M, Rahmani F, Delirezh N. CuO nanoparticles induce cytotoxicity and apoptosis in human K562 cancer cell line via mitochondrial pathway, through reactive oxygen species and P53. Iran J Basic Med Sci. 2015;18(10):993-1000.
- Dang TM, Le TT, Fribourg-Blanc E, Dang MC. Synthesis and optical properties of copper nanoparticles prepared by a chemical reduction method. Advances in Natural Sciences: Nanoscience and Nanotechnology. 2011; 2(1):015009.
Crossref - Wang Q, Liu ZF, Kong L, Liu SP. Absorption and resonance Rayleigh scattering spectra of the interaction for copper nanoparticles with vitamin B1. Chin J Anal Chem. 2007;35(3):365-369.
Crossref - Rajeshkumar S, Rinitha G. Nanostructural characterization of antimicrobial and antioxidant copper nanoparticles synthesized using novel Persea americana seeds. Open Nano. 2018;3:18-27.
Crossref - Khan A, Rashid A, Younas R, Chong R. A chemical reduction approach to the synthesis of copper nanoparticles. Int Nano Lett. 2016;6:21-26.
Crossref - Shiny KS, Sundararaj R, Mamatha N, Lingappa B. A new approach to wood protection: Preliminary study of biologically synthesized copper oxide nanoparticle formulation as an environmental friendly wood protectant against decay fungi and termites. Maderas: Ciencia y TecnologIa. 2019;21(3):347-356.
Crossref - Ouidad A, Sara C, Samir D. Biological properties and Acute Toxicity Study of Copper oxide nanoparticles prepared by aqueous leaves extract of Portulaca oleracea (L). Asian J Pharm Res. 2020;10(2):89-94.
Crossref - Sivaraj R, Rahman PK, Rajiv P, Salam HA, Venckatesh R. Biogenic copper oxide nanoparticles synthesis using Tabernaemontana divaricate leaf extract and its antibacterial activity against urinary tract pathogen. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2014;133:178-81.
Crossref - Patel BH, Channiwala MZ, Chaudhari SB, Mandot AA. Biosynthesis of copper nanoparticles; its characterization and efficacy against human pathogenic bacterium. J Environ Chem Eng. 2016;4(2):2163-2169.
Crossref - Sharmila V, Mounica NV, Anusha S, et al. Regulatory requirements of Similar Biologics for marketing authorization in India. Int J Drug Regul Aff. 2017;5:20-24.
Crossref - Usman MS, Zowalaty ME, Shameli K, Zainuddin N, Salama M, Ibrahim NA. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int J Nanomedicine. 2013;8(1):4467-4479.
Crossref - Khashan KS, Sulaiman GM, Abdulameer FA. Synthesis and antibacterial activity of CuO nanoparticles suspension induced by laser ablation in liquid. Arabian J Sci Eng. 2016;41:301-310.
Crossref - Meghana S, Kabra P, Chakraborty S, Padmavathy N. Understanding the pathway of antibacterial activity of copper oxide nanoparticles. RSC Adv. 2015;5(16):12293-12299.
Crossref - Punniyakotti P, Panneerselvam P, Perumal D, Aruliah R, Angaiah S. Anti-bacterial and anti-biofilm properties of green synthesized copper nanoparticles from Cardiospermum halicacabum leaf extract. Bioprocess Biosyst Eng. 2020;43(9):1649-1657.
Crossref - Das P, Ghosh S, Nayak B. Phyto-fabricated nanoparticles and their anti-biofilm activity: Progress and current status. Front Nanotechnol. 2021;3:739286.
Crossref - Adjimani JP, Asare P. Antioxidant and free radical scavenging activity of iron chelators. Toxicol Rep. 2015;2:721-728.
Crossref - Velika B, Kron I. Antioxidant properties of benzoic acid derivatives against superoxide radical. Free Radicals and Antioxidants. 2012;2(4):62-67.
Crossref - Jahan I, Erci F, Cakir-Koc R, Isildak I. Microwave-irradiated green synthesis of metallic silver and copper nanoparticles using fresh ginger (Zingiber officinale) rhizome extract and evaluation of their antibacterial potentials and cytotoxicity. Inorganic and Nano-Metal Chemistry. 2020; 51(5):722-732.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.