ISSN: 0973-7510
E-ISSN: 2581-690X
Candida albicans is the most common fungal pathogen in humans. Antimicrobial resistance in C. albicans is increasingly reported. The antifungal activity of eugenol against clinically relevant fungi including C. albicans has been recently described. In this study, the antifungal effects of eugenol on major virulence factors of C. albicans were evaluated. C. albicans isolates were exposed to sub-MIC of eugenol. The crystal violet-based method was used to quantify the germ tube formation. Extracellular enzymatic activity (proteinase, phospholipase, and lipase) was determined using the agar plate test. The adhesion to buccal epithelial cells was monitored microscopically. Our result showed that eugenol possesses fungicidal activity against C. albicans. At sub-inhibitory doses, eugenol significantly suppressed germ tube formation and cell adhesion of C. albicans (p<0.05). However, the enzymatic activity of yeast cells exposed to eugenol was slightly reduced. Our data indicate the inhibitory effect of eugenol on the growth and the pathogenicity of C. albicans in terms of germ tube formation and adhesion.
Eugenol, Germ Tube Formation, Hydrolytic Enzymes, Adhesion, Candida albicans
Candida albicans is commonly found as a harmless commensal in the gastrointestinal tract, oral cavity, and genital area. It is responsible for various clinical manifestations in immunocompromised patients varying from mucocutaneous infection to invasive systemic infection.1 Several virulence factors including adhesion to host tissue, dimorphic transition, and production of extracellular enzymes may contribute to the transformation from harmless commensal to virulent pathogen.2 Adhesion to the host tissue is a crucial initiation step of colonization and infection. Short hyphal elements called “germ tubes” are important to the adhesion of Candida to the host epithelium. Moreover, the organism’s hyphal forms contribute to invasion and tissue destruction.3 Extracellular hydrolytic enzymes play an important role in nutrition acquisition, invasion, tissue injury, and host defense evasion.4-7 The treatments of candidiasis with polyenes, azole, and echinocandins are satisfactory. However, these antifungal drugs also have many disadvantages regarding their host toxicity, spectrum of activity, and pharmacokinetic properties. Furthermore, infections by multidrug-resistant C. albicans have been recently reported.8 Thus, antibiotics with a new mode of action are urgently needed.9
Previous studies showed that eugenol, the major active ingredient of clove oil, has antimicrobial activity against many microorganisms including Candida sp. It was found that eugenol was not only able to reduce the growth but also the virulence of Candida sp. such as cell surface hydrophobicity and biofilm formation.10,11 Here, we investigated the effect of eugenol on germ tube formation, extracellular hydrolytic enzyme, and adhesion to epithelial cells of C. albicans.
Strains of tested microorganisms
Two reference strains (ATCC 10231 and 90028) and twenty clinical C.albicans isolates obtained from blood, urine, genital swab, and body fluids were used. Tested microorganisms were identified by phenotypic analysis including germ tube and chlamydoconidia production, culture on CHROMagar™ Candida, growth at 45°C, and carbohydrate assimilation and fermentation test. Before use, yeast cells were grown for 48 h at 37°C on a Sabouraud dextrose agar plate (SDA) (Oxoid®, USA).
MIC and MFC assay
To determine the MIC of eugenol, the broth microdilution method was performed according to a method of EUCAST (EDef 7.1) with some modifications. Eugenol was purchased from Fluka (Steinheim, Germany). Briefly, eugenol was serially diluted in double-strength RPMI 1640 (Biochrom AG, Germany) containing 4% glucose and 2% DMSO (2 x RPMI-GD) to achieve a range of concentration from 1.95 to 1,000 µg/ml. Yeast suspension (1 x 104 CFU/ml) was added with an equal volume of serially diluted eugenol. After incubation at 37°C for 24 hours, yeast growth was determined by using spectrophotometry. The lowest concentration of eugenol inhibiting yeast cells growth was considered the MIC value. MFC was determined by sub-culturing 100 µl from the optically clear well onto SDA. The MFC was considered as the lowest eugenol concentration that kill ≥99.9% of the initial inoculum. All analyses were repeated three times with duplicate determinations.
Time-kill assay
Time-kill assay was conducted as previously described with some modifications.12 Yeast cells (5 x 104 CFU/ml) of C. albicans ATCC 10231 were grown in RPMI-GD in the presence of eugenol at a final concentration of 0, 1/4, 1/2, and 1 x MIC at 37°C. The viability of the yeast cells (CFU/ml) at a different time point was determined by the colony count method. The analysis was independently repeated two times with duplicate determinations.
Inhibitory effect of eugenol on germ tube formation
Inhibitory effect of eugenol on germ tube formation was determined by method described elsewhere with modification.13 Cell suspension (1 x 105 CFU/ml) of fresh grown C. albicans was prepared and then a 100-µl aliquot was added to the wells of a 96-well, flat-bottom plate containing RPMI-GD (100 µl) in the presence of 10% fetal bovine serum (Gibco BRL, USA) and different eugenol concentration ( 0, 0.25, or 0.5 x MIC). A blank RMPI-GD well without yeast cells was included. After incubation for 2 h at 37°C, the medium was discarded and wells were washed once with 100 µl of 70% ethanol. A 200-µl aliquot of 0.25% sodium dodecyl sulfate (SDS) was added to each well to removed yeast cells without a germ tube. Then, the plates were washed thrice with distilled water. Germ tube formation was quantified by using the crystal violet-based technique. To minimize background interference, the absorbance values of 570 nm of the blank were subtracted from the values of the test wells. Three independent experiments with duplicate determinations were performed.
Effect of eugenol on hydrolytic enzyme activity
The effects of eugenol on activities of hydrolytic enzymes including proteinase, phospholipase, and lipase were determined by using agar containing bovine serum albumin,14 egg yolk,15 and Tween-80.16 Five microliters of 1 x 106 CFU/ml cell suspension were spotted on assay agar containing either 0 (control), 0.25, or 0.5 x MIC of eugenol. The agar plates were incubated in a humidity chamber at 37°C for 5, 2, and 7 days, respectively. Enzymatic activity (PZ) was determined by the ratio of colony diameter to diameter of colony plus the precipitation or degradation zone. Experiment was performed in triplicate and repeated twice.
Effect of eugenol on candidal adhesion to buccal epithelial cell
Yeast cells (5 x 105 CFU/ml) of C. albicans ATCC 10231 were incubated in RPMI-DG with eugenol at concentrations of 0, 0.25, and 0.5 × MIC at 37°C for 1 h. Treated cells were harvested by then centrifugation and washed twice with NSS. Yeast cells were resuspended in NSS and adjusted to yield approximately 1×107 CFU/ml. Buccal epithelial cells (BEC) were collected by gently rubbing on the mucosal surface of the cheeks of three healthy volunteers with a sterile swab and pooled in sterile NSS. The BEC was washed three times and adjusted to a concentration of 1×105 CFU/ml in NSS. Then, the candidal adhesion to the human buccal epithelial cell was performed according to the previously described method.17 The number of yeast cells attached to 100 BECs was counted using light microscopy at 400x magnification. Experiment was performed in triplicate and repeated thrice.
Statistical analysis
To evaluate the difference between the control and eugenol treated groups, Wilcoxon Signed Rank Test was used to analyze the effect of eugenol on virulence factors. A p-value < 0.05 was considered significant.
MIC, MFC, and antifungal activity of eugenol
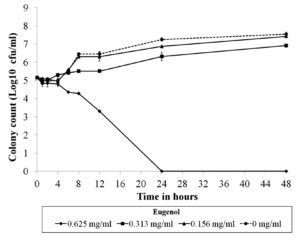
The MICs and MFCs of eugenol against twenty-two C. albicans strains were equal to 625 µg/ml. Due to the well-known genetic background of C. albicans ATCC 10231, this strain was selected for time-kill analysis. The obtained result demonstrated that eugenol at MIC was fungicidal within 24 hours because cell viability was decreased by more than 3 log10 CFU/ml relative to the initial inoculum (Figure 1).
Inhibitory effect of eugenol on germ tube formation
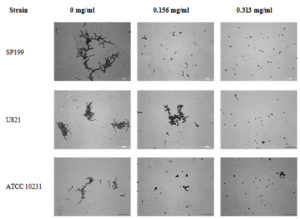
The crystal violet-based method was used to evaluate the effect of eugenol on germ tube formation of C. albicans. Eugenol cause a significant decrease in OD570 from 0.31 (range: 0.19–0.42) of control to 0.09 (range: 0.01–0.31) and 0.06 (range: 0.01–0.20) at 156 and 313 µg/ml eugenol, respectively (p< 0.05). The inhibitory effect of eugenol on germ tube formation was dose-dependent manner (Table 1). The photograph depicting the germ formation of yeast cells treated with various concentrations of eugenol is shown in Figure 2.
Table (1):
Germ tube formation of C. albicans (n=22) in the presence of eugenol determined by the crystal violet technic measured at OD570.
| Eugenol (µg/ml) | Germ tube formation (OD570) | |
|---|---|---|
| Mean ± SD | Range | |
| 0 | 0.31 ± 0.06 | (0.19–0.42) |
| 156 | 0.09 ± 0.08a | (0.01–0.31) |
| 313 | 0.06 ± 0.05a | (0.01–0.20) |
a p<0.01, SD, standard deviation; OD570, optical density at 570 nm.
Effect of eugenol on proteinase, phospholipase, and lipase activities
Hydrolase activities of C. albicans exposed to varying concentrations of eugenol were determined using hydrolase-specific testing agar. Twenty-one and nineteen strains including two reference strains exhibiting proteinase and phospholipase activities were used in this experiment, respectively. The Pz for C. albicans unexposed to eugenol was 0.68 ± 0.09 for proteinase and 0.71± 0.07 for phospholipase. When the yeast was exposed to 156 and 313 µg/ml eugenol, the activities of these enzymes were slightly but statistically significantly reduced to 0.70 ± 0.08 and 0.70 ± 0.07 for proteinase and 0.73 ± 0.08 and 0.75 ± 0.08 for phospholipase, respectively (p<0.05). In addition, the Pz of lipase activity of twenty-two strains of C. albicans not exposed to eugenol was 0.52 ± 0.09. Lipase activity of the tested strains was not reduced when exposed to eugenol at 0.25 and 0.5 × MIC (0.52 ± 0.09 and 0.53 ± 0.09, respectively, Table 2).
Table (2):
Enzymatic activity of C. albicans in the presence of eugenol. Ratio of colony diameter to diameter of the colony plus degradation or precipitation zone was determined and defined as enzyme activity (Pz).
| Eugenol (µg/ml) |
Proteinase activity (n=21) | Phospholipase activity (n=19) | Lipase activity (n=22) | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | |
| 0 | 0.68 ± 0.09 | 0.43–0.82 | 0.71 ± 0.07 | 0.57–0.90 | 0.52 ± 0.09 | 0.42–0.72 |
| 156 | 0.70 ± 0.08a | 0.48–0.76 | 0.73 ± 0.08 a | 0.60–1.00 | 0.52± 0.09 | 0.42–0.76 |
| 313 | 0.70 ± 0.07a | 0.51–0.77 | 0.75 ± 0.08 a | 0.62–1.00 | 0.53 ± 0.09 | 0.43–0.76 |
a p<0.05
Effect of eugenol on C. albicans adhesion to the BEC
Compared with the control, eugenol at 0.25 x and 0.5 x MIC inhibited the adhesion activity of C. albicans from 180 ± 10.8 yeast cells/100 BEC to 67 ± 5.2 and 36 ± 3.9, respectively (p<0.05). The result showed that the reduction of BEC adhesion of C. albicans by eugenol was concentration-dependent (Table 3).
Table (3):
Adhesion of C. albicans ATCC 10231 to buccal epithelial cells in the present of eugenol.
| Eugenol (µg/ml) | Yeast cells/100 BEC | |
|---|---|---|
| Mean ± SD | Range | |
| 0 | 180 ± 10.8 | 168–188 |
| 156 | 67 ± 5.2a | 62–72 |
| 313 | 36 ± 3.9a | 33–40 |
a p<0.05; BEC: buccal epithelial cell; SD: standard deviation
Candidiasis has become increasingly significant due to the growing population of immunocompromised patients. Most of the azole drugs are fungistatic. So, treatment provides the chance for acquired resistance to develop in the presence of this drug. New antifungal drugs, especially with a fungicidal mode of action are urgently needed. Eugenol, which is a monohydric phenol compound widely used as a local antiseptic and anesthetic, has been found to have antimicrobial activity against both planktonic and sessile Candida sp.10,11 As previously reported, our result showed that eugenol has fungicidal activity against C. albicans.
Adhesion was considered as the first step in the process leading to persistent colonization, biofilm formation, and establishment of the disease.19 Eugenol has been reported to reduce cell surface hydrophobicity and adhesion of C. tropicalis and C. dubliniensis to polystyrene and Hep2 cells.11 In our study, eugenol at sub-inhibitory concentrations significantly suppressed the adhesion of C. albicans to buccal epithelial cells. This result emphasized that eugenol could interfere with the adhesion properties of Candida. Eugenol may inhibit the synthesis of adhesins or it may cause disruption of adhesion molecules to present on the cell wall and thereby prevent adhesion of the yeast cells.
The secretion of hydrolytic enzymes such as aspartyl proteinases, phospholipases, and lipases during infection is required for the acquisition of nutrients, tissue damage, invasion, and immune system evasion.4-7 When eugenol was added to the test media, the zones of phospholipase and proteinase activities were slightly reduced. However, no effect of eugenol on lipase activity was observed. A previous study revealed that Candida proteinase also plays an auxiliary role of proteinase in cellular adhesion.20 However, this mechanism can be excluded from our study because eugenol had a limited effect on proteinase activity.
Germ tube formation is a morphological transition process from yeast to hyphal form, leading to tissue invasion, evasion of phagocytosis by macrophage, and biofilm formation.21-23 C. albicans mutants locked in yeast form were avirulent in an animal model.24-26 In this study, eugenol suppressed the germ tube formation in the presence of serum, which is a hyphae-inducing agent, indicating a significant role of eugenol in hyphal growth inhibition and thereby invasive and biofilm formation capacity.
It has been found that eugenol inhibits ergosterol biosynthesis in Candida,27 also perturbs the activity of amino acid permeases in yeast resulting in cell death and subsequent cytoplasmic leakage.28 In addition, it has been proposed that eugenol may disturb cellular fluidity and permeability by inserting itself between the fatty acyl chains of the membrane lipid bilayer.29 By that proposed mechanism, eugenol may suppress yeast-to-hypha transition and alter the cell surface to mask adhesins. Nevertheless, this is speculative and requires further clarification.
Because azole drugs and eugenol have different targets. The latter was found to be still effective against different species of Candida sp. with intrinsic or acquired resistance to fluconazole. Eugenol also exhibited in vitro synergy with fluconazole and amphotericin B against Candida sp.30-32 Moreover, the efficacy of eugenol as antifungal prophylaxis and treatment of oral and vaginal candidiasis in an immunosuppressed rat model has been reported.33 The results obtained in this study demonstrated that eugenol possesses fungicidal activity against C. albicans and has inhibitory effects on significant virulence factors of C. albicans involved in germ tube formation and adhesion to epithelial cells.
ACKNOWLEDGMENTS
The authors would like to thank the Department of Medical Technology for technical support and Thammasat University Hospital for providing C. albicans isolates used in this study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
AP designed the study, performed the experiments, analyzed the data, and wrote the manuscript. NM and BN carried out the sampling and culture method. CC and PJ performed the experiments. All authors read and approved the final manuscript for publication.
FUNDING
This study was supported by the Faculty of Allied Health Sciences, Thammasat University with grant number AHS 01/2016.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Ethical Committee of Thammasat University, Project no. 046/2015.
- Eggimann P, Garbino J, Pittet D. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis. 2003;3(11):685-702.
Crossref - Hube B, Naglik J. Candida albicans proteinases: resolving the mystery of a gene family. Microbiology. 2001;147(8):1997-2005.
Crossref - Jacobsen ID, Wilson D, Wachtler B, Brunke S, Naglik JR, Hube B. Candida albicans dimorphism as a therapeutic target. Expert Rev Anti Infect Ther. 2012;10(1):85-93.
Crossref - Ghannoum MA. Potential role of phospholipases in virulence and fungal pathogenesis. Clin Microbiol Rev. 2000;13(1):122-143.
Crossref - Naglik JR, Challacombe SJ, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev. 2003;67(3):400-428.
Crossref - Schaller M, Borelli C, Korting HC, Hube B. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses. 2005;48(6):365-377.
Crossref - Gacser A, Stehr F, Kroger C, Kredics L, Schafer W, Nosanchuk JD. Lipase 8 affects the pathogenesis of Candida albicans. Infect Immun. 2007;75(10):4710-4718.
Crossref - Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1-e50.
Crossref - Campoy S, Adrio JL. Antifungals. Biochem Pharmacol. 2017;133:86-96.
Crossref - He M, Du M, Fan M, Bian Z. In vitro activity of eugenol against Candida albicans biofilms. Mycopathologia. 2007;163(3):137-143.
Crossref - de Paula SB, Bartelli TF, Di Raimo V, et al. Effect of eugenol on cell surface hydrophobicity, adhesion, and biofilm of Candida tropicalis and Candida dubliniensis isolated from oral cavity of HIV-infected patients. Evid Based Complement Alternat Med. 2014;2014:505204.
Crossref - Canton E, Peman J, Gobernado M, Viudes A, Espinel-Ingroff A. Patterns of amphotericin B killing kinetics against seven Candida species. Antimicrob Agents Chemother. 2004;48(7):2477-2482.
Crossref - Abe S, Satoh T, Tokuda Y, Tansho S, Yamaguchi H. A rapid colorimetric assay for determination of leukocyte-mediated inhibition of mycelial growth of Candida albicans. Microbiol Immunol. 1994;38(5):385-388.
Crossref - Staib F. Serum-proteins as nitrogen source for yeastlike fungi. Sabouraudia. 1966;4(3):187-193.
Crossref - Anil S, Samaranayake LP. Brief exposure to antimycotics reduces the extracellular phospholipase activity of Candida albicans and Candida tropicalis. Chemotherapy. 2003;49(5):243-247.
Crossref - Slifkin M. Tween 80 opacity test responses of various Candida species. J Clin Microbiol. 2000;38(12):4626-4628.
Crossref - Taweechaisupapong S, Choopan T, Singhara S, Chatrchaiwiwatana S, Wongkham S. In vitro inhibitory effect of Streblus asper leaf-extract on adhesion of Candida albicans to human buccal epithelial cells. J Ethnopharmacol. 2005;96(1):221-226.
Crossref - Ellepola ANB, Samaranayake LP. Investigative Methods for Studying the Adhesion and Cell Surface Hydrophobicity of Candida Species: An Overview. Microb Ecol Health Dis. 2001;13(1):46-54.
Crossref - Silva-Dias A, Miranda IM, Branco J, Monteiro-Soares M, Pina-Vaz C, Rodrigues AG. Adhesion, biofilm formation, cell surface hydrophobicity, and antifungal planktonic susceptibility: relationship among Candida spp. Front Microbiol. 2015;6.
Crossref - Consolaro MEL, Gasparetto A, Svidzinski TIE, Peralta RM. Effect of pepstatin A on the virulence factors of Candida albicans strains isolated from vaginal environment of patients in three different clinical conditions. Mycopathologia. 2006;162(2):75-82.
Crossref - Sudbery PE. Growth of Candida albicans hyphae. Nat Rev Microbiol. 2011;9(10):737-48.
Crossref - Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol. 2001;9(7):327-335.
Crossref - Heinsbroek SE, Kamen LA, Taylor PR, Brown GD, Swanson J, Gordon S. Actin and phosphoinositide recruitment to fully formed Candida albicans phagosomes in mouse macrophages. J Innate Immun. 2009;1(3):244-53.
Crossref - Lo H-J, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90(5):939-949.
Crossref - Berman J, Sudbery PE. Candida albicans: a molecular revolution built on lessons from budding yeast. Nat Rev Genet. 2002;3(12):918-30.
Crossref - Vediyappan G, Dumontet V, Pelissier F, d’Enfert C. Gymnemic acids inhibit hyphal growth and virulence in Candida albicans. PLOS ONE. 2013;8(9):e74189.
Crossref - Ahmad A, Khan A, Manzoor N, Khan LA. Evolution of ergosterol biosynthesis inhibitors as fungicidal against Candida. Microbial Pathogenesis. 2010;48(1):35-41.
Crossref - Darvishi E, Omidi M, Bushehri AA, Golshani A, Smith ML. The antifungal eugenol perturbs dual aromatic and branched-chain amino acid permeases in the cytoplasmic membrane of yeast. PLoS One. 2013;8(10):e76028.
Crossref - Latifah-Munirah B, Himratul-Aznita WH, Mohd Zain N. Eugenol, an essential oil of clove, causes disruption to the cell wall of Candida albicans (ATCC 14053). Front Life Sci. 2015;8(3):231-240.
Crossref - Ahmad A, Khan A, Khan LA, Manzoor N. In vitro synergy of eugenol and methyleugenol with fluconazole against clinical Candida isolates. J Med Microbiol. 2010;59(10):1178-1184.
Crossref - Ahmad A, Wani MY, Khan A, Manzoor N, Molepo J. Synergistic interactions of eugenol-tosylate and its congeners with fluconazole against Candida albicans. PLOS ONE. 2015;10(12):e0145053.
Crossref - Khan MSA, Malik A, Ahmad I. Anti-candidal activity of essential oils alone and in combination with amphotericin B or fluconazole against multi-drug resistant isolates of Candida albicans. Med Mycol. 2012;50(1):33-42.
Crossref - Chami N, Chami F, Bennis S, Trouillas J, Remmal A. Antifungal treatment with carvacrol and eugenol of oral candidiasis in immunosuppressed rats. Braz J Infect Dis. 2004;8:217-226.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.