ISSN: 0973-7510
E-ISSN: 2581-690X
This study analysed Pseudomonas aeruginosa and Staphylococcus aureus burn wound infections, in order to evaluation their incidence, histological change and antimicrobial susceptibilities. Out of 39 burn wound cases admitted to surgery department, 20 and 12 strains of P. aeruginosa and S. aureus respectively were isolated from pyogenic burned skin lesions. Antibiotic resistance sketches of these strains to antibiotics were strategized. All the tested strains were multiple antibiotic resistance. Developed rates of susceptibility were confirmed for P. aeruginosa isolates against cefotoxim, gentamicin and nitrofurantoin. Also this study was to examine relation between the demonstrated antibiotic resistance and the occurrence of plasmids. Molecular sizes of the noticed plasmids were 24,321 kbp in P. aeruginosa and 23,25 kbp in S. aureus. Plasmid curing in grouping with MIC purpose revealed that resistance of P. aeruginosa and S. aureus isolates was plasmid allied. Histological analysis of burn wound infection is created on the thought of microorganisms attacking viable tissue below the eschar surface. The great MAR recognised marks it required for antibiotic resistance testing to be piloted former to antibiotics remedy for burn wound infection.
P. aeruginosa, S. aureus; Burns, Antimicrobial Agents, Plasmid; Histology.
Burns hurt is a chief community health delinquent in many nations in the world. In case of a burn injury, the organism experiences complex changes in homeostasis; these changes are rarely comparable to changes in case of any other trauma or disorder. For this reason, mortality in the early phase of burns in such traumas is common1,2. Contamination in the burn injury stretches the therapeutic of the wound in all phases of healing; for this reason, it is important, that the treatment of the burn infection injury includes antibiotic therapy, deletion of necrotic tissues in time, ensuring the blood and oxygen source to the wound, the augmentation of the resistance of the organism, and the adequate diet3.
Bacterial contamination is one of the greatest severe difficulties in burn basis serious complications and death following thermal injury4. P. aeruginosa and S. aureus are the furthermost chief contagious and risky bacteria in injury patient5. P. aeruginosa is one of the maximum significant and greatest reasons of grave contamination in injury patients6-8. Treatment of these infections is problematical by antibiotic resistance9-12.
In this study examined the histologic examination of biopsy and antimicrobial resistance of bacterial isolates from Burns. Likewise, the persistence of this study was to study any relative between antibiotic-resistance of bacteria and the company of plasmids.
Sample Collection and Identification of Bacteria
Thirty-nine cases were selected from patients carried out at the Surgery Department, General Hospital, Port Said City, Egypt (Bioethics agreement according to the Ethical Committee, Suez Canal University, Egypt). The average age was 25 years. A swab of each pus sample was suspended in 3 mL of water peptone. Drops of the prepared postponements were banquet on surface of plates holding Pseudomonas selective medium and Staphylococcus 110 medium13. All plates were incubated face down and the bacteria were allowed to grow at 37°C for 24-48 hours prior to enumeration and further identification. Pure well-isolated colonies were preceded for their biochemical tests: Glucose oxidation/fermentation, lactose fermentation, oxidase test, nitrate reduction, indole reaction, urease production, coagulase test, Voges-Proskaure, arginine and ornithine utilization tests14.
Antibiotic Susceptibility Studies
All trials were piloted using the inventive typical cultures to escape the unstructured hurt of antibiotic resistance. Antibiotic resistance was tested using a modified Kirby-Bauer disc diffusion method15-17.
Plasmid Analysis
The plasmid isolated by mini-prep alkaline extraction method [18]. Concentrations and purity of DNA were assessed spectrophotometrically using Spectro 22, Labo Med, Inc., USA. Gels were prepared by adding 1% agarose and 5 µL ethidium bromide (10 mg/mL) to the TBE buffer. Pure DNA sample (3µL) was added to 12µL deionized water and 1µL endonuclease (EcoR I, Hind III (Sigma Production), BamH I (Roche Diagnostics GmbH). Sequential dilutions of acridine orange were used for curing of isolated plasmids18.
Histopathological Study
Twenty-five tissue biopsies were collected, all biopsies were practise and stains according to basic histopathological technique. Specimen were taken in 10% Buffered Neutral Formalin 2h and fixed immediately at 37°C. All specimens were taken and processed manually in which all specimens were dehydrate in 70%, 80% for 2hours, and then 90% alcohol for overnight and absolute alcohol for 2 hours. Then cleared in xylene for 3 hours for two coplin jar. Then tissues were saturate with molten paraffin wax 2 changes for 2hours each. Then fixed with paraffin wax in mould and left to harden at room temperature and then chilled in refrigerator. Twenty-five blocks were prepared, albumenized slides were set and with rotary microtome. Five-micrometre paraffin tissue sections of skin were examined to evaluate the Burn infection. haematoxylin and eosin staining method was used to evaluate the changes in skin 19.
Thirty-two isolates were recovered from pyogenic lesions burned skin over the course of this period. P. aeruginosa (20 strains) accounted for 51% of total isolates (Fig. 1). This trailed by S. aureus (12 strains, 31 %) and other organisms (18 %). As can be seen in table I, the rate of cephradine and kanamycin were similar among both P. aeruginosa and S. aureus isolates, at 100%. Advanced degrees of susceptibility were confirmed for P. aeruginosa isolates against cefotoxim, gentamicin and nitrofurantoin. All S. aureus strians were sensitive to vancomycin.
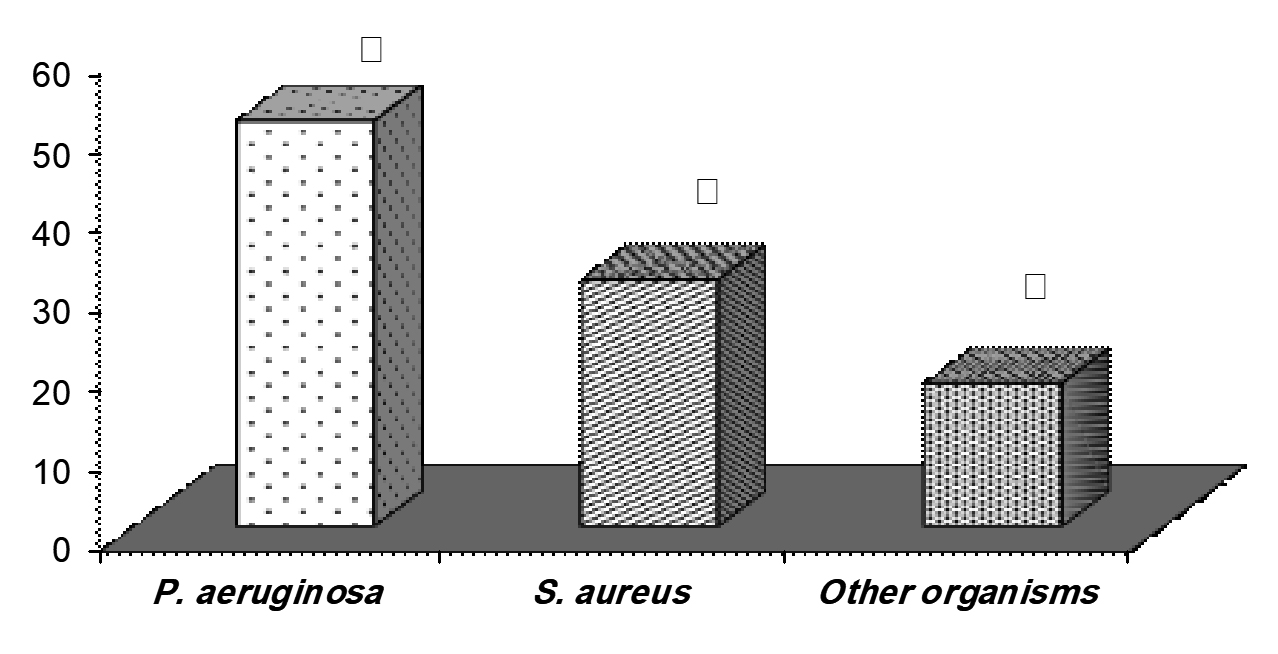 Fig. 1. Frequency of P. aeruginosa and S. aureus bacterial strains form studied wound burn infection
Fig. 1. Frequency of P. aeruginosa and S. aureus bacterial strains form studied wound burn infection Fig. 2. Skin tissue contain bacterial contamination fixed in Buffered Neutral Formalin [hematoxyline and eosin Stain (x40)]
Fig. 2. Skin tissue contain bacterial contamination fixed in Buffered Neutral Formalin [hematoxyline and eosin Stain (x40)]Plasmid profiles of the two bacterial isolates under study were detected. Only six out of 10 isolates ( 5 P. aeruginosa and 5 S. aureus) were found to contain plasmids. No plasmids could be detected for other 4 isolates. Two isolates (one P. aeruginosa and one S. aureus) were selected for further study as representative of plasmid-bearing isolates. Each of them was found contain only one plasmid (Table II). Molecular sizes of the detected plasmids were 24,321 kbp in P. aeruginosa and 23,25 kbp in S. aureus. Concentration and degree of purity of the plasmid DNA(S) were as in table II. Plasmid curing in combination with MIC determination revealed that resistance to ampicillin was plasmid linked. Digestion of the two isolated plasmids, singly with Hind III, EcoR I and Bam H1 restriction enzymes showed in table III. Number of recognition sites, number of fragments and the approximate of molecular size of restricted fragments as shown in table III. The histologic analysis of the paraffin sections with the haematoxylin and eosin staining method showed significant increase in bacterial counts per counted high power field (40׳).
Table (1):
Antimicrobial resistance profile of P. aeruginosa and S. aureus isolates from studied wound burn infection
P. aeruginosa |
S. aureus |
|
|---|---|---|
Isolates (n) |
20 |
12 |
Ampicillin (10 μg) |
100 |
33 |
Amoxycillin (10 μg) |
60 |
50 |
Cephradine (30 μg) |
100 |
100 |
Cefotoxim (30 μg) |
0 |
ND |
Cephalexin (30 μg) |
59 |
Nd |
Streptomycin (10 μg) |
ND |
10 |
Colistin sulfate (10 μg) |
100 |
25 |
Chloramphenicol(30 μg) |
ND |
0 |
Velosef (30 μg) |
75 |
25 |
Tetracycline (10 μg) |
100 |
ND |
Erythromycin (30 μg) |
ND |
100 |
Kanamycin (30 μg) |
100 |
100 |
Rifampicin (5 μg) |
25 |
0 |
Gentamicin (30 μg) |
0 |
43 |
Nitrofurantoin (300 μg) |
0 |
ND |
Vancomycin (30 μg) |
ND |
0 |
ND: Not Determined
Table (2):
Characterisation of isolated plasmids including numbers, concentration, purity, curing and size
Plasamid Characterization |
P. aeruginosa |
S. aureus |
|---|---|---|
No. of Plasmids |
1 |
1 |
Conc.(μg/mL)/ Purity |
320/2.1 |
320/2.0 |
% Curing |
100% |
100% |
Total plasmid Size (Kbp) |
24.321 |
23.25 |
Table (3):
The restriction patterns of plasmids from P. aeruginosa and S. aureus isolates from studied burned skin
Plasmids of: |
Restriction Enzymes |
No. of Recognition Sites |
No. of Fragments |
Size of Fragments (Kbp) |
|---|---|---|---|---|
P. aeruginosa |
Hind III EcoR I BamH I |
3 3 0 |
2 2 0 |
13.3 and11.021 17.3 and 7.02 0 |
S. aureus |
Hind III EcoR I BamH I |
4 0 0 |
3 0 0 |
9.2, 7.75 and 6.3 0 0 |
Contagion is one of the supreme thoughtful worries in burn patients, and Pseudomonas, particularly P. aeruginosa, is the record vital, hardy, and hazardous organism10-12. P. aeruginosa blossoms on the humid burn wound external8,19 and is extremely pathogenic in thermally wounded immunosuppressed patients. Notwithstanding developments in therapeutic and surgical overhaul, the scenario residues deprived, with a death rate of about 80% in such patients8. In several cautiously unindustrialized nations such as Iran20-21, Zimbabwe22, South Korea4, Jordan23, Libya24, Nigeria25-26, India1,27-29, Turkey6,30 and Syria31. P. aeruginosa was described to be public bacteria between burn patients. Though P. aeruginosa is not a typical microbe of injury wound contagions in the industrialised countries, a few burn centres in Canada and the USA32, France33, and Italy34 have informed P. aeruginosa as significant bacterium in burn divisions.
The second best shared separate in this study was S. aureus, in studies from cautiously unindustrialized nations4,6,8,10-11,24-28. In the Surgery Department, S. aureus was initiate in 31% of sections occupied from the burn damages. In case of a severe burn, the chance of S. aureus taint rises, subsequently the patients expend more time in the Sector, and suffer more regular dressings. Thus, surgery should be achieved as primary as conceivable, hence reducing the period of hospital stay, infection-related treatment costs, and taming the value of cure2.
Antibiotics is single of the highest hazard influences for antibiotic resistance. Extreme custom of antibiotics excites the progress of antibiotics- resistant microbes, developments treatment expenses, and causes side possessions2. Antibiotics are only approved if the causal agent is found to be sensitive to antibiotics, preliminary with minor group antibiotics, and in the occurrence of the signs of wound infection. Throughout surgery, in case of average to severe burns, a sole amount of cefuroxime and vancomycin are suggested in case of P. aeruginosa and S. aureus infection correspondingly.
In many studies plasmid related resistance, particularly for pathogenic bacterial strains, are still of dangerous position35-38. Amount of gratitude locations, quantity of wreckages and the estimated of molecular size of restricted fragments few. As of the partial total of endonuclease used in this study. Digestion with EcoR I provided two fragments with P. aeruginosa while no fragments were observed for S. aureus isolate. Comprehensive description of these plasmids is required in future for more indulgent about gene appearance and constancy. Histological investigation of a burn wound biopsy documents the needed variation and is the greatest technique for finding of burn wound infection. We achieve that to make meticulous staining of skin tissue with haematoxylin and eosin staining.
Finally, it is recommending that officials in our burn units take into account recover workers’ sanitation; reflect suitable cleansing for all tools; found a novel and actual antibiotic strategy and avoid unnecessary use of antibiotics.
Acknowledgements
The author would like to thank the Surgery Department Staff General Hospital, Egypt, for providing the urine samples which was the source of bacterial strains.
Conflict of Interest
The authors declares that there is no conflict of interest.
Authors’ Contribution
Author listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
None
Data Availability
All datasets generated and analyzed during this study are available in the NCBI database repository, Accession No: MH161378 and is included in the manuscript.
Ethics Statement
The study was approved from the Universities Ethical Committee (ENREC).
- Revathi G., Puri J., Jain B. K. Bacteriology of burns. Burns, 1998, 24:347-349.
Crossref - Rokas B., Algimantas T., Rytis R. Staphylococcus aureus infection in the surgery of burns. MEDICINA, 2003, 39: 1078-1081.
- Bowler P.G., Duerden B.I., Armstrong D.G. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev, 2001; 14: 240-244.
Crossref - Song W., Lee K.M., Kang H.J., Shin D.H., Kim D.K. Microbiologic aspects of predominant bacteria isolated from burn patients in Korea. Burns, 2001; 27: 136-139.
Crossref - Trafny E.A. Susceptibility of adherent organisms from Pseudomonas aeruginosa and Staphylococcus aureus strains isolated from burn wounds to antimicrobial agents. ֽiter J of Antimicro Agents, 1998; 10: 223-228.
Crossref - Oncul O., Yksel F., Altunay H., Aחikel C., Elikz B., Avuslu S. The evaluation of nosocomial infection during 1-year period in the burn unit of a training hospital in Istanbul, Turkey. Burns, 2002; 28: 738-744.
Crossref - Xue B., Liu X., Tang M. The change in bacterial flora and antibiotic resistance of bacteria of burn patients in our hospital during 1986-1996. Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi, 1999; 15: 309-312.
- Rastegar L.A.R., Alaghehbandan R., Akhlaghi L. Burn wound infections and antimicrobial resistance in Tehran, Iran: an increasing problem. Annals of Burns and Fire Disasters, 2005; 2: 17-19.
- Alaghehbandan R., Rossignol A.M., Rastegar L. A. Paediatric burn injuries in Tehran, Iran. Burns, 2001; 27: 115-118.
Crossref - Rastegar L.A., Alaghehbandan R., Nikui R. Epidemiological study of 3341 burns patients during three years in Tehran, Iran. Burns, 2000; 26: 49-53.
Crossref - Rastegar L.A., Bahrami H.H., Alaghehbandan R. Pseudomonas infections in Tohid Burn Centre, Iran. Burns, 1998; 24: 637-641.
Crossref - Karimi E.H., Pour K.P., Ghanaatpisheh F. Frequency of Pseudomonas aeruginosa serotypes in burn wound infections and their resistance to antibiotics. Burns, 2002; 28: 340-348.
Crossref - Oxoid Maual for Culture Media, Ingredients and other Laboratory Services. Eighth edition, Oxoid Limited, UK, 1998.
- Holt J.G., Krieh N.R., Sneath P.A., Staley J., Williams S. T. Bergey,s manual of determinative bacteriology. Ninth edition, Williams and wilkins Co., Baltimore, MD, 1994.
- Selim S.A., El Alfy S.M., Abdel Aziz M.H., Mohamed H.M., Alasbahi A.A. Effective of metronidazole to bacterial flora in vagina and the impact of microbes on live birth rate during intracytoplasmic sperm injection (ICSI)”, Archives of Gynecology and Obstetrics, 2001; 20: 11-15.
- Robert S., Anders R.L., Niels F., Frank E. Evaluation of different disk diffusion/media for detectionof methicillin resistance in Staphylococcus aureus and coagulase-negative staphylococci. APMIS, 2003; 111: 905-914.
Crossref - Wardhana A., Djan R., Halim Z. Bacterial and antimicrobial susceptibility profile and the prevalence of sepsis among burn patients at the burn unit of Cipto Mangunkusumo Hospital. Ann Burns Fire Disasters, 2017; 30(2): 107–115.
- Sambrook J., Fritsch E.F., Maniatis T. Molecular cloning: A laboratory manual. Second edition, Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, 1989.
- Brown R.C., Hopps H.C. Staining of bacteria in tissue sections: A reliable Gram stain method. Am J Clin Pathol, 1973; 60: 234–240.
Crossref - Gang R.K., Bang R.L., Sanyal S.C., Mokaddas E., Lari A.R. Pseudomonas aeruginosa septicaemia in burns. Burns, 1999; 25: 611-616.
Crossref - Rastegar L.A., Panjeshahin M.R., Talei. A.R., Rossignol A.M., Alaghehbandan, R. Epidemiology of childhood burn injuries in Fars province, Iran J Burn Care Rehabil, 2002; 23: 39-45.
Crossref - Panjeshahin M.R., Rastegar L.A., Talei A.R., Shamsnia J., Alaghehbandan R. Epidemiology and mortality of burns in the southwest of Iran. Burns, 2001; 27: 219-26.
Crossref - Igumbor E., Gwanzura L., Chirara M., Obi C., Muza D., Chihara M. Antibiotic sensitivity and plasmid profiles of Pseudomonas aeruginosa. Cent Afr J. Med., 2001; 47; 84.
Crossref - Al-Akayleh A.T. Invasive burn wound infection. Ann Burns and Fire Disasters, 1999; 12: 204- 206.
- Husain M.T., Karim Q.N., Tajuri S. Analysis of infection in a burn ward. Burns, 1989; 15: 299-302.
Crossref - Atoyebi O.A., Sowemimo G.O.A., Odugbemi T. Bacterial flora of burn wounds in Lagos, Nigeria: A prospective study. Burns, 1992; 18: 448-451.
Crossref - Ozumba U.C., Jiburum B.C. Bacteriology of burn wounds in Enugu, Nigeria. Burns, 2000; 26: 178-180.
Crossref - Kaushik R., Kumar S., Sharma R., Lal P. Bacteriology of burn wounds – the first three years in a new burn unit at the Medical College Chandigarh. Burns, 2001; 27: 595-597.
Crossref - Pandit D.V., Gore M.A., Saileshwar N., Deodhar L.P. Laboratory data from the surveillance of a burns ward for the detection of hospital infection. Burns, 1993; 19: 52-55.
Crossref - Sharma S., Hans C. Bacterial infections in burns patients: A three years’ study at RML hospital, Delhi. J Commun Disease, 1996; 28: 101-106.
- Arslan E., Dalay C., Yavuz M., Gcenler L., Acart rk S. Gram-negative bacterial surveillance in burn patients. Ann Burns and Fire Disasters, 1999; 12: 81-83.
- Dayoub A., Zeidan F., Radidy S. Infection in burns: Experience of a teaching hospital in Syria. Ann Burns and Fire Disasters, 1995; 8: 17-19.
- Shankowsky H.A., Callioux, L.S., Tredget E.E. North America survey of hydrotherapy in modern burn care. J Burn Care Rehabil, 1994; 15: 143-146.
Crossref - Cremer R., Ainaud P., Le Bever H., Faber N., Carsin H. Nosocomial infections in a burns unit. Results of a prospective study over a year. Ann Fr Anesth Reanim, 1996; 15: 599-607.
Crossref - Calvario A., Di Lonardo A., Larocca A.M.V., Parisi D., Montagna M.T., Ressa M., Silvestri A., Maggio G. Microbiological monitoring of severely burned patients admitted to the burns centre in Bari (Italy) in the period 1989-92. Ann Burns and Fire Disasters, 1994; 7: 73-79.
- Bekowitz , F. E. Antibiotic resistance in bacteria. South Med J, 1995; 88: 797-804.
Crossref - Demain A.L., Davies J.E. Manual of industrail microbiology and biotechnology. Second edition, ASM Press: Washington, D. C.1999.
- Lai R. Insecticide microbiology. Springer-Verlag, New york, 1984, 150-151.
© The Author(s) 2019. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


