ISSN: 0973-7510
E-ISSN: 2581-690X
Entomopathogenic bacterium, Xenorhabdus has a mutualistic relationship with entomopathogenic nematode of the genus Steinernema and produces several bio-agent compounds with antimicrobial and nematicidal activities. Root-knot nematodes are considered one of the most important pests facing the cultivation of grapevine worldwide. A micro-plot field trial was conducted in naturally infested soil with Meloidogyne incognita to evaluate the potential of two strains of entomopathogenic bacteria namely Xenorhabdus budapestensis DSM 16342 (EMA) and X. szentirmaii DSM 16338 (EMC) applied separately or integrated with neem cake and/or furadan at half of recommended dose on nematode development and growth improvement of Taify grapevine. Data of nematode populations, number of galls and egg-masses, eggs/g root, plant lengths and weights and number of leaves were recorded four months after application. Results appeared significant differences between treatments and control. The triple application was more effective than dual and single applications in reducing nematode infestation and improving plant growth. Combined application of EMC or EMA with furadan or neem cake increased the efficacy (64.6-68.6%) and improved plant fresh weight (27.4-69.5%). Conclusively, utilization of such bacterial filtrates with either neem cake and/or nematicide could gain a successful approach in integrated nematode management programs.
Entomopathogenic bacteria, neem cake, nematicide, Meloidogyne incognita, integrated management, Grapevine.
Xenorhabdus, gram-negative bacterium of the family Enterobacteriaceae, is symbiotic bacteria with entomopathogenic nematode (EPN) of the genus Steinernema1. This bacterium (EPB) produces many bioactive compounds which demonstrate insecticidal, nematicidal, cytotoxic and anti-microbial activities2,3,4. These compounds are evolutionary products developed under strict selective pressure, and comprise a potent chemical against a large scale of eukaryotic and prokaryotic organisms5. Many EPN-EPB complexes occur, and many antimicrobial peptide profiles could be established. X. budapestensis DSM 16342 (EMA) and X. szentirmaii DSM 16338 (EMC) are the unique sources of highly efficient antimicrobial peptides against plant pathogens6,7. Root-knot nematodes, Meloidogyne spp. were amongst pathogens that cause serious commercial damage to fruit trees including grapevines8. Grapevines infested by nematodes ultimately exhibit destroyed roots, leading to the compulsory replacement of the plants9,10. Multiples classical methods and strategies as crop rotations, resistant rootstocks, nematicides and bio-agents are used for the management of phytonematodes infecting grapevine worldwide. The use of entomopathogenic bacterium, Xenorhabdus sp. has been evaluated and employed against root-knot nematodes infecting Taify grapevine11. In laboratory and field trials, S. feltiae–X. bovienii complex had suppressive effects on M. incognita infecting tomato plant12. Numerous research papers mentioned that for best nematode management on plants, more compatible materials should be applied. Combined applications of the fungus Verticillium chlamydosporium plus Heterorhabditid mutalistic bacterium, Photorhabdus luminescens and compost significantly reduced M. incognita infection and improved cucumber plant growth13. Over the past three decades, basic and applied research has shown that the use of neem (Azadirachta indica A. Juss.) products can provide a key component in guarantying integrated pest management14. Neem cake decreased the root galling when combined with bacterium, Pasteuria penetrans in a field infested with Meloidogyne incognita15. In numerous studies, entomopathogenic bacteria were evaluated alone or in combination with other microorganisms as fungi on root-knot nematodes, but no previous study has been conducted to use entomopathogenic bacteria combined with neem cake against M. incognita infecting grapevine. Therefore, this investigation was planned to evaluate the effectiveness of entomopathogenic bacteria, Xenorhabdus spp. (EMA and EMC) integrated with neem cake and/or nematicide (furadan) on performance of Taify grapevine cuttings and managing M. incognita under micro-plot conditions.
Bacterium strains
Xenorhabdus strains, X. budapestensis DSM 16342 (EMA) and X. szentirmaii DSM 16338 (EMC) which had been isolated from the entomopathogenic nematodes Steinernema bicornutum and S. rarum, are originated from the Fodor laboratory, Pannonia University, Keszthely, Hungary. The bacterial isolates were routinely grown in the dark on LBTA (Luria Bertani Agar) indicator plates at 25°C (trypton 10 g/L, yeast extract 10 g/L, sodium chloride 10 g/L, agar 15 g/L, and supplementing with bromothymol blue 25 mg/L, and 2,3,5-triphenyltetrazolium chloride 40 mg/L, 1L of distilled water [pH 6.8]). For preparing bacterial filtrate, single black – dark blue colonies of each bacterium was added separately into test tubes containing 5-mL of LB liquid medium as an inoculum for 100 mL culture. 100 mL aliquots of culture in 500 mL Erlenmeyer flasks were shaken overnight at 25°C, and then transferred to flasks containing 400 mL of the same media, shaking at 200 rpm for 5 days. The multiplied bacterial culture was centrifuged (13,000 rpm for 30 min) at 4°C. A supernatant was filtered through 0.22 μm Millipore filter to obtain cell free filtrate. The filtrate was stored at 4°C until required.
Neem cake
It is a by-product of the cold pressing from the neem seeds and kernels. Fresh seeds and kernels were collected from ripe fruits of 10 years old neem (Azadirachta indica A. Juss) trees growing in Taif region, Saudi Arabia, then cleaned and dried in the shade for one week. Neem cake was obtained by using cold-pressing vegetable oil machine from compressing neem seed and kernel16. After proper drying the formulations were crushed and converted into fine powder using grinder and stored in tin containers at 4°C.
Experimental layout and design
A micro-plot field experiment was conducted in a grapevine farm located at Taif region, Saudi Arabia, to determine the influence of entomopathogenic bacteria (EMA and EMC) and/or neem cake integrated with furadan at half of the recommended dose on nematode reproduction and the resulting effect on grapevine plant growth. The experimental area (35 m2) was heavy naturally infested with Meloidogyne incognita. The area was designed as a randomized complete block (RCB) and replicated five times. Each block included 12 treated plots and untreated check. A plot consisted of one row, 50 ׳ 90 cm was practiced. Roots of two-month-old grapevine seedlings var. Taify with two leaves were soaked in bacterial filtrate or LB medium for 15 min before transplanting. Plots were then planted with three seedlings each. An additional volume of 10 mL bacterial suspension or LB medium was introduced to the surface of the soil per plant and allowed to soak in. Two weeks later, neem cake was added around plants at a rate of 2 g/seedling in a single treatment and 1 g/seedling in concomitant applications, incorporated into the soil and then watered to allow decomposition. At the same time furadan as nematicide was applied singly at 0.6 g/seedling and at half dose (0.3 g/seedling) in integrated treatments. Five untreated plots were served as control. Therefore, a total of 13 treatments including a control viz. (1) EMC, (2) EMA, (3) Neem cake @ 2 g/plant, (4) Furadan 10 G @ 0.6 g/plant, (5) EMC+ Neem cake @ 1 g/plant, (6) EMA+ Neem cake @ 1 g/plant, (7) EMC+ Furadan 10 G @ 0.3 g/plant, (8) EMA+ Furadan 10 G @ 0.3 g/plant, (9) Neem cake @ 1 g/plant+ Furadan 10 G @ 0.3 g/plant, (10) EMC+ Neem cake @ 1 g/plant+ Furadan 10 G @ 0.3 g/plant, (11) EMA+ Neem cake @ 1 g/plant+ Furadan 10 G @ 0.3 g/plant, (12) LB medium (negative control), (13) Check (nematode only) were maintained in this experiment. Seedlings were harvested 4 months after planting and roots were washed free from adhering soil with tap water. Lengths and fresh weights of shoot and root, dry weights and number of leaves were measured. From each plot, a composite soil (250 g) was processed for J2s extraction by sieving and decanting method17. At each treatment, Roots were stained in acid fuchsin18 and examined for recording the number of galls, egg-masses and nematode in roots under a stereomicroscope at 40–100X magnification. Eggs were collected using sodium hypochloride technique19. The efficacy of treatments on nematode population was calculated with the equation of Henderson and Tilton. Efficacy % = [1- (Total nematode population of treated plants after application x Total nematode population of check plants before application) / (Total nematode population of treated plants before application x Total nematode population of check plants after application)] x 10020. Rate of nematode build-up = Pf/ Pi, where Pf is the final population and Pi is the initial population. The index for root galling (GI) and egg-mass (EI) were assessed on a 0-5 scale, where 0 = 0 galls or egg-masses and 5 > 10021.
Statistical analysis
Data was subjected to one-way analysis of variance (ANOVA)22 followed by Duncan’s multiple range test (P < 0.05) using COSTATE software package and treatment means were compared with the control plants infested with nematodes, according to the Dunnet’s test at P < 0.05 [ns (p < 0.12), * (p < 0.033), ** (p < 0.002) *** (p < 0.001), GraphPad Prism version 7.0). The experiment was performed once.
The influence of Taify grapevine seedlings treatment under micro-plot conditions with bacterial filtrate (EMC and EMA) alone or in combination with neem cake and/or nematicide compared to control treatment were studied. Results indicated that all treatments of bacterial filtrates, neem cake and furadan significantly reduced nematode infestation (Fig. 1 and Table 1). Treatments with three integrated components showed the least numbers of J2s population/250 g soil, nematode in root, galls, egg-masses and eggs/g root. Treatment with filtrate of EMC combined with neem cake and furadan significantly revealed the highest effect on the numbers of J2s population in soil [F (12, 52) = 389.8; P<0.05] (Fig. 1A), nematode in roots [F = 700.2] (Fig. 1B), galls [F = 933.9] (Fig. 1C) and egg-masses [F = 456] (Fig. 1D) when compared with other treatments and control. EMA+ neem+ furadan treatment ranked second to the previous treatment in the same nematode parameters, then application of furadan with EMC or EMA and neem cake plus bacterial filtrates. Single treatments also performed intermediate suppression in the total nematode populations in soil and root, galls and egg-masses. Treatment with furadan, resulted the minimum counts of all nematode infestation criteria followed by EMC treatment, whereas, treatment with neem alone resulted the lowest effect on nematode development (Fig. 1). The population of nematode in both soil and root were suppressed in all treated seedlings to be ranging between 805.6 and 2851.8 in comparison with the control that reached up to 4864.4 (Table 1). The highest efficacies on nematode population were observed when EMC or EMA filtrate was applied in combined form with neem cake and/or furadan as compared to separate allocations. Both treatments of EMC+ neem+ furadan and EMA+ neem+ furadan resulted in the maximum efficacy percentage of 83.9% and 80.7% as well as reduction percentage reached to 83.4% and 80.2%, respectively. Dual application of EMC or EMA with furadan or neem cake occupied the remarkable efficacy that averaged to 76.1%, 73.6% and 66.5%, 64.6% and reduction that valued to 76.4%, 74.3% and 62.5%, 59.7%, respectively. On the other hand, the lowest efficacy treatment was neem cake alone that reached to 54.2% with reduction percentage of 41.4%. While both EMC and EMA applied singly have 57.9% and 55.7% efficacy with reasonable reduction that averaged 48.2% and 43.9%, respectively (Table 1). Data in Table 1 also clarify that all treatments decreased the rate of nematode build-up ranging between 0.35 to 0.99, root galling index (2 to 4), egg-mass index (1.2 to 3.8) and number of eggs/g root (29.8 to 795).
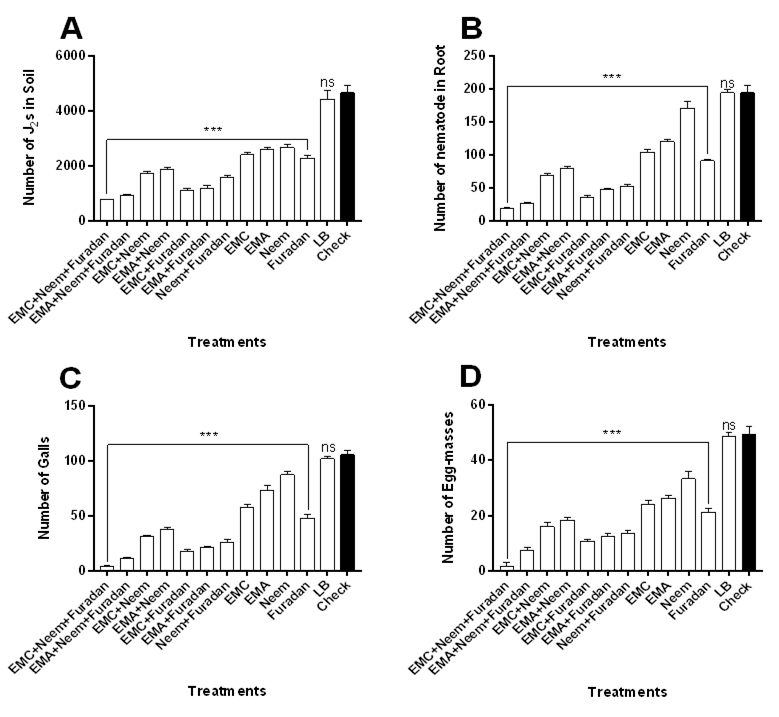 Fig. 1. Influence of treatments with bacterial filtrates, neem cake and furadan in single or concomitant applications on the infection of Taify grapevine seedlings with Meloidogyne incognita under micro-plot field conditions. Error bars represent SD.
Fig. 1. Influence of treatments with bacterial filtrates, neem cake and furadan in single or concomitant applications on the infection of Taify grapevine seedlings with Meloidogyne incognita under micro-plot field conditions. Error bars represent SD. Table (1):
Suppressive effect of entomopathogenic bacteria alone or combined with neem cake and/or furadan on the development of Meloidogyne incognita infested Taify grapevine seedlings under micro-plot field conditions
* Treatments |
Initial J2s/ 250 g soil |
aFinal nematode population |
Efficacy % |
bReduction % |
Rate of build-up |
Root gall index |
Egg-mass index |
No. of Eggs/ g root |
|---|---|---|---|---|---|---|---|---|
EMC |
2770 |
2520.4 d |
57.9 |
48.2 |
0.91 |
4 c |
3 b |
401.4 d |
EMA |
2850 |
2728.8 c |
55.7 |
43.9 |
0.96 |
4 c |
3 b |
494.4 c |
Neem |
2880 |
2851.8 c |
54.2 |
41.4 |
0.99 |
4 c |
3.8 a |
795 b |
Furadan |
2640 |
2377 d |
58.4 |
51.1 |
0.90 |
4 c |
3 b |
297.4 e |
EMC + Neem |
2520 |
1826 e |
66.5 |
62.5 |
0.72 |
3.8 c |
3 b |
203.4 f |
EMA + Neem |
2560 |
1961.8 e |
64.6 |
59.7 |
0.77 |
4 c |
3 b |
282.6 e |
EMC + Furadan |
2220 |
1149.2 g |
76.1 |
76.4 |
0.52 |
3 d |
2.6 b |
129.8 gh |
EMA + Furadan |
2190 |
1249.2 g |
73.6 |
74.3 |
0.57 |
3 d |
3 b |
157.6 fg |
Neem+ Furadan |
2430 |
1648.2 f |
68.6 |
66.1 |
0.68 |
3 d |
3 b |
181.6 fg |
EMC + Neem + Furadan |
2320 |
805.6 h |
83.9 |
83.4 |
0.35 |
2 e |
1.2 d |
29.8 i |
EMA + Neem + Furadan |
2310 |
965 h |
80.7 |
80.2 |
0.42 |
2.8 d |
2 c |
71.8 hi |
LB |
2340 |
4635.4 b |
8.4 |
4.7 |
1.98 |
4.6 b |
4 a |
1043 a |
Check (Nematode only) |
2250 |
4864.4 a |
— |
— |
2.16 |
5 a |
4 a |
1070.4 a |
*Each treatment was represented by five replicates (Plots), each with three plants. Numbers in each column followed by the same letter are not significantly different (P < 0.05 using Duncan’s Multiple Range Test in COSTATE statistical program). aFinal nematode population= Number of J2s/250 g soil + Number of nematode in root. bReduction % = Total nematode population of check plants – Total nematode population of treated plants/ Total nematode population of check plants x 100.
The effect of treatments on growth criteria of the grapevine seedlings including length and weight of shoot and root and number of leaves was recorded (Fig. 2 and Table 2). Results indicated that immersing seedling root in bacterial filtrates of EMC and EMA then treated with neem cake and furadan significantly provided best result in all growth parameters compared to other treatments and control (Fig. 2). Based up on lengths of harvested plants, treatment of seedlings with EMC filtrate prior to cultivation then amended with neem cake plus furadan resulted in significant largest plants relative to other treatments and control as measured by their shoot length (F= 60.1) (Fig. 2A) and root length (F= 23.5) (Fig. 2B). EMA+ neem cake+ furadan treatment ranked second in the same criteria, followed by combined application of EMC or EMA plus neem cake, then EMC alone treatment. When seedlings treated with EMA or neem cake plus furadan or neem cake alone, not significant difference was noted in shoot length (Fig. 2A). Separate application of furadan gave the smallest plants when compared to other treatments and nematode alone. The same trend was recorded in root length (Fig. 2B). Data also showed that the greatest shoot and root fresh weights were obtained with the seedlings exposed to EMC or EMA concomitant with neem cake and furadan with significant difference between them and other treatments as well as control. Statistically, there was no significant difference in shoot weight (F= 79.3) among treatments (EMC with EMA+ neem cake), (EMA with EMC+ furadan) and (neem cake with neem cake+ furadan) (Fig. 2C). Regarding root fresh weight, except for single furadan treatment, there was a significant difference between the nematode treatment and other treatments (F= 39.7) (Fig. 2D). Data presented in Table 2 showed that the tallest plants (60.2 cm) were observed from the combined application of EMC with neem cake and furadan with percentage of increase averaged to 90.5%, followed by EMA+ neem cake+ furadan treatment (82.9%), then dual applications of neem cake with EMC (79.7%) or EMA (71.5%). However, single application of EMC recorded 67.7% increase in plant length. The shortest seedling height was measured from the LB medium (negative control) treatment (32.4 cm, 2.5%) and furadan treatment (34 cm, 7.6%). Likewise, integrated treatment of EMC or EMA with neem cake plus furadan surpassed the other tested treatments in increasing percentages of increase in plant fresh and dry weights with values of 89% and 88.5% or 76.2% and 76.9%, respectively (Table 2). There was no statistically significant difference between EMC and EMA+ neem cake treatments in improving the previous plant growth measurements. For number of leaves, the results mirrored those from plant length and weight, maximum number of leaves was observed by the application of EMC (83.3%) or EMA (56.4%) with neem cake and furadan. However, number of leaves was minimum in case of single application of furadan (9%) and LB medium (5.1%) treatments.
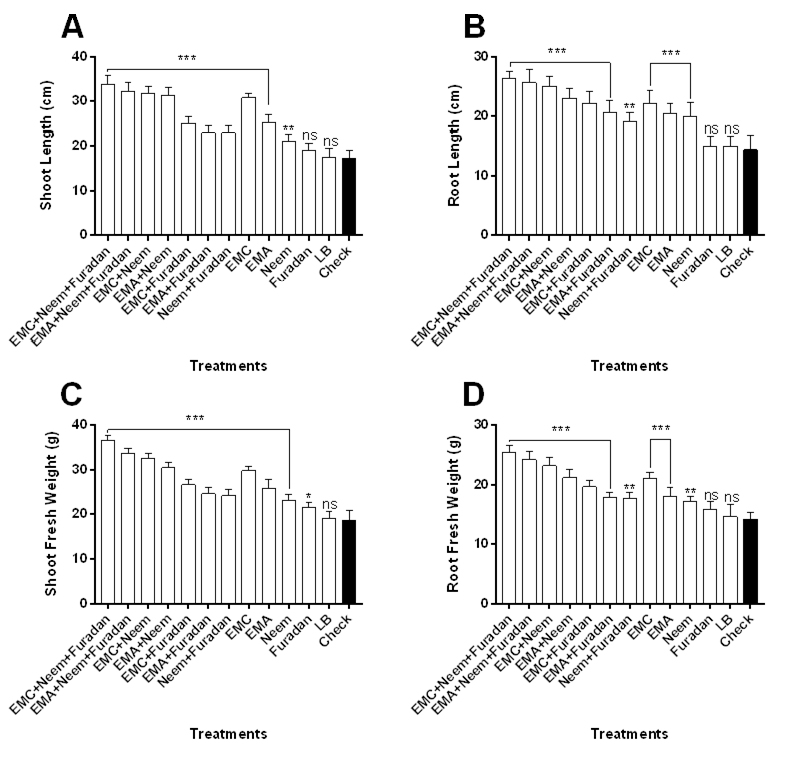 Fig. 2. Plant growth response of Taify grapevine to M. incognita infection as influenced by application of treatments. Error bars represent SD.
Fig. 2. Plant growth response of Taify grapevine to M. incognita infection as influenced by application of treatments. Error bars represent SD.Table (2):
Influence of entomopathogenic bacteria alone or integrated with neem cake and/or furadan on growth parameters of Taify grapevine seedlings infested with M. incognita under micro-plot field conditions
* Treatments |
Plant Length(cm) |
a Increase % |
Plant Fresh weight(g) |
Increase % |
Plant Dry weight(g) |
Increase % |
No. of Leaves |
Increase % |
|---|---|---|---|---|---|---|---|---|
EMC |
53 c |
67.7 |
50.8 c |
54.9 |
30.2 de |
45.2 |
20 cd |
28.2 |
EMA |
45.6 de |
44.3 |
43.8 de |
33.5 |
27.8 fg |
33.7 |
19.4 de |
24.4 |
Neem |
41 f |
29.7 |
40.4 f |
23.2 |
23.4 ij |
12.5 |
17.2 fg |
10.3 |
Furadan |
34 g |
7.6 |
37.4 g |
14 |
22.4 jk |
7.7 |
17 fg |
9 |
EMC + Neem |
56.8 a-c |
79.7 |
55.6 b |
69.5 |
32.8 c |
57.7 |
23 b |
47.4 |
EMA + Neem |
54.2 bc |
71.5 |
51.6 c |
57.3 |
31.2 cd |
50 |
21.2 c |
35.9 |
EMC + Furadan |
47.2 d |
49.4 |
46.2 d |
40.9 |
28.2 ef |
35.6 |
19.6 de |
25.6 |
EMA + Furadan |
43.6 d-f |
38 |
42.4 ef |
29.3 |
26 gh |
25 |
18.8 de |
20.5 |
Neem+ Furadan |
42 ef |
32.9 |
41.8 ef |
27.4 |
24.8 hi |
19.2 |
18.2 ef |
16.7 |
EMC + Neem + Furadan |
60.2 a |
90.5 |
62 a |
89 |
39.2 a |
88.5 |
28.6 a |
83.3 |
EMA + Neem + Furadan |
57.8 ab |
82.9 |
57.8 b |
76.2 |
36.8 b |
76.9 |
24.4 b |
56.4 |
LB |
32.4 g |
2.5 |
33.6 h |
2.4 |
22 jk |
5.8 |
16.4 g |
5.1 |
Check (Nematode only) |
31.6 g |
— |
32.8 h |
— |
20.8 k |
— |
15.6 g |
— |
* Each treatment was represented by five replicates (Plots), each with three plants. Numbers in each column followed by the same letter are not significantly different (P < 0.05 using Duncan’s Multiple Range Test in COSTATE statistical program). a Increase % = Growth measurement of treated plants – Growth measurement of check plants / Growth measurement of check plants × 100.
The use of certain natural or synthetic materials that have already been involved in integrated management of such pathogenic nematodes is a desire trend. Of these materials, there are bacterial and plant products. Utilization of such compounds singly or mixing has earned much more benefits in excreting nematode development and diminishing grapevine damage under greenhouse or outdoor conditions11,23,24. However, results of the present micro-plot field investigation initiate the novel phenomenon in suppressing root-knot nematode associated with improving grapevine growth parameters using entomopathogenic bacterial filtrate separately or mixing with either neem cake and/or furadan as a nematicide at the minimum rates to avoid environmental pollution. Apparently, results from the present experiment indicated that triple concomitant applications included bacterial filtrates gave the maximum reduction of nematode population, root galling and egg-mass formation as well increasing grapevine growth over the control and the other treatments. Meanwhile, dual applications of bacteria with furadan at half of recommended dose ranked second in reducing previous nematode parameters, although they not acted as a good growth promotors for grapevine seedlings. On the other hand, plants receiving bacterial filtrates then amended with neem cake improved plant growth and gave reasonable reductions in nematode criteria. Exposing grapevine roots to bacterial filtrates before treating with neem cake may possibly undergo physiological changes stimulating a certain degree of resistance in plants against nematode penetration and development. These results agree with those of25, who mentioned that Xenorhabdus sp. filtrate suppressed M. incognita penetration into groundnut roots. Several researchers recorded that entomopathogenic symbiotic bacteria, Xenorhabdus and Photorhabdus are environmentally benign and produce some of the active compounds include xenorhabdins and xenocoumacins, bacteriocins, proteinaceous chitinases and non-protein indoles and stilbene derivatives. These metabolites have shown different bioactivities against pests and pathogens including nematodes26,27,28. Recently, seven compounds were isolated from X. budapestensis SN84 and tested for their nematicidal properties against J2s of M. incognita. Among tested compounds, Rhabdopeptide J, 2 showed strong inhibitory activity29. The toxicity and repellency effects of cell-free bacterial suspensions of Xenorhabdus on the second-stage juveniles of M. incognita were almost entirely due to ammonium30. The present results are also agreed with those of31 who reported that combined application of Pasteuria penetrans and neem extract maximized shoot length and weight of babchi plant and minimized number of juveniles per root system. Neem cake plus Glomus fasciculatum increased the plant growth of tomato and reduced Meloidogyne incognita reproduction and root-galling32. The metabolites released during the decomposition of neem including azadirachtin, carotenoids, phenolic compounds, triterpenoids, salannin, limonoids and steroids and ketones stimulated and change the physiology of plant cells to release abnormal compounds which repel the nematodes from the uninfected cells and tissues of plant33,34. In the present study, among single applications, furadan at full recommended dose was the uppermost treatment achieving the highest nematode suppressive rates, while a phytotoxic effect may occur since it gave the least values of growth characters. Here we investigated entomopathogenic bacterial filtrate as a possible sustainable adjuvant for use with neem cake and nematicide. Neem cake was possibly increase activity of antagonistic microorganisms by releasing mineral elements into soil, increasing osmotic potential of soil solutions35 and thereby nematode control was enhanced36 and plant growth was improved. Concomitant application of bacterial filtrate with neem cake plus furadan decreased rate of nematode build-up 6-fold, whereas, double application included furadan (4-fold) and neem cake (3-fold). Obviously, the present findings indicated that the bacterial filtrate applied either singly or integrated with neem cake and/or furadan at a half of recommended dose was the best applications in improving growth of grapevine and suppressing M. incognita development and reproduction in the naturally infested soil. Although several investigations recorded the nematicidal activity of entomopathogenic symbiotic bacterial filtrates singly in laboratory and greenhouse but this is the first report that EMC and EMA could fit well to the principles of integrated nematode management, thanks to their safety to environment, humans and animals and absence of nematode resistance. The results also support our hypothesis that bacterial filtrates can act additively or synergistically with other agricultural inputs in sustainable management programs of nematode.
It can be concluded from the present investigation that the use of EMC or EMA, neem cake and nematicide in integrations represents a promising novel approach for the integrated management of root-knot nematode infecting Taify grapevine and enhances the growth of plant.
Acknowledgements
The authors would like to thank Dr. Fodor Andras for providing EMA and EMC bacteria.
Conflicts of Interest
The authors declares that there is no conflict of interest.
Authors’ Contribution
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
The authors gratefully acknowledge Taif University, Kingdom of Saudi Arabia [grant number 1-438-5762] for the financial support.
Data Availability
All datasets generated or analyzed during this study are included in the manuscript.
Ethics Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
- Forst S., Dowds B., Boemare N.E., Stackebrandt E. Xenorhabdusspp. and Photorhabdus spp.: bugs that kill bugs. Annu. Rev. Microbiol., 1997; 51: 47–72.
Crossref - Bode, H.B. Entomopathogenic bacteria as a source of secondary metabolites. Curr. Opin. Chem. Biol., 2009; 13: 224–230.
Crossref - Andalo V., Rocha F.S., Maximiniano C., Moino A., Campos V.P. In vivo and in vitro study of the effects of entomopathogenic bacteria and their filtrates on Meloidogyne incognita. Int. Research J. Microbiol., 2012; 3: 005-009.
- Nour El-Deen A.H., Andras F., Al-Barty A.F. Nematicidal activity of entomopathogenic bacteria against root-knot nematodes, Meloidogyne incognita in-vitro. Int. J. Adv. Res., 2014; 2: 708-713.
- Houard J., Aumelas A., Noal T., Pages S., Givaudan A., Fitton-Ouhabi V., VillainGuillot P., Gualtieri M. Cabanillasin, a new antifungal metabolite, produced by entomopathogenic Xenorhabdus cabanillasii JM26. J Antibiot., 2013; 66 (10): 617-620.
Crossref - Fodor A., Varga I., Hevesi M., Mathe-Fodor A., Racsko J., Hogan J.A. 2012. Antimicrobial peptides of Xenorhabdusorigin against multidrug resistant plant pathogens, pp. 147–195. In Bobbarala V. (ed.), A search for antibacterial agents. Rijeka.
Crossref - Vozik D, Bélafi-Bakó K, Hevesi M, Böszörményi E, Fodor A. Effectiveness of a peptide-rich fraction from Xenorhabdus budapestensis culture against fire blight disease on apple blossoms. Not. Bot. Horti. Agrobo., 2015; 43 (2): 547-553.
- Kesba H.H. 1999. Ecological and pathological studies on some plant parasitic nematodes infecting grape, Vitis vinifera L. MSc Thesis, Cairo Univ., Egypt.
- Pinkerton J.N., Forge T.A., Ivors K.L., Ingham R.E. Plant-parasitic nematodes associated with grapevines, Vitis vinifera, in Oregon vineyards. Supplement to the J. Nematol., 1999; 31: 624–634.
- Montealegre J., Aballay E., Sanchez S., Rivera L., Fiore N., Pino A. Hongos y nematodos fitopatףgenos asociados al sistema radical en uva de mesa en la III Regiףn de Chile. Aconex, 2009; 103: 5-9.
- Nour El-Deen A.H., Alghamdi A.S., Al-Barty A.F., Darwish H.Y., Samra B.N., Al-Qurashi A., Al-Malki K., AL Osaimi H. Management of root-knot nematode, Meloidogyne incognita by novel Bio-methods on grapevine. Biosci. Res., 2018; 15: 3033-3039.
- Lewis E.E., Grewal P.S., Sardanelli S. Interactions between the Steinernema feltiae–Xenorhabdus bovienii insect pathogen complex and the root-knot nematode Meloidogyne incognita. Biol. Control, 2001; 21: 55–62.
Crossref - Zakaria H.M., Kassab A.S., Shamseldean M.M., Oraby M.M., El-Mourshedy M.M. Controlling the root-knot nematode, Meloidogyne incognita in cucumber plants using some soil bioagents and some amendments under simulated field conditions. Ann. Agric. Sci., 2013; 58: 77–82.
Crossref - Saxena, R.C. Neem for sustainable pest management and environmental conservation. ECHO Asia Notes, 2015; 24: 1-17.
- Reddy P., Nagesh M., Devappa V. The effect of integration of Pasteuria penetrans, Paecilomyces lilacinus and neem cake for the management of root-knot nematodes infecting tomato. Pest Manage. Hortic. Ecosyst., 1997; 3: 100-104.
- Duong D.H., Ngo X.Q., Do D.G., Le T.A., Nguyen V.T., Nic S. Effective control of neem (Azadirachta indica A. Juss) cake to plant parasitic nematodes and fungi in black pepper diseases in vitro. J. Viet. Environ., 2014; 6: 233-238.
- Barker K.R. 1985. The application of microplot techniques in nematological research, pp. 127–134. In BakerK.R., Carter C.C., Sasser J.N. (eds.), An Advanced Treatise on Meloidogyne: Methodology. North Carolina State University Graphics, Raleigh, USA.
- Byrd D.W., Kirkpatrick J.T., Barker K.R. An improved technique for clearing and staining plant tissue for detection of nematodes. J. Nematol., 1983; 14: 142-143.
- Hussey R.S., Barker K.R. A comparison on methods of collecting inocula of Meloidogyne spp. including a new technique. Plant Dis. Reptr., 1973; 57: 1925-1928.
- Puntener W. 1981. Manual for field trials in plant protection. Agric. Div., Ciba-Geigy Limited, Basle, Switzerland.
- Taylor A.L., Sasser J.N. 1978. Biology, Identification and Control of Root-knot Nematodes (Meloidogyne Species), p. 111. Dep. Plant Pathol., North Carolina State Univ. and U.S. Agency Int. Dev., Raleigh, USA.
- Gomez K.A., Gomez, A.A. 1984. Statistical procedures for agricultural research, 2nd Ed. John Wiley & Sons Inc., New York, USA.
- El-Saedy M.A., El-Sayed M.E., Hammad S.E. Efficacy of boron, silicon, jojoba and four bio-products on controlling Meloidogyne incognita infecting thompson seedless grapevines. American-Eurasian J. Agric. Environ. Sci., 2015; 15: 1710–1720.
- El-Nagdi W.M., Hafez O.M., Taha R.A. Efficiency of jojoba oil and bio-nematicide on Meloidogyne incognita and performance of flame seedless grapevine cuttings. Agric. Eng. Int., 2017: 118-124.
- Vyas R.V., Patel B., Maghodia A., Patel D.J. Significance of metabolites of native Xenorhabdus, a bacterial symbiont of Steinernema, for suppression of collar rot and root-knot disease of groundnut. Ind. J. Biotechnol., 2008; 7: 371–377.
- Samaliev H.Y., Andreoglou F.I., Elawad S.A., Hague N.G. The nematicidel effects of the bacteria Pseudomonas oryzihabitans and Xenorhabdus nematophilus on the root knot nematode Meloidogyne javanica. Nematology, 2000; 2: 507-514.
Crossref - Brachmann A.O., Bode H.B. Identification and bioanalysis of natural products from insect symbionts and pathogens. Adv. Biochem. Eng. Biotechnol., 2013; 135: 123″155.
Crossref - Challinor V.L., Bode H.B. Bioactive natural products from novel microbial sources. Ann. Acad. Sci., 2015; 1354: 82″97.
Crossref - Bi Y., Chunzhi G., Zhiguo Y. Rhabdopeptides from Xenorhabdus budapestensis SN84 and their nematicidal activities against Meloidogyne incognita. J. Agric. Food Chem., 2018; 66: 3833″3839.
Crossref - Grewal P.S. 1999. Insecticidal nematode laboratory, pp. 15–24. In Polavarapu S. (ed.), Optimal Use of Insecticidal Nematodes in Pest Management. Workshop Proceedings, Rutgers University, USA.
- Mehtab A., Javed N., Khan S.A., Gondal A.S. Combined effect of Pasteuria penetrans and neem extract on the development of root-knot nematode in medicinal plants. Pak. J. Nematol., 2013; 31: 55-59.
- Rizvi R., Geeta S., Rizwan A.A., Sartaj A.T., Irshad M. Sustainable management of root-knot disease of tomato by neem cake and Glomus fasciculatum. Cogent Food Agric., 2015; 1: 1-13.
Crossref - Jacobson M. 1990. Review of neem research in the United States, pp. 4-14. In Locke L., Lawson R. (eds.), Proceedings of a workshop on neem’s potential in pest management programs. USDA-ARS, Beltsville, USA.
- Akhtar M., Malik A. Roles of organic amendments and soil organisms in the biological control of plant parasitic nematode. Bioresour. Technol., 2000; 74: 35-47.
Crossref - Stirling G.R. 1991. Biological control of plant parasitic nematodes. CAB International, Oxon, UK.
- Wang K.H., Sipes B.S., Schmitt D.P. Management of Rotylenchulus reniformisin pineapple, Ananas comosus, by intercycle cover crops. J. Nematol., 2002; 34: 104-114.
© The Author(s) 2019. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


