The use of Omega-3 fatty acids is garnering increasing interest due to its substantial effects on human health. Fish are a major dietary source of ω3FA; however, they are not the primary producers. The primary producers are microalgae and other microorganisms, which serve as the original source of omega-3s in the food chain. The marine fish, however, has not been able to meet the global demands. Recently, the generation of fatty acids has been investigated from another source, namely microorganisms. Omega-3 fatty acids are naturally produced by the microorganisms. However, the use of metabolic engineering has provided evidence for improvements in production. The current article reviews research on microorganisms using engineering ways to accumulate ω3FA such as docosahexaenoic acid or DHA and eicosapentaenoic acid or EPA. These studies have demonstrated that modulation of existing pathways, as well as reconstitution of biosynthetic pathways, has a high potential for increasing the fatty acid yield. However, certain bottlenecks limit the yield of fatty acids in various host organisms. These may include the fatty acid flux of intermediates that exists between various lipid pools. Even though the heterologous and native microbes show fatty acid flux under acyltransferases control, there is evidence that modulation of even a single acyltransferase by genetic approach can provide significant alteration for producing fatty acids. The microbes with oleaginous properties are being identified rigorously and are expected to further advance the process of engineering which leads to enhanced production of fatty acids in microbes.
Omega-3 Fatty Acids, Eicosapentaenoic Acid (EPA), Docosahexaenoic Acid (DHA), Metabolic Engineering, Desaturase and Elongase Pathway, Polyketide Synthase (PKS) Pathway
Omega-3 fatty acids, their source, and use
Omega-3 fatty acids (ω3FA) contribute significantly to human cell membranes and are extensively dispersed in nature.1 α-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) are the main long-chain polyunsaturated omega-3 fatty acids.2 These fatty acids are essential for lipid metabolism. The fatty acids impart energy and form vital functional components in body functions like immunity, endocrine system, lungs, heart and blood vessels.3,4 ALA is not produced in the human body, making it a vital component that must be obtained from meals and beverages. The transformation of ALA gives rise to EPA and DHA that are mandatory for retina and brain function.2 Thus, the supplementation of such essential fatty acids is vital for humans.
The researchers have proposed alternative sources such as fish oil, transgenic plants, microalgae, etc.3 The fatty acids production by microbes is presently considered for applications in commercial industries in the world.5 The oil from microbes has various advantages including benefits to health due to the presence of squalene or phytosterols and does not have odor or cholesterol.6 Another advantage of using these microbes is that they can be cultivated easily, they are independent of season and available full-year.6-8 It is because of these features that fatty acid production using microbes is an effective and attractive approach for application in the industry.
Studies on various kinds of microbes have reported anabolic pathways for fatty acids, and there are several studies performed on the cultivation strategies. The technique of single-cell oil production is also reported previously for the manufacture of DHA and ARA.9 Various oleaginous fungus and microalgae have been used to commercialize DHA and ARA production.10 To fulfill the worldwide demand for these fatty acids, it is critical to design an efficient method for their manufacturing.3 The application in the industry can be boosted by the metabolic engineering process that can improve production of fatty acid by using oleaginous microorganisms.
Microbial source of omega-3-fatty acid
The various types of fatty acids can be synthesized from a wide range of microbes that include yeast, diatom, fungi, and algae. The microbe species that can synthesize high levels of ARA, DHA, EPA include Thraustochytrium, Mortierella alpina, Phaeodactylum tricornutum, and Chlorella minutissima.11,12 DHA producers are Crypthecodinium cohnii, Thraustochytrium sp., Aurantiochytrium sp., and Ulkenia sp., while ARA producer includes M. alpine.13,14
Genetic Engineering to Increase Omega-3 Fatty Acid Yield
The ALA production was observed to be increased when Yarrowia lipolytica, Mortierella alpina, Schizochytrium, and Crypthecodinium cohnii strains were grown using agro-industrial wastes.15 The genetic engineering of Yarrowia lipolytica yeast yielded a high amount of DGLA, ARA, and EPA.16 The genetic engineering of Rhodosporidium toruloides was found to boost ALA production to nearly 49% by introduction of Δ12 desaturase (FAD2) and ω3 desaturase (FAD3) gene.
The pollutants made by humans such as polychlorinated biphenols, dioxins, and methyl mercury are present in some fish oils and due to the decline of stocks of fish worldwide, there is an issue of their utility for DHA and EPA production.2 The stability and sustainability of microbial oils with high amount of DHA and EPA in comparison to oil from fish is more. In addition, the oils from microbes are enrich in naturally produced antioxidants that aids in the prevention of oxidative damage.2,9 Thus, the utilization of various microbes is possible for the special oil production. For example, omega-3 oils from the microbial source like Schizochytrium and Crypthecodinium species.17 The DHA and ARA are breast milk components that are involved in the development of neonates.
The use of high-throughput technology like whole-genome mutagenesis have improved fatty acid synthesis successfully in traditional strain.2 The whole-genome mutagenesis was performed for M. alpine for production of fatty acids and Di-homo-γ-linolenic acid (DGLA) rich oil.18 The process of mutagenesis has been applied to Thraustochytrid strains for an alteration of fatty acids through mutations.1
IMPORTANCE OF OMEGA-3-FATTY ACID
There are varieties of health benefits that ω3FA have. These include anxiety and depression, eye, development of brain during the time of pregnancy and early age, heart related disease, Attention deficit hyperactivity disorder, inflammation, metabolism related syndrome, autoimmune diseases, age-related mental diseases, mental abnormalities, and cancer, Alzheimer’s disease, child asthma, liver fat, bone and joint health, pain during periods, and skin health.
Anxiety and depression are fairly common disorders across the globe. Depression causes lethargy, sadness, and loss of interest in life,19 whereas anxiety causes nervousness and constant worry.20 It is interesting to note that the consumption of ω3FA helps in decrease of depression and anxiety. The supplementation to individuals suffering from anxiety showed improvement in symptoms.19,21 EPA is one of the fatty acids that was shown to have significant effects against depression and was able to work as an antidepressant drug.22
The fatty acids can also aid in the improvement of eye health. The DHA is observed as a structural component in the eye, particularly in the retina. The lack of DHA can result in vision problems. The intake of appropriate quantities of ω3FA is related to the reduction in macular degeneration risk which is a main reason of blindness and eye damage across the globe.23,24
The use of ω3FA is vital for the development and growth of the in infant’s brain. In the brain, 40% of polyunsaturated fatty acids (PUFA) are attributed to DHA and in the eye, 60% are attributed to DHA.25,26 Thus, the eyesight of infants that take DHA-fortified formula is better in comparison to those infants that don’t take DHA.27 Omega-3 fatty acids provide several advantages during pregnancy, including improved social and communication abilities, increased IQ, healthy development, a lower chance of autism, cerebral palsy, and attention deficit hyperactivity disorder, and fewer behavioral disorders.28
The heart diseases related risks such as stroke, heart attack, which are leading death cause in the world, can also be reduced with the consumption of omega-3 fatty acids. It had been long since the prevalence of these diseases was found to be lower in communities with fish consumption that was further related to consumption of fatty acids.29 Heart health is related to fatty acids due to many benefits. The omega-3 fatty acids are proved to reduce triglycerides by 15%-30% in individuals. Omega-3 fatty acid ingestion was also found to reduce blood pressure. Moreover, omega-3 fatty acids were shown to increase the level of HDL cholesterol.30 The effect of omega-3 fatty acid was also observed on the aggregation of platelets that prevents clot formation in the blood.31,32 The omega-3 fatty acids prevent the damage to arteries and keep them smooth by preventing the formation of plaque that is the major cause of hardening of arteries. Omega-3 fatty acids decrease inflammation and have a significant role in the inflammation related response. In certain individuals, Omega-3 fatty acid’s use can reduce LDL cholesterol levels, but the indications provided by studies are mixed in nature.33,34 Although there are many beneficial effects on the risk factors for heart issues; however, the evidence on the prevention of strokes or heart attacks is still not conclusive with some investigations showing no benefits.35
Attention deficit hyperactivity disorder is another disease that has been linked to omega-3 fatty acid usage. The disease is behavioral and is characterized by impulsivity, hyperactivity, and inattention.36 The children with the disease were observed to exhibit lower levels of omega-3 fatty acid in comparison to healthy peoples. Studies have indicated that supplementation with omega-3 can improve the symptoms of attention deficit hyperactivity disorder, aid in the completion of tasks, improve attention, decrease aggression, restlessness, impulsiveness, and hyperactivity.37,38 Fish oils have also been shown to be an effective therapy for attention deficit hyperactivity disorder.39
Metabolic syndrome is another condition that can benefit from supplementation of omega-3 fatty acid. The metabolic syndrome includes a group of diseases like obesity, insulin resistance, higher level of LDL cholesterol, lower levels of HDL cholesterol and higher blood pressure. They enhance the danger for diabetes and heart diseases and gain a major health concern. The use of omega-3 fatty acid was shown to reduce inflammation, insulin resistance, and risk of heart problems in individuals with metabolic syndrome.40
Inflammation, on the other hand, is the response to damage or infection in humans and is very crucial for the health of an individual. In certain cases, however, it can last for a longer duration without injury or infection. This condition is referred to as long-term or chronic inflammation. Omega-3 fatty acids have been shown to reduce the synthesis of cytokines and eicosanoids, which are associated with inflammation.41
Moreover, ω3FA can fight against autoimmune diseases as well, where the body recognizes the own cells as foreign and attacks them. During early life, it is essential to use the ω3FA to fight certain diseases. Availability of ample omega-3 fatty acids at early stages have been linked to lowered disease risk such as multiple sclerosis, type 1 diabetes and autoimmune diabetes. It may also aid in the treatment of Crohn’s disease, psoriasis, rheumatoid arthritis, lupus and ulcerative colitis.42
Reports have shown that psychotic individuals have reduced amounts of ω3 fatty acids.21 The supplementation of these has been suggested to lower mood swing frequency and in the case of bipolar disorder and schizophrenia, it can lower relapse in individuals.21 The violent behavior was also observed to be decreased with the intake of ω3FA.43
The brain’s function declines as we age. Various studies have related ω3FA with reduction of decline in mental health and risk of Alzheimer’s disease.44,45 An investigation highlighted that supplementation of ω3FA at the time of onset of disease may be beneficial due to mildness of Alzheimer’s disease.46
In the western world, cancer contributes to the death rates significantly. The claim that ω3FA lower cancer risk was investigated. In cancer related studies, it is highlighted that ω3FA reduces the 55 percent risk of having cancer in the colon.47 Consuming ω3FA has been linked to lower risks of prostate and breast cancer. These results, however, were not consistent across all studies.48,49
Asthma is another health condition that is characterized by wheezing, shortness of breath, and cough and forms a dangerous threat to life. The cause includes swelling and inflammation of lung airways. The frequency of asthma has been rising in past years across the world including the US.50 The relation of ω3FA with reduced risk of asthma has been reported by various studies on young adults and children.51
ω3FA can also cure non-alcoholic fatty liver disease. In the Western world, rising obesity has resulted in an increase in non-alcoholic fatty liver disease.52 Using ω3FA may decrease inflammation associated with fatty liver and non-alcoholic fatty liver disease.52
Skeletal system is defected by two common diseases known as arthritis and osteoporosis. The use of ω3FA has the potential to increase the strength of the bone through calcium boosting in bones that alleviate osteoporosis risk.53 The individuals on ω3FA show the pain reduction in joints and an increase in strength of the grip.54
The quality of life can be significantly altered by the pain radiating in the thighs and lower back from the pelvis and lower abdomen due to menstruation. Research indicates that women with ω3FA have less discomfort during menstruation compared to those without.55 A research found that using ω3FA instead of ibuprofen was more effective for treating menstruation discomfort.56
The skin contains DHA as a structural element. The cell membrane of the skin is maintained by DHA that is a major part. The cell membrane remains moist, soft, supple, and without wrinkle, if it is healthy. The EPA is known to impart several benefits to the skin. These include skin hydration and management of production of oil, prevention of the red bumps observed on upper arms due to follicular hyperkeratinization of hairs, reduction of acne risk, and premature aging. The skin damage from the sun can also be protected by ω3FA. EPA aids in blocking collagen damage observed after exposure to the sun.57
Microbial biosynthesis of omega (ω)-3 fatty acids
For lipid production, acetyl-CoA and NADPH are continuously required with an excess of carbon.58 The generation of acetyl-CoA (Figure) is continually produced in oleaginous organisms through a series of processes initiated by an environment devoid of nutrients and causes the citrate pool to expand in the mitochondria. The dependent enzyme on Adenosine monophosphate (AMP) in the Kreb’s cycle is the isocitrate dehydrogenase (IDH) enzyme. The IDH is responsible for performing oxidative decarboxylation reaction in the oleaginous organisms. The AMP is converted to ammonia and IMP under nitrogen-deficient conditions because of increased AMP deaminase activity in the mitochondria. The consequence of this reaction is the blockage of isocitrate to citrate conversion and citrate translocation to cytoplasm.59
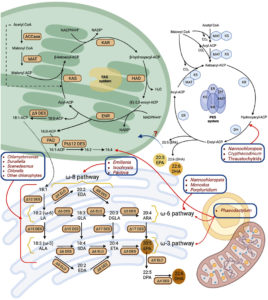
Figure. Pathways of ω (omega)-fatty acid biosynthesis in microorganisms.106 In microalgae, saturated (C16:0) and monounsaturated (C18:1) fatty acids are initially produced within chloroplast and subsequently transported to endoplasmic reticulum, where further desaturation and elongation occur. The specific desaturation and elongation pathways-namely omega-3, omega-6, or omega-8 differ among species. Additionally, certain microalgae possess a cytoplasmic PKS (polyketide synthase) pathway capable of synthesizing DHA and EPA through iterative chain extension and desaturation. The lines, red in color, highlight the presence of these biosynthetic routes in various algal taxa, blue boxes denote species-specific pathways, dashed arrows represent multi-step enzymatic conversions, and question marks indicate unidentified mechanisms for DHA/EPA incorporation into the endoplasmic reticulum.
Abbreviations used include: ACCase (acetyl-CoA carboxylase), ACP (acyl carrier protein), ALA (alpha-linolenic acid), ARA (arachidonic acid), DES (desaturase), DGLA (dihomo-gamma-linolenic acid), DPA (docosapentaenoic acid), EDA (eicosadienoic acid), ELO (elongase), ENR/ER (enoyl reductase), ETA (eicosatetraenoic acid), FAS (fatty acid synthase), GLA (gamma-linolenic acid), HAD/DH (hydroxyacyl dehydratase), KAR/KR (ketoacyl reductase), KAS/KS (ketoacyl synthase), LA (linoleic acid), MAT (malonyl/acetyl transferase), PAD (palmitoyl-ACP ִΔ9 desaturase), PtִΔ12 DES (plastidial ִΔ12 desaturase), and SDA (stearidonic acid)
ATP: citrate lyase (ACL) enzyme cleaves acetyl-CoA and oxaloacetate (OAA) from citrate, resulting in a constant source of acetyl-CoA for lipid synthesis.60 Other than acetyl-CoA, NADPH is also an important parameter for fatty acid synthesis. According to reports, malic enzyme’s decarboxylation activity is ascribed to the conversion of malate to pyruvate, which processes NADPH supply.58 In certain microbes such as Yarrowia lipolytica, the NADPH regeneration of is possibly catalyzed by malic enzyme. Pentose phosphate pathway also forms an alternative pathway for the production of NADPH.61 Stearic acid or palmitic acid form the end products for biosynthesis of lipids in microbes.62 Desaturase and elongase enzymes function sequentially to produce polyunsaturated fatty acids through desaturation and elongation activities. Hence, amount and type of the fatty acid are dependent on elongases and desaturases genes in the microbial genome.58
The humans can either take DHA as a dietary component or can use it from the transformation of DPA or EPA in a lesser amount as intermediate. The Δ4-desaturase is required for this elongation step. It has been observed that “Sprecher’s shunt” is more likely to produce DHA through C24 intermediate.63 Microbes including mosses, fungus, and microalgae use a series of enzyme processes to synthesize DHA. These involve two major pathways namely PKS pathway (polyketide synthase pathway) which is anaerobic and elongase/desaturase pathway which is aerobic.
Key Determinants of Microbial Production Efficiency
A variety of variables impact the formation of ω3FA. These include temperature, pH, aeration, media composition, incubation time, stabilization of ω3FA. It was observed, cell growth is favored by high temperature, whereas production of fatty acids is favored by lower temperature.64 Psychrophiles create small amounts of fatty acids at optimum temperatures. Thus, cyanobacteria and bacteria rarely produce fatty acids. The EPA production is favored by low growth temperatures. For instance, the Mortierella species are known to produce EPA at high concentrations in 12-15 °C temperature. The change in temperature from 25 to 11 °C was observed as the triggering factor for the production of EPA. But post cultivation for 4 days at 12 °C when 25 °C temperature was maintained, there was no increase in production of EPA.65 It was shown that 21.5 °C was the optimum temperature for P. tricornutum to enhance the production of EPA whereas M. alipina showed a rapid decrease in EPA production.66
pH is also known to affect the synthesis of fatty acids. The pH is recommended to be maintained optimum as per the microbes used, as the change in pH will require energy to restore it to normal. Fungi produce more fatty acids when the pH of their culture medium rises. For EPA production in algae and fungi, the optimum pH is 6.0-7.6. The pH of 6.0 is required by Thraustochytrium aureum, whereas Mortierella fungi and red alga Porphyridium requires 6-7 pH initially.66 The pH value of 8-9 is required for the production of ARA by Mortierella species.67
The presence of molecular oxygen in the medium influences the composition of fatty acids. The level of unsaturation is governed by the availability of oxygen, which is required in the desaturation pathway by bacteria. Gyronidium cohnii is a dinoflagellate that exhibits an increase in fatty acid content with oxygen level increment.68
The components like carbon source, cell density, light intensity, metal ions, media salinity influence the composition of fatty acids. The Mortierella is known to use linseed oil for the enhancement of EPA production. Using glucose, maltose, or starch as carbon source, the Thraustochytrium aureum can produce an increased amount of DHA.69
Incubation period is another element that influences fatty acid synthesis. Extension of time for fermentation can amplify EPA. It was observed that after 20 days of incubation, maximum lipid content and biomass was recovered from fungal species. The EPA concentration was elevated even after 36 days.70
The susceptibility of fatty acids towards oxidation is high that can further cause the production of toxic substances like peroxides or volatile substances.2 There is a complex mechanism that underlies fatty acid oxidation and is dependent on the form of the lipid. The oxidation reaction can be catalyzed by temperature, light, or the trace metals present. Thus, optimum storage, processing, and packaging are required for the microbial oil. There are antioxidants like Ascorbic acid, gallic acid, lactoferrin, ascorbyl palmitate, tocopherols, propyl gallate that can be added.3 Another approach of microencapsulation has been proposed with the potential to increase stability of oil and control of-flavors.3
Aerobic Desaturase and Elongase Pathway
Microalgae, thraustochytrids, fungi, bacteria, mammals, plants, and other microorganisms use an aerobic enzyme encoded route to synthesize ω3FA.4 The enzymes can be divided into two categories: chain elongation and fatty acid desaturation (Figure). The desaturation process is catalyzed by desaturase enzyme by the introduction of a double bond in the carboxyl end of the substrate fatty acid. In microbes, the front-end desaturase namely, Δ8, Δ6, Δ5, and Δ4 desaturases, and membrane-bound desaturases namely Δ15, Δ12, and Δ3 desaturases are present.71 The elongation complex is for fatty acid chain elongation and consists of 4 subunits namely, Acyl-CoA elongase, enoyl-CoA reductase, ketoacyl-CoA reductase, hydroxyacyl-CoA dehydratase.72
The steps involved in the synthesis of DHA are as follows:
- Step I: Generation of stearidonic acid (SDA) from Alpha-linolenic acid catalyzed by Δ6 desaturase on 6th carbon.
- Step II: Generation of eicosatetraenoic acid (ETA) from SDA by action of Δ6 elongase.
- Step III: Generation of EPA from ETA by the action of Δ5 desaturase on 5th carbon.
- Step IV: Generation of docosapentaenoic acid (DPA) from EPA by action of Δ5 elongase.
- Step V: Production of DHA from DPA by the action of Δ4 desaturase on 4th carbon.
Photosynthetic and non-photosynthetic microorganisms produce de novo fatty acids in their plastids and cytoplasm. Endoplasmic reticulum-associated enzymes, on the other hand, are primarily responsible for fatty acid desaturation and elongation.4 Stearic acid is the end product for fatty acid synthase and is formed by a reaction catalyzed by an enzyme known as acyl carrier protein Δ9-desaturase. The ω3 desaturases (mainly Δ12 and Δ15) are present in microbes, some cyanobacteria and plants for the formation of linoleic acid and ALA. These enzymes, however, are lacking in animals and humans.4 The microbes thus need the insertion of desaturases and elongases to overcome the deficiency of such enzymes.4 The use of the genetic engineering approach has been combined with the conventional methods to scale up fatty acid production.
The ω3FA are formed in microbes through 2 convergent and different pathways. These are Δ8-pathway and Δִ6-pathway. The Δ6-pathway involves the action of Δ6-desaturase on linoleic acid and ALA. This leads to the formation of SDA and γ-linolenic acid.73 After this, the ETA and DGLA are generated by Δ6-elongase action. The ETA and Dihomo-γ-linolenic acid are further converted into EPA and ARA by the enzyme Δ5-desaturase. The Δ8-pathway, an alternate mechanism, initiates ω3FA production by Δ9-elongation of substrates, similar to the Δ6-pathway. This is catalyzed by Δ9-elongases that is responsible for the generation of eicosatrienoic acid (ERA) and eicosadienoic acid (EDA). The fatty acids (Δ9-elongated) are catalyzed by desaturase (Δ8) and the reaction is desaturation that leads to the production of ETA and DGLA. Later, ETA and DGLA form EPA and ARA through Δ5-desaturation in a manner similar to that in Δ6-pathway. Biosynthesis of DHA in microbes includes elongation of EPA via Δ5 elongases to form DPA (docosapentaenoic acid) that at last produces DHA by the process of desaturation in Δ4 position. ω3-desaturases convert n-6 PUFAs (e.g., linoleic acid) to n-3 PUFAs (e.g., ALA), thereby linking the n-6 and n-3 pathways.4
In eukaryotic organisms, the ω3FA synthesis occurs through conventional 6-pathway. In certain protists like Euglena gracilis, Acanthamoeba spp., and Tetrahymena pyroformis and microalgae like Pavlova salina and Isochrysis galbana, ω3FA synthesis takes place via Δ8-pathway. The ω3FA with long-chain were created by cloning the genes that encode for elongation and desaturation process identified from various organisms. The approach has successfully integrated both standard Δ6 and alternative Δ8 pathways in microorganisms.4
Anaerobic polyketide synthase pathway
The fatty acid synthesis also occurs without the use of the elongase/desaturase system through an alternate route called as the anaerobic polyketide synthase pathway (Figure) to form EPA or DHA. The PKS pathway (polyketide synthase pathway-anaerobic) was explained for the first time in Shewanella pneumatophore which is a marine bacterium.74 It was sometime later that de novo synthesis was also discovered in the species for production of C18 and C16 fatty acids. The enzymes in the polyketide synthase pathway (anaerobic) are multifunctional and can undertake EPA synthesis without needing desaturase, elongase, and fatty acid synthase.
The fatty acyl chain extension requires double bond insertion with the help of dehydratase-isomerase module in the anaerobic polyketide synthase pathway. The aerobic desaturase/elongase pathway is distinct and needs oxygen-dependent desaturation steps for the elongation of the fatty acyl chain and insertion of double bonds.4 In Thraustochytrium and Schizochytrium, the existence of both systems has been reported. The Schizochytrium is known to lack Δ12-desaturase activity in their aerobic desaturase/elongase system and is not competent to utilize FAS (fatty acid synthase) products for long-chain polyunsaturated fatty acids (LCPUFAs) (long-chain polyunsaturated fatty acids) long-chain fatty acid synthesis. Gene involved in desaturases has been recognized in anerobic and aerobic pathways of Thraustochytrium and Schizochytrium. The findings demonstrated the presence of an incomplete elongase/desaturase system in Schizochytrium sp. Thraustochytrium possesses Δ12-desaturase, which produces long-chain polyunsaturated fatty acids (LCPUFAs) through the desaturase/elongase and polyketide synthase pathways.4 Thus, microbes have either aerobic desaturase/elongase or anaerobic polyketide synthase pathway and in some cases both the systems for the synthesis of fatty acid.
Metabolic engineering of microorganisms for fatty acid production
Microbial biosynthetic pathway reconstitution
The compositions of fatty acids vary from strains to species to genus in microbes and are based on the conditions of cultivation and stages of growth. The fatty acid synthesis by de novo pathway in photosynthetic cyanobacteria and microalgae, is catalyzed by dissociated set of enzymes that form FAS II (fatty acid synthase type II).75 In contrast, the yeast-like eukaryotic microorganisms utilizes FAS I (fatty acid synthase type I) for fatty acids synthesis.76 These microbes have C16 or C18 SFA (saturated fatty acid) and MUFA (mono-unsaturated fatty acids). The desaturation and elongation reactions help some microbes to achieve synthesis of GLA, ALA and LA. Many microbes have the potential to produce C18 monounsaturated fatty acid but are not capable of performing desaturation and elongation reactions. The process of reconstitution in such microbes requires the introduction of polyketide synthase (PKS) pathway which is anaerobic, gene set or assembly of the entire aerobic Δ9-elongase or Δ6-desaturase system. In E. coli, attempts were initially made to introduce the anaerobic polyketide synthase-like pathway due to the growth rate and synthesis of fatty acids. Such investigations used heterologous expression polyketide synthase-like pathway and were not optimized for the strain or titer or yield. The DHA and EPA were 0.7%-22% and 0.4%-5.2% of the total fatty acids produced by the genetically modified organism’s expression, respectively (Table 1). The gene cluster from the polyketide synthase-like pathway was successful in producing EPA in E. coli. There was 6% EPA production by the introduction of 38 kb DNA fragment from S. pneumatophore strain. The EPA yield was found to be low in the case of gene cluster isolated from Shewanella oneidensis.77 Another research showed that the transgenic strain could generate 0.1%-0.4% DHA and 8%-14% EPA.78 With the progress in the field, the unnecessary genes were filtered, and the new gene cluster of 20 kb was used for transfection in E. coli. The results showed a 3.5-6 fold increase in EPA production.79 These reports were the first to show the possibility of producing EPA/DHA using engineered microbes as an alternate to the ω3FA found in the food. The higher DHA levels were also obtained by using DHA biosynthesis genes and expressing them in E. coli. 1.3%-5.2% of the fatty acids were found to be DHA in the transgenic E. coli.80 In other studies, cyanobacteria have been used for EPA production. The engineered E. coli with EPA synthesis cluster showed a major impact on cultivation temperature and production was found to be 0.12-0.64 mg of EPA/gm dry weight of cell.81 In general, the yield of DHA and EPA is high with there is low temperatures.
Table (1):
The summary of ω3FA production from engineered microorganism strains
Study |
Omega -3 fatty acid |
Genes |
Host strain |
Yield (%) |
Time (hours) |
Temperature (ºC) |
|---|---|---|---|---|---|---|
Orikasa et al.80 |
DHA |
DHA gene cluster (pfaA–E) from Moritella marina MP-1 |
Escherichia coli DH5α |
5.2 |
96 |
15 |
Lee et al. 77 |
EPA |
EPA gene cluster (pfaA–E) from Shewanella oneidensis MR-1 |
Escherichia coli XL1-Blue |
0.689 |
34 |
20 |
Orikasa et al.82 |
EPA |
EPA gene cluster (pfaA–E) from Shewanella sp. SCRC-2738 |
Escherichia coli JM109 |
22 |
ND |
15 |
Amiri-Jami et al.78 |
EPA |
EPA gene cluster (pfaA–E) from Shewanella baltica MAC1 |
Escherichia coli EPI 300T1 |
14 |
120 |
15 |
Amiri-Jami et al.78 |
DHA |
DHA gene cluster (pfaA–E) from Shewanella baltica MAC1 |
Escherichia coli EPI 300T1 |
0.4 |
120 |
15 |
Amiri-Jami et al.79 |
EPA |
EPA gene cluster (pfaA–E) from Shewanella baltica MAC1 |
Lactococcus lactis subsp. cremoris |
0.12 mg/gm of DCW |
24 |
15 |
Amiri-Jami et al.79 |
DHA |
DHA gene cluster (pfaA–E) from Shewanella baltica MAC1 |
Lactococcus lactis subsp. cremoris |
1.35 mg/gm of DCW |
24 |
15 |
Takeyama et al.81 |
EPA |
EPA biosynthetic gene cluster from Shewanella putrefaciens SCRC-2738 |
Synechococcus sp. NKBG042902 |
0.5 |
24 |
17 |
Tavares et al.83 |
EPA |
Δ6-Desaturase (D6-Des), Δ9-Desaturase (D9-Des), Δ12-Desaturase (D12-Des), ω3-Desaturase (ω3-Des), Δ6-Elongase (D6-Elo) |
Saccharomyces cerevisiae |
0.49 |
48 |
30 |
Xue et al.16 |
EPA |
The metabolic process involves a range of enzymes such as Δ9-elongase (D9-Elo), Δ8-desaturase (D8-Des), Δ5-desaturase (D5-Des), Δ17-desaturase (D17-Des), and Δ12-desaturase (D12-Des), as well as carnitine palmitoyltransferase 1 (CPT1) |
Yarrowia lipolytica |
56.6 |
168 |
30 |
Damude et al.84 |
DHA |
The enzymes involved include Δ6-desaturase (D6-Des), C18/20-elongase (C18/20-Elo), Δ5-desaturase (D5-Des), Δ17-desaturase (D17-Des), C20/22-elongase (C20/22-Elo), and Δ4-desaturase (D4-Des), all of which play crucial roles in the biosynthetic conversion of essential fatty acids. |
Yarrowia lipolytica |
5.6 |
120 |
30 |
Hamilton et al.85 |
EPA and DHA |
D5-Elo, CoA-D6-Des |
Phaeodactylum tricornutum |
18% EPA and 11% DHA |
ND |
20 |
Sugihara et al.86 |
DHA |
The bacterial genotype includes mutations and markers such as deoR, endA1, gyrA96, hsdR17 (rK⁻ mK⁺), recA1, phoA, relA1, thi-1, a deletion of lacZYA-argF (ΔlacZYA-argF), U169ϕ80dlacZΔM15, F⁻, k⁻, and supE44. |
E. coli DH5α |
2% |
48 |
15 |
Kajikawa et al.87 |
EPA |
MpDES6, MpELO1 and MpDES5 |
Pichia pastoris |
0.03 |
72 |
30 |
Li et al.88 |
ARA |
The coding sequences for Δ5- and Δ6-desaturases along with Δ6-elongase were identified |
Pichia pastoris GS115 |
0.3 |
– |
– |
Li et al.88 |
EPA |
The gene segments encoding Δ5- and Δ6-desaturase enzymes, as well as Δ6-elongase, were analyzed. |
Pichia pastoris GS115 |
1 |
– |
– |
Allen et al.89 |
EPA |
EPA-overproducing chemical mutant |
Photobacterium Profundum EA2 |
25-30 |
– |
– |
Abbreviations: ND- Not determined, DCW- dry cell weight
Several researchers have informed the modification of biosynthetic pathways in yeast for the synthesis of ω3FA. In a study, 3 genes named D5-desaturase, D6-desaturase, and an ELO-like elongase were used for reconstitution of EPA synthesis pathway.87 Although EPA levels were found to be low in transgenic yeast, they were able to utilize C18 fatty acids for production of EPA. Later diatom Phaeodactylum tricornutum was used to increase those genes copy number in P. pastoris yeast to increase EPA yield.88 This discovery revealed that gene expression may not be the only factor influencing EPA synthesis in yeast. In contrast, the introduction of polyketide synthase-like pathway led to rising in DHA production significantly in yeast. 1.3% of total fatty acids (of total lipids) was observed to be DHA in yeast expressing polyketide synthase-like gene clusters. Further, two more modifications were introduced in yeast. These include co-expression of long-chain acyl-CoA synthetase and 3-ketoacyl-ACP synthase inhibition. The resultant DHA production was increased to 8% in modified yeast.
The commercial production of EPA has witnessed the engineering of Yarrowia lipolytica.16 The wild strain of Y. lipolytica mainly consists of palmitoleic acid (16:1), LA, palmitic acid (16:0), and monounsaturated fatty acid (18:1). The high levels of GLA were found to be accumulating upon engineering D6-desaturase pathway in yeast. The accumulation of intermediates in D6-desaturase pathway was avoided using assembly of 7 genes from D9-pathway namely, D17-desaturase, D5-desaturase, D8-desaturase, D9-elongase, D12-desaturase, and C16-elongase from various microbes. Subsequently, the wild strain was transformed in 4 different cassettes of expression harboring heterologous genes under the promoter’s regulatory control which was present in Y. lipolytica. The production of EPA was found to be 9.8% from the resultant strain of yeast. To maximize EPA accumulation further, engineering was performed to conjugate the expression of elongases and desaturases with choline phosphotransferase and PEX10 deletion that is required for perioxisomal proliferation.90 The end result of engineering was a strain with 30 copies of 9 heterologous genes with 56.6% EPA production and 30% of lipids from dry cell weight in a high-glucose medium after 6 days.16 The further genetic analysis highlighted that along with the deletion of PEX10 gene, disruption of other 3 ORFs SCP2 gene which encoded sterol carrier protein, LIP1 gene which encoded lipase 1 and nonessential gene ORF also occurred. The functional loss of these genes was associated with the high EPA yield and lipid content observed in the engineered strain of yeast. The biochemical analysis performed in detail revealed that triacylglycerol formed was 85% of the total fatty acids. The triacylglycerol’s sn-1 and sn-3 positions were predominantly involved in the production of EPA. The yeast engineering for the accumulation of ω3FA was the first example of its kind and was shown to exceed EPA production in terms of productivity as well as yield in comparison to the previously engineered microbes.
Microbial genetic modification
The production of ω3FA occurs naturally in microbes. The genetic transformation has the potential to boost the output of fatty acids in microorganisms using a technique known as metabolic engineering. The genetic approach in comparison to the reconstitution process of biosynthetic pathway is simpler and involves fewer heterologous genes. The D15 and D6 desaturase genes transgenic expression in Synechocystis sp. which is photosynthetic cyanobacteria, was performed by cloning from Mortierella alpine, Gibberella fujikuroi, and Synechocystis sp. It was observed 8.9 mg/L ALA production and 4.1 mg/L SDA production at 5-times higher than the wild strain.91 Engineering using genetic and metabolic approaches also improved the generation of extremely long-chain fatty acids. There was 3.5-fold enhancement in the EPA production in S. baltica MAC1 mutants generated using transposon Tn5 mutagenesis. The mutant was able to yield EPA of 0.3 mg per g dry cell weight, whereas the wild strain did not produce any EPA.92 The marine microalgae were also modified by introducing mutation by ethyl methane sulfonate for EPA production scaling up in Nannochloropsis oculate. This method generated two mutated strains that were capable of producing 36.5 and 31.6 mg EPA respectively per gram of dry cell weight.93 The success in increasing the yield of EPA showed that genetic mutagenesis has the potential to improve strain towards more production of fatty acids with long-chain.
A fungus named M. alpina has proven to be used for ARA production in the industries. The Agrobacterium tumefaciens-mediated transformation for the x3-desaturase gene overexpression has shown 4-fold increment in yield of EPA. The EPA levels were highest in transgenic strains and accounted for 40% of fatty acids.94 The fungus with mutant D12-desaturase could convert ALA to EPA. The mutation was able to achieve EPA content of 20% from the fatty acids produced in 10 days of growth in presence of 3% linseed oil at 20 °C. 95 The Aurantiochytrium limacinum is a marine protist and has been used for EPA production through modulation of D5-desaturase gene expression under the influence of thraustochytrid ubiquitin promoter. DHA, DPA, and palmitic acid are naturally produced by Thraustochytrids along with low levels of EPA and ARA. The expression of D5 desaturase gene in A. limacinum extracted from T. aureum led to increment in ARA and EPA levels in transgenic thraustochytrids. The levels were elevated by 13.2-fold in comparison to mock transfects.96 The expression of heterologous genes was also shown to alter the fatty acid content in photosynthetic diatom. Thirty percent of the fatty acids present in diatom Phaeodactylum tricornutum is EPA and DHA is only found in trace amounts. However, the picoalga Ostreococcus tauri used for acyl-CoA D6-desaturase overexpression was not able to show a significant increment in DHA or EPA levels. This shows that D6-desaturation is the only rate-limiting bottleneck in fatty acid synthesis. Genetically modified P. tricornutum showed a 17.7% and 8.2% decrease in EPA levels at the exponential phase and stationary phase of growth. In transgenic strain, DHA was found to be 10.4% in the stationary phase and this accounted for an 8-fold increment in P. tricornutum cells.85 The metabolic engineering used in this study was the first to report modulation in diatoms for fatty acid content. These instances support the idea that metabolic engineering can boost ω3FA levels.
Summary of strategies taken so far
In a nutshell, the FAS, A desaturase/elongase, and anaerobic PKS pathways detailing the development of EPA and DHA production demonstrate the efforts made towards metabolic engineering. Each pathway has distinct merits: the FAS approach (Table 2) provides the freedom of controlling metabolism of precursor and redox cycling; the desaturase/elongase pathway (Table 3) fine tunes the enzymatic steps of PUFA conversion, and the PKS pathway (Table 4) is a more direct means of producing long-chain PUFAs that does not require oxygen. Precursor amplification, stacking of pathways, redox adjusting, and optimizing sinks lipids have been shown to work with many host systems. The addition of heterologous gene cluster integration silences the sequential control logic of a given organism’s genetic backbone, and frame shifting codons with optimization that control expression deepens the influence of synthetic biology on host limitations. All these pieces of research deepen the understanding the engineering of strains, providing a foundation that seeks to render the biosynthesis of ω3FAs economical and widespread amidst their undeniable nutritional importance.
Table (2):
Specific metabolic engineering steps in FAS pathway for enhanced EPA and DHA production
Step |
Target/Enzyme |
Modification |
Microorganism |
Effect |
Reference |
|---|---|---|---|---|---|
1 |
fabH (β-ketoacyl-ACP synthase III) |
Deletion |
Escherichia coli expressing pfa genes |
Increased DHA production from 7.5% to 17% of total fatty acids |
Giner-Robles et al.107 |
2 |
fabF (β-ketoacyl-ACP synthase II) |
Over-expression |
Synechococcus elongatus PCC 7942 |
Enhanced α-linolenic acid (ALA) production up to 22.6% of total FA |
Santos-Merino et al.108 |
3 |
ACCase (Acetyl-CoA Carboxylase) |
Overexpression |
Schizochytrium sp. |
DHA content increased from 36.4% to 37.6% of total FA |
Han et al.109 |
4 |
MCAT (Malonyl-CoA:ACP Transacylase) |
Overexpression |
Schizochytrium sp. |
Total lipid content increased by 39.6% and DHA by 53.7% (from 27.9% to 42.9%) |
Li et al.110 |
5 |
ATP-Citrate Lyase |
Co-overexpression with ACCase |
Schizochytrium sp. |
Further increased DHA content to 37.9% of all FA |
Wang et al.111 |
6 |
Malic Enzyme |
Overexpression |
Schizochytrium sp. |
Enhanced NADPH availability, leading to increased DHA yield from 19.2% to 26.7% of DCW |
Wang et al.111 |
7 |
FAS Pathway Suppression |
Downregulation |
Schizochytrium sp. |
diverted carbon flux towards synthesis of PUFA via PKS pathway, increasing DHA proportion in total fatty acids |
Liu et al.112 |
8 |
Pfa Gene Cluster |
Introduction |
Lactococcus lactis subsp. cremoris MG1363 |
Enabled de novo DHA and EPA synthesis; DHA production reached to 1.35 mg/gm DCW |
Amiri-Jami et al.79 |
9 |
Pfa Gene Cluster |
Introduction |
Escherichia coli Nissle 1917 |
Achieved EPA synthesis up to 31.36 mg/gm DCW at 15 °C; DHA was less than 0.2% of total FA |
Amiri-Jami et al.113 |
10 |
Desaturases and Elongases |
Introduction of Δ6, Δ5, Δ17 desaturases and C18/20 elongase |
Yarrowia lipolytica |
Produced EPA at 40% of total lipids; further engineering led to 57% EPA in fatty acid methyl esters |
Xue et al.114 |
11 |
Peroxisome Biogenesis Gene (PEX10) |
Inactivation |
Yarrowia lipolytica |
Increased EPA yield to 15% of DCW; reduced SFA to less than 5% |
Xue et al.114 |
12 |
FAA1 and GPD1 |
Overexpression |
Yarrowia lipolytica |
Converted excess free fatty acids into triacylglycerols, increasing EPA production by 18%–110% |
Qin et al.115 |
13 |
TGL3 and TGL4 |
Deletion |
Yarrowia lipolytica |
Prevented degradation of triacylglycerols into free fatty acids; TGL4 deletion led to a 300% increase in EPA production |
Qin et al.115 |
Table (3):
Specific metabolic engineering steps in aerobic Desaturase/Elongase pathway (O₂-dependent) for enhanced EPA and DHA production
Step |
Enzyme |
Modification |
Microorganism |
Effect |
Reference |
|---|---|---|---|---|---|
1 |
Δ6-desaturase |
Overexpression of endogenous or heterologous gene |
Phaeodactylum tricornutum, Yarrowia lipolytica |
Enhances ALA → SDA or LA → GLA |
Zhu et al.116 |
2 |
Elongase (C18 → C20) |
Expression of Δ6-elongase |
Schizochytrium, Y. lipolytica |
SDA → ETA or GLA → DGLA |
Xue et al.114 |
3 |
Δ5-desaturase |
Overexpression from Ostreococcus tauri |
Phaeodactylum tricornutum |
Enhances EPA production |
Domergue et al.117 |
4 |
Δ5-elongase |
Overexpression |
Y. lipolytica |
Shifts EPA to DPA |
Xie et al.118 |
5 |
Δ4-desaturase |
Expression to produce DHA from DPA |
Y. lipolytica, |
Final DHA step |
Gemperlein et al.119 |
6 |
Pathway Optimization |
Codon optimization, synthetic operons |
Y. lipolytica |
Balanced flux |
Celińska et al.120 |
7 |
Co-factor supply |
↑ NADPH via malic enzyme |
Schizochytrium, Mucor circinelloides |
Supports desaturation steps |
Zhang et al.121 |
8 |
Lipid sink |
Overexpression of DGAT |
Nannochloropsis oceanica |
↑ EPA in TAGs |
Xin et al.122 |
9 |
Lipase deletion |
Knockout of TAG lipase (NoTGL1990) |
Nannochloropsis |
Prevents EPA degradation |
Miao et al.123 |
Table (4):
Specific metabolic engineering steps in anaerobic Poly-Ketide Synthase (PKS) pathway (O‚ -independent) for enhanced DHA and EPA production
Step |
PKS Subunit/Domain |
Modification |
Microorganism |
Effect |
Reference |
|---|---|---|---|---|---|
1 |
PKS_B (AT domain) |
Domain swap with EPA-specific AT |
Schizochytrium sp. |
↑ EPA 5-fold |
Zhang et al.124 |
2 |
C16 elongase |
Overexpression |
Schizochytrium |
Enhances EPA & DHA |
Ma et al.125 |
3 |
Full PKS cluster |
From Shewanella japonica |
E. coli |
Enables EPA synthesis |
Metz et al.126 |
4 |
PPTase |
Co-expression with PKS |
Y. lipolytica |
PKS activation |
Bejenari et al.127 |
5 |
ACCase |
Overexpression → ↑ malonyl-CoA |
Schizochytrium |
Precursor boost |
Wang et al.128 |
6 |
Carbon flux |
Overexpression of ME & G6PDH |
Thraustochytrids |
↑ acetyl-CoA & NADPH |
Muthu et al.129 |
7 |
Codon optimization |
Gene refactoring for PKS |
Y. lipolytica |
↑ Expression |
Schmidt et al.130 |
8 |
Modular platform |
2A peptide for PKS expression |
Aurantiochytrium sp. |
Balanced protein levels |
Wang et al.131 |
Metabolic bottlenecks
Even though metabolic engineering has been used to reconstitute biosynthetic fatty acid pathways, only a few strains have produced the required outcomes in terms of increased DHA or EPA levels. The modulation and transformation of microbes using various genes is not a hurdle technically; however, the complexity at the metabolic level has led to many bottlenecks in the engineering for high-level production.
The substrate dichotomy is the first bottleneck that is recognized in yeast and plants. In the initial investigations, attempts were made to determine the elongases and desaturases substrates in biosynthetic pathway of fatty acid. The study involved the transformation of yeast S. cerevisiae by the introduction of D12 desaturases, D5 desaturases, and D6 desaturases from other organisms and analysis of composition of fatty acid and acyl-CoA pool in culture. The research highlighted that the desaturases have an affinity for phosphatidylcholine sn-2 position in fungi, lower plants, worms, and algae. D12 desaturase was found to use lipid-linked acyl chains and was active on sn-positions of all glycerolipids.97 The reconstituted ARA pathway in yeast demonstrated the role of acyl carriers with co-expression of D5 and D6 desaturase from algae and the impact of the yield of ARA was found to be low with 0.4% of fatty acids contributing to ARA. The incorporation of GLA into phosphatidylcholine highlighted that transfer of D6-desaturated product was inefficient from phosphatidylcholine to acyl-CoA pool. This is a bottleneck that limits synthesis of fatty acids in yeast. Research has identified 3 acyl-CoA dependent D6 desaturases in microalgae with a 71%-73% of conversion rate which was very high in comparison to other D6-desaturases.98
The reconstitution of EPA and ARA pathways was successfully achieved with the utilization of ALA as substrates as mentioned before. There was 20 times more production that was achieved using EPA or ARA end products. The comparatively large yields of long-chain fatty acids were attributed to the effectiveness of elongation of desaturated intermediates between phosphatidylcholine and the acyl-CoA pool. In contrast, another enzyme was shown to only transform ALA to SDA in Mantoniella squamata. The reconstitution of EPA pathway was also performed using co-expression of D6-elongase (PSE1) with acyl-CoA D5-desaturase and MsD6. There was a 2%-3% increase in fatty acids observed from the pathway. Although the yield of EPA was low; however, the interaction between elongase and acyl-CoA desaturases have the ability to overcome acyl-exchange bottleneck. In Micromonas pusilla, a marine microalga, identification of acyl-CoA D6-desaturase was recently made and it was used for expression in yeast that resulted to the 71% conversion of ALA to SDA.99 The crucial thing to note here is that, while the substrate specificities of desaturases have been effectively identified in recent investigations, their identification is hampered by the quick activity of acyltransferases, which induce the transfer of acyl groups in several host species, including yeast. In vivo investigations may not accurately detect the specificities of acyl carriers. However, the application of acyl-CoA desaturases for engineering in higher plants has shown a high accumulation of fatty acids with long-chain.100 This shows that employing acyl-CoA desaturases than lipid-linked desaturases can increase the output of ω3 long-chain fatty acids.
The competition among other intermediates in fatty acid synthesis may form a second bottleneck. The yeast that has been transformed harbors pools of phosphatidylcholine, phospholipids, and acyl-CoA. These form substrates for many acyltransferases. The transgenic yeast was analyzed in detail highlighted part of the biosynthetic pathway of fatty acid is channeled out for TAG biosynthesis.97,100 Elongation of intermediates is prematurely ended due to fatty acid flow. Subcellular components and enzymatic activities play critical roles in regulating the fatty acid transit of end products and intermediates, such as TAG pools, phosphatidylcholine and acyl-CoA. The biochemical studies formed the basis for the formulation of a simplified model in yeast for lipid synthesis accumulated TAGs. The engineering of microbes for ideal flux of fatty acid may be achieved by controlling the exchange of acyl intermediates, channeling of fatty acid end products, and minimal flux towards TAG pathway. In most of the fatty acid-producing microalgae, the DHA and EPA are bound to storage TAGs or membrane lipids.101 The ultimate fatty acids composition in engineered strains is modulated by various acyltransferase activities that use end products as well as fatty acid intermediates.
The acyl group exchange between phosphatidylcholine and acyl-CoA pool is bidirectional and can be catalyzed by acyltransferase in plants, animals, and yeast.102,103 In yeast, the transfer system is very efficient, and the forward reaction is significantly higher than the reverse activity highlighting the low efficiency of removal of fatty acid from phosphatidylcholine. The enzymatic process in yeast may use a wide spectrum of acyl-CoAs. Although the PC turnover is important in lysophosphatidylcholine acyltransferase, it remains to be explored whether this activity can alleviate the bottleneck that is acyl exchange in recombinant yeast to produce more fatty acids. The characterization and identification of lysophosphatidylcholine acyltransferase enzymes in microbes has the potential to highlight role of acyl transferases in the fatty acids flux and acyl exchange between acyl-CoA pool and phosphatidylcholine. The intermediates are directly transferred to diacylglycerol to phosphatidylcholine on the sn-2 position. This can be separately catalysed by acyl-CoA enzymes, which eventually combine them into triacylglycerol. The LRO1 gene encodes for an enzyme in the yeast that also contributes to the triacylglycerol production in cell division.104 In microalgae and plants, the enzymes for triacylglycerol biosynthesis and lipid turnover have been identified.105 However, there is very little evidence for phospholipid: diacylglycerol acyltransferase (PDAT) contributing towards the flux of fatty acid intermediates. The redirection of carbon flux is centrally controlled by the membrane-associated Kennedy pathway and that directs the flux to triacylglycerol synthesis. Diacylglycerol acyltransferase (DGAT), glycerol-3-phosphate acyltransferase (GPAT) and lysophosphatidic acid acyltransferase (LPAAT) are the three acyltransferases. The DGAT enzyme in comparison to the other two enzymes is crucial for triacylglycerol accumulation in microbes. The DGAT enzymes are part of acyltransferase protein family and are known to exist in membrane-bound and soluble forms. The DGAT2 and DGAT1 are the membrane-bound whereas DGAT3 is mainly soluble. The DGAT2 subfamily is crucial for triacylglycerol production during the stationary phase of growth.104 The existence of multiple isoforms of DGAT in a single microbial species highlights the complexity of triacylglycerol synthesis via DGAT action.
In various microbes, the triacylglycerol content and composition of fatty acids are the determinants for reactions catalyzed by PDAT and Kennedy pathway activities. The engineered Y. lipolytica was analyzed for EPA in triacylglycerols and it was observed that EPA enrichment occurred at positions of sn-1 and sn-3 mainly.16 The reactions involving desaturase mostly occur at phosphatidylcholine’s sn-2 position that may point to the remodeling of lipids and triacylglycerols before the EPA incorporation. The aforementioned acyltransferases may contribute to the remodeling of lipids in strains that have been engineered. The elucidation of the part played by different acyltransferases in the efficient fatty acids flux is essential for the identification of the mechanism underlying acyl exchange in such strains. This in turn will provide highlights for overcoming the various bottlenecks in fatty acid production in engineered microbes. The PKS pathway, unlike desaturase/elongase pathway, is identified to produce lesser intermediates that in various pools of substrate can skip acyl exchange. The polyketide synthase system has substrates common to fatty acid synthase, which signifies the importance of polyketide synthase substrates in the fatty acid yield of microbial hosts. Since the end products of polyketide synthase pathway are free fatty acids, so their transfer from the synthesis site to triacylglycerol may be another bottleneck in the expression and assembly of fatty acid polyketide synthase gene clusters in host cells.
Improvement strategies
Although the microbial engineering has great potential for enhanced production of ω3FA, there are still several bottlenecks that impact the productivity and yield of the strains. These need to be evaluated before the engineering of strains and their utilization for industrial production. Many approaches exist to bypass some of the bottlenecks and enhance the end product yield. These may include acyl exchange reduction between phosphatidylcholine and acyl-CoA pool, improvement of fatty acid flux, increment in the precursor pool, and inhibition of competing pathways.
Future prospects
In the future, metabolic engineering of ω3FAs, particularly DHA and EPA, would be of great interest to meet the world nutritional demands in a sustainable biotechnological approach. Rapid development in synthetic biology and CRISPR/Cas mediated genome editing creates new possibilities to control desaturase/elongase and PKS pathways where titre and selectivity are improved in microbial and algal hosts. In the future, it would be interesting to develop optimal-host chassis such as oleaginous yeasts, thraustochytrids or genetically amenable microalgae, and construct multi-gene pathways, as well as utilize dynamic regulatory systems facilitating flux of pathway. The rational design and optimization of cost-efficient fermentation processes built on high-throughput screening techniques and systems biology might close the gap between laboratory scale and commercial scale. Environmentally tolerant strains (tolerant to pH, temperature, or salinity) engineered for industrial conditions are expected to be essential for outdoor and heterotrophic production at large scale. Multidisciplinary efforts are needed to adapt molecular advancements to commercial nutraceutical, pharmaceutical, and aquafeed products, making the long-awaited, sustainable, and inexpensive supply of health-promoting ω3FA possible.
The growing global demand for omega-3 fatty acids necessitates sustainable alternatives to fish-derived sources. Microbial production, enhanced through metabolic engineering and genetic modification, offers a promising and eco-friendly approach to meet this need. By reconstructing biosynthetic pathways and optimizing enzymatic steps in both aerobic desaturase/elongase and anaerobic PKS systems, microorganisms such as Yarrowia lipolytica, Schizochytrium, and Phaeodactylum tricornutum have demonstrated significantly improved EPA and DHA yields. Continued advances in systems biology, strain optimization, and process engineering are expected to further enhance productivity, paving the way for large-scale, commercially viable microbial omega-3 production that supports both human health and environmental sustainability.
ACKNOWLEDGMENTS
The authors acknowledge the Department of Life Sciences, School of Biosciences and Technology, Galgotias University and the Department of Pharmacology, Maharana Pratap College of Pharmacy, for the infrastructure facility.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
AD performed supervision. SS performed formal analysis. DS and MK investigated the study.AD and MM wrote the manuscript. CM and AD reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
Not applicable.
ETHICS STATEMENT
Not applicable.
- Adarme-Vega TC, Lim DKY, Timmins M, Vernen F, Li Y, Schenk PM. Microalgal biofactories: a promising approach towards sustainable omega-3 fatty acid production. Microb Cell Fact. 2012;11:1-10.
Crossref - Kralovec JA, Zhang S, Zhang W, Barrow CJ. A review of the progress in enzymatic concentration and microencapsulation of omega-3 rich oil from fish and microbial sources. Food Chem. 2012;131(2):639-44.
Crossref - Rubio-Rodriguez N, Beltran S, Jaime I, de Diego SM, Sanz MT, Carballido JR. Production of omega-3 polyunsaturated fatty acid concentrates: A review. Innov Food Sci Emerg Technol. 2010;11(1):1-12.
Crossref - Gong Y, Wan X, Jiang M, Hu C, Hu H, Huang F. Metabolic engineering of microorganisms to produce omega-3 very long-chain polyunsaturated fatty acids. Prog Lipid Res. 2014;56:19-35.
Crossref - Ward OP, Singh A. Omega-3/6 fatty acids: Alternative sources of production. Process Biochem. 2005;40(12):3627-3652.
Crossref - Deeba F, Pruthi V, Negi YS. Converting paper mill sludge into neutral lipids by oleaginous yeast Cryptococcus vishniaccii for biodiesel production. Bioresour Technol. 2016;213:96-102.
Crossref - Deeba F, Patel A, Arora N, Pruthi V, Pruthi PA, Negi YS. Amaranth seeds (Amaranthus palmeri L.) as novel feedstock for biodiesel production by oleaginous yeast. Environ Sci Pollut Res. 2018;25(1):353-362.
Crossref - Deeba F, Pruthi V, Negi YS. Aromatic hydrocarbon biodegradation activates neutral lipid biosynthesis in oleaginous yeast. Bioresour Technol. 2018;255:273-80.
Crossref - Armenta RE, Valentine MC. Single-cell oils as a source of ω3FA: An overview of recent advances. JAOCS, J Am Oil Chem Soc. 2013;90(2):167-182.
Crossref - Ratledge C. Fatty acid biosynthesis in microorganisms being used for Single Cell Oil production. Biochimie. 2004;86(11):807-815.
Crossref - Khan WA, Chun-Mei H, Khan N, Iqbal A, Lyu SW, Shah F. Bioengineered Plants Can Be a Useful Source of Omega-3 Fatty Acids. Biomed Res Int. 2017;2017(1):7348919.
Crossref - Abedi E, Sahari MA. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci Nutr. 2014;2(5):443-463.
Crossref - Mendes A, da Silva TL, Reis A. DHA concentration and purification from the marine heterotrophic microalga Crypthecodinium cohnii CCMP 316 by winterization and urea complexation. Food Technol Biotechnol. 2007;45:38-44.
- Shimiziu S, Kawashima H, Shinmen Y, Akimoto K, Yamada H. Production of eicosapentaenoic acid by Mortierella fungi. J Am Oil Chem Soc. 1988;65:1455-9.
Crossref - Qi B, Fraser T, Mugford S, et al. Production of very long-chain polyunsaturated omega-3 and omega-6 fatty acids in plants. Nat Biotechnol. 2004;22(6):739-45.
Crossref - Xue Z, Sharpe PL, Hong SP, et al. Production of omega-3 eicosapentaenoic acid by metabolic engineering of Yarrowia lipolytica. Nat Biotechnol. 2013;31(8):734-740.
Crossref - Yeiser M, Harris CL, Kirchoff AL, et al. Growth and tolerance of infants fed formula with a new algal source of docosahexaenoic acid: Double-blind, randomized, controlled trial. Prostaglandins Leukot Essent Fat Acids. 2016;115:89-96.
Crossref - Sakuradani E, Shimizu S. Single cell oil production by Mortierella alpina. J Biotechnol. 2009;144(1):31-36.
Crossref - Ginty AT, Conklin SM. Short-term supplementation of acute long-chain omega-3 polyunsaturated fatty acids may alter depression status and decrease symptomology among young adults with depression: A preliminary randomized and placebo-controlled trial. Psychiatry Res. 2015;229(1-2):485-489.
Crossref - Kessler RC, Wai TC, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
Crossref - Grosso G, Galvano F, Marventano S, et al. Omega-3 fatty acids and depression: Scientific evidence and biological mechanisms. Oxid Med Cell Longev. 2014;2014(1):313570.
Crossref - Jazayeri S, Tehrani-Doost M, Keshavarz SA, et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008;42(3):192-198.
Crossref - Merle BMJ, Benlian P, Puche N, Bassols A, Delcourt C, Souied EH. Circulating ω3FA and neovascular age-related macular degeneration. Investig Ophthalmol Vis Sci. 2014;55(3):2010-2019.
Crossref - Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728-1738.
Crossref - Singh M. Essential fatty acids, DHA human brain. Indian J Pediatr. 2005;72(3):239-242.
Crossref - Horrocks LA, Yeo YK. Health benefits of docosahexaenoic acid (DHA). Pharmacol Res. 1999;40(3):211-225.
Crossref - Tai EKK, Wang XB, Chen ZY. An update on adding docosahexaenoic acid (DHA) and arachidonic acid (AA) to baby formula. Food Funct. 2013;4(12):1767-1775.
Crossref - Strickland AD. Prevention of cerebral palsy, autism spectrum disorder, and attention deficit-hyperactivity disorder. Med Hypotheses. 2014;82(5):522-528.
Crossref - Leaf A. Historical overview of n-3 fatty acids and coronary heart disease. Am J Clin Nutr. 2008;87(6):1978S-80S.
Crossref - Bernstein AM, Ding EL, Willett WC, Rimm EB. A meta-analysis shows that docosahexaenoic acid from algal oil reduces serum triglycerides and increases HDL-cholesterol and LDL-cholesterol in persons without coronary heart disease. J Nutr. 2012;142(1):99-104.
Crossref - Roysland R, Masson S, Omland T, et al. Prognostic value of osteoprotegerin in chronic heart failure: The GISSI-HF trial. Am Heart J. 2010;160(2):286-293.
Crossref - Singh RB, Dubnov G, Niaz MA, et al. Effect of an Indo-Mediterranean diet on progression of coronary artery disease in high-risk patients (Indo-Mediterranean Diet Heart Study): A randomized single-blind trial. Lancet. 2002;360(9344):1455-1461.
Crossref - Satoh N, Shimatsu A, Kotani K, et al. Purified eicosapentaenoic acid reduces small dense LDL, remnant lipoprotein particles, and C-reactive protein in metabolic syndrome. Diabetes Care. 2007;30(1):144-146.
Crossref - Oelrich B, Dewell A, Gardner CD. Effect of fish oil supplementation on serum triglycerides, LDL cholesterol, and LDL subfractions in hypertriglyceridemic adults. Nutr Metab Cardiovasc Dis. 2013;23(4):350-357.
Crossref - Kwak SM, Myung SK, Lee YJ, Seo HG. Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: A meta-analysis of randomized, double-blind, placebo-controlled trials. Arch Intern Med. 2012;172(9):686-694.
Crossref - Giacobini MB, Medin E, Ahnemark E, Russo LJ, Carlqvist P. Prevalence, patient characteristics, and pharmacological treatment of children, adolescents, and adults diagnosed with ADHD in Sweden. J Atten Disord. 2018;22(1):3-13.
Crossref - Bloch MH, Qawasmi A. Omega-3 fatty acid supplementation for the treatment of children with attention-deficit/hyperactivity disorder symptomatology: Systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2011;50(10):991-1000.
Crossref - Milte CM, Parletta N, Buckley JD, Coates AM, Young RM, Howe PRC. Eicosapentaenoic and docosahexaenoic acids, cognition, and behavior in children with attention-deficit/hyperactivity disorder: A randomized controlled trial. Nutrition. 2012;28(6):670-677.
Crossref - Rytter MJH, Andersen LBB, Houmann T, et al. Diet in the treatment of ADHD in children-A systematic review of the literature. Nord J Psychiatry. 2015;69(1):1-18.
Crossref - Ebrahimi M, Ghayour-Mobarhan M, Rezaiean S, et al. Omega-3 fatty acid supplements improve the cardiovascular risk profile of subjects with metabolic syndrome, including markers of inflammation and auto-immunity. Acta Cardiol. 2009;64(3):321-327.
Crossref - Calder PC. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 Suppl):1505S-1519S.
Crossref - Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21(6):495-505.
Crossref - Benton D. The impact of diet on anti-social, violent and criminal behaviour. Neurosci Biobehav Rev. 2007;31(5):752-774.
Crossref - Fotuhi M, Mohassel P, Yaffe K. Fish consumption, long-chain ω3FA and risk of cognitive decline or Alzheimer disease: A complex association. Nat Clin Pract Neurol. 2009;5(3):140-152.
Crossref - Mohajeri MH, Troesch B, Weber P. Inadequate supply of vitamins and DHA in the elderly: Implications for brain aging and Alzheimer-type dementia. Nutrition. 2015;31(2):261-275.
Crossref - Canhada S, Castro K, Perry IS, Luft VC. Omega-3 fatty acids’ supplementation in Alzheimer’s disease: A systematic review. Nutr Neurosci. 2018;21(8):529-538.
Crossref - Theodoratou E, McNeill G, Cetnarskyj R, et al. Dietary fatty acids and colorectal cancer: A case-control study. Am J Epidemiol. 2007;166(2):181-195.
Crossref - Terry PD, Terry JB, Rohan TE. Long-chain (n-3) fatty acid intake and risk of cancers of the breast and the prostate: Recent epidemiological studies, biological mechanisms, and directions for future research. J Nutr. 2004;134(12 Suppl):3412S-3417S.
Crossref - Kaizer L, Boyd NF, Kriukov V, Tritchler D. Fish consumption and breast cancer risk: An ecological study. Nutr Cancer. 1989;12(1):61-68.
Crossref - Centers for Disease Control and Prevention (CDC). Vital signs: asthma prevalence, disease characteristics, and self-management education: United States. 2001-2009. MMWR Morb Mortal Wkly Rep. 2011;60:547-52.
- Li J, Xun P, Zamora D, et al. Intakes of long-chain omega-3 (n-3) PUFAs and fish in relation to incidence of asthma among American young adults: The CARDIA study. Am J Clin Nutr. 2013;97(1):173-178.
Crossref - Bouzianas DG, Bouziana SD, Hatzitolios AI. Potential treatment of human nonalcoholic fatty liver disease with long-chain omega-3 polyunsaturated fatty acids. Nutr Rev. 2013;71(11):753-771.
Crossref - Kruger MC, Horrobin DF. Calcium metabolism, osteoporosis and essential fatty acids: A review. Prog Lipid Res. 1997;36(2-3):131-51.
Crossref - Danao-Camara TC, Shintani TT. The dietary treatment of inflammatory arthritis: case reports and review of the literature. Hawaii Med J. 1999;58(5):126-131.
- Deutch B. Menstrual pain in Danish women correlated with low n-3 polyunsaturated fatty acid intake. Eur J Clin Nutr. 1995;49(7):508-516.
- Zafari M, Behmanesh F, Mohammadi AA. Comparison of the effect of fish oil and ibuprofen on treatment of severe pain in primary dysmenorrhea. Casp J Intern Med. 2011;2(3):279-282.
- Spencer EH, Ferdowsian HR, Barnard ND. Diet and acne: A review of the evidence. Int J Dermatol. 2009;48(4)339-347.
Crossref - Ochsenreither K, Gluck C, Stressler T, Fischer L, Syldatk C. Production strategies and applications of microbial single cell oils. Front Microbiol. 2016;7:1-26.
Crossref - Patel A, Arora N, Sartaj K, Pruthi V, Pruthi PA. Sustainable biodiesel production from oleaginous yeasts utilizing hydrolysates of various non-edible lignocellulosic biomasses. Renew Sustain Energy Rev. 2016;62:836-855.
Crossref - Patel A, Matsakas L, Hruzova K, Rova U, Christakopoulos P. Biosynthesis of Nutraceutical Fatty Acids by the Oleaginous Marine Microalgae Phaeodactylum tricornutum Utilizing Hydrolysates from Organosolv-Pretreated Birch and Spruce Biomass. Mar Drugs. 2019;17(2):119.
Crossref - Zhang H, Zhang L, Chen H, et al. Regulatory properties of malic enzyme in the oleaginous yeast, Yarrowia lipolytica, and its non-involvement in lipid accumulation. Biotechnol Lett. 2013;35(12):2091-2098.
Crossref - Martins DA, Custodio L, Barreira L, et al. Alternative sources of n-3 long-chain polyunsaturated fatty acids in marine microalgae. Mar Drugs. 2013;11(7):2259-2281.
Crossref - De Caterina R, Basta G. n-3 Fatty acids and the inflammatory response – Biological background. Eur Hear J Suppl. 2001;3(suppl_D):D42-D49.
Crossref - Winwood RJ. Recent developments in the commercial production of DHA and EPA rich oils from micro-algae. OCL – Oilseeds Fats, Crop Lipids. 2013;20(6):1-5.
Crossref - Bajpai PK, Bajpai P, Ward OP. Optimisation of culture conditions for production of eicosapentaenoic acid by Mortierella elongate NRRL 5513. J Ind Microbiol. 1992;9(1):11-17.
Crossref - Yongmanitchai W, Ward OP. Growth of and omega-3 fatty acid production by Phaeodactylum tricornutum under different culture conditions. Appl Environ Microbiol. 1991;57(2):419-25.
Crossref - Bajpai P, Bajpai PK. Arachidonic acid production by microorganisms. Biotechnol Appl Biochem. 1992;15(1):1-10.
Crossref - Harrington GW, Holz GG. The monoenoic and docosahexaenoic fatty acids of a heterotrophic dinoflagellate. Biochim Biophys Acta (BBA)/Lipids Lipid Metab. 1968;164(1):137-139.
Crossref - Bajpai P, Bajpai PK, Ward OP. Production of docosahexaenoic acid by Thraustochytrium aureum. Appl Microbiol Biotechnol. 1991;35:706-710.
Crossref - Bajpai P, Bajpai PK. Eicosapentaenoic acid (EPA) production from microorganisms: a review. J Biotechnol. 1993;30(2):161-183.
Crossref - Shanklin J, Cahoon EB. Desaturation and related modifications of fatty acids. Annu Rev Plant Biol. 1998;49:611-641.
Crossref - Leonard AE, Pereira SL, Sprecher H, Huang YS. Elongation of long-chain fatty acids. Prog Lipid Res. 2004;43(1):36-54.
Crossref - Michaelson LV, Lazarus CM, Griffiths G, Napier JA, Stobart AK. Isolation of aDִ5-fatty acid desaturase gene from Mortierella alpina. J Biol Chem. 1998;273(30):19055-19059.
Crossref - Yazawa K. Production of eicosapentaenoic acid from marine bacteria. Lipids. 1996;31(1-2):S297-S300.
Crossref - Campbell JW, Cronan J. Bacterial fatty acid biosynthesis: Targets for antibacterial drug discovery. Annu Rev Microbiol. 2001;55:305-32.
Crossref - Schweizer E, Hofmann J. Microbial Type I Fatty Acid Synthases (FAS): Major Players in a Network of Cellular FAS Systems. Microbiol Mol Biol Rev. 2004;68(3):501-517.
Crossref - Lee SJ, Jeong YS, Kim DU, Seo JW, Hur BK. Eicosapentaenoic acid (EPA) biosynthetic gene cluster of Shewanella oneidensis MR-1: Cloning, heterologous expression, and effects of temperature and glucose on the production of EPA in Escherichia coli. Biotechnol Bioprocess Eng. 2006;11:510-515.
Crossref - Amiri-Jami M, Griffiths MW. Recombinant production of omega-3FA in Escherichia coli using a gene cluster isolated from Shewanella baltica MAC1. J Appl Microbiol. 2010;109(6):1897-1905.
Crossref - Amiri-Jami M, Lapointe G, Griffiths MW. Engineering of EPA/DHA omega-3 fatty acid production by Lactococcus lactis subsp. cremoris MG1363. Appl Microbiol Biotechnol. 2014;98(7):3071-80.
Crossref - Orikasa Y, Nishida T, Yamada A, et al. Recombinant production of docosahexaenoic acid in a polyketide biosynthesis mode in Escherichia coli. Biotechnol Lett. 2006;28(22):1841-1847.
Crossref - Takeyama H, Takeda D, Yazawa K, Yamada A, Matsunaga T. Expression of the eicosapentaenoic acid synthesis gene cluster from Shewanella sp. in a transgenic marine cyanobacterium, Synechococcus sp. Microbiology. 1997;143(8):2725-31.
Crossref - Orikasa Y, Yamada A, Yu R, et al. Characterization of the eicosapentaenoic acid biosynthesis gene cluster from Shewanella sp. strain SCRC-2738. Cell Mol Biol. (Noisy-Le-Grand) 2004;50(5):625-30.
- Tavares S, Grotkjær T, Obsen T, Haslam RP, Napier JA, Gunnarsson N. Metabolic engineering of Saccharomyces cerevisiae for production of eicosapentaenoic acid, using a novel D5-Desaturase from Paramecium tetraurelia. Appl Environ Microbiol. 2011;77(5):1854-1861.
Crossref - Damude HG, Zhang H, Farrall L, et al. Identification of bifunctional D12/w3 fatty acid desaturases for improving the ratio of w3 to w6 fatty acids in microbes and plants. Proc Natl Acad Sci U S A. 2006;103(25):9446-9451.
Crossref - Hamilton ML, Haslam RP, Napier JA, Sayanova O. Metabolic engineering of Phaeodactylum tricornutum for the enhanced accumulation of omega-3 long chain polyunsaturated fatty acids. Metab Eng. 2014;22(100):3-9.
Crossref - Sugihara S, Orikasa Y, Okuyama H. An EntD-like phosphopantetheinyl transferase gene from Photobacterium profundum SS9 complements pfa genes of Moritella marina strain MP-1 involved in biosynthesis of docosahexaenoic acid. Biotechnol Lett. 2008;30(3):411-414.
Crossref - Kajikawa M, Yamato KT, Kohzu Y, et al. Isolation and characterization of ִD6-desaturase, an ELO-like enzyme and ִD5-desaturase from the liverwort Marchantia polymorpha and production of arachidonic and eicosapentaenoic acids in the methylotrophic yeast Pichia pastoris. Plant Mol Biol. 2004;54:335-352.
Crossref - Li YT, Li MT, Fu CH, Zhou PP, Liu JM, Yu LJ. Improvement of arachidonic acid and eicosapentaenoic acid production by increasing the copy number of the genes encoding fatty acid desaturase and elongase into Pichia pastoris. Biotechnol Lett. 2009;31(7):1011-1017.
Crossref - Allen EE, Bartlett DH. Structure and regulation of the omega-3 polyunsaturated fatty acid synthase genes from the deep-sea bacterium Photobacterium profundum strain SS9. Microbiology. 2002;148(Pt 6):1903-1913.
Crossref - Prestele J, Hierl G, Scherling C, et al. Different functions of the C3HC4 zinc RING finger peroxins PEX10, PEX2, and PEX12 in peroxisome formation and matrix protein import. Proc Natl Acad Sci U S A. 2010;107(33):14915-14920.
Crossref - Chen G, Qu S, Wang Q, et al. Transgenic expression of delta-6 and delta-15 fatty acid desaturases enhances omega-3 polyunsaturated fatty acid accumulation in Synechocystis sp. PCC6803. Biotechnol Biofuels. 2014;7(1):32.
Crossref - Amiri-Jami M, Wang H, Kakuda Y, Griffiths MW. Enhancement of polyunsaturated fatty acid production by Tn5 transposon in Shewanella baltica. Biotechnol Lett. 2006;28(15):1187-1192.
Crossref - Chaturvedi R, Fujita Y. Isolation of enhanced eicosapentaenoic acid producing mutants of Nannochloropsis oculata ST-6 using ethyl methane sulfonate induced mutagenesis techniques and their characterization at mRNA transcript level. Phycol Res. 2006;54(3):208-219.
Crossref - Ando A, Sumida Y, Negoro H, et al. Establishment of Agrobacterium tumefaciens-mediated transformation of an oleaginous fungus, Mortierella alpina 1S-4, and its application for eicosapentaenoic acid producer breeding. Appl Environ Microbiol. 2009;75(17):5529-5535.
Crossref - Jareonkitmongkol S, Shimizu S, Yamada H. Production of an eicosapentaenoic acid-containing oil by a Dִ12 desaturase-defective mutant of Mortierella alpina 1S-4. J Am Oil Chem Soc. 1993;70(2):119-123.
Crossref - Kobayashi T, Sakaguchi K, Matsuda T, et al. Increase of eicosapentaenoic acid in thraustochytrids through thraustochytrid ubiquitin promoter-driven expression of a fatty acid ִD5 desaturase gene. Appl Environ Microbiol. 2011;77(11):3870-3876.
Crossref - Domergue F, Abbadi A, Ott C, Zank TK, Zahringer U, Heinz E. Acyl carriers used as substrates by the desaturases and elongases involved in very long-chain polyunsaturated fatty acids biosynthesis reconstituted in yeast. J Biol Chem. 2003;278(37):35115-35126.
Crossref - Domergue F, Abbadi A, Zahringer U, Moreau H, Heinz E. In vivo characterization of the first acyl-CoA ִD6-desaturase from a member of the plant kingdom, the microalga Ostreococcus tauri. Biochem J. 2005;389(Pt 2):483-90.
Crossref - Petrie JR, Shrestha P, Mansour MP, Nichols PD, Liu Q, Singh SP. Metabolic engineering of omega-3 long-chain polyunsaturated fatty acids in plants using an acyl-CoA ִD6-desaturase with ω3-preference from the marine microalga Micromonas pusilla. Metab Eng. 2010;12(3):233-240.
Crossref - Hoffmann M, Wagner M, Abbadi A, Fulda M, Feussner I. Metabolic engineering of ω3-very long chain polyunsaturated fatty acid production by an exclusively acyl-CoA-dependent pathway. J Biol Chem. 2008;283(33):22352-2262.
Crossref - Muhlroth A, Li K, Rokke G, et al. Pathways of lipid metabolism in marine algae, co-expression network, bottlenecks and candidate genes for enhanced production of EPA and DHA in species of chromista. Mar Drugs. 2013;11(11):4662-4697.
Crossref - Kazachkov M, Chen Q, Wang L, Zou J. Substrate preferences of a lysophosphatidylcholine acyltransferase highlight its role in phospholipid remodeling. Lipids. 2008;43(10):895-902.
Crossref - Stahl U, Stalberg K, Stymne S, Ronne H. A family of eukaryotic lysophospholipid acyltransferases with broad specificity. FEBS Lett. 2008;582(2):305-309.
Crossref - Sandager L, Gustavsson MH, Stahl U, et al. Storage lipid synthesis is non-essential in yeast. J Biol Chem. 2002;277(8):6478-6482.
Crossref - Fan J, Yan C, Zhang X, Xu C. Dual role for phospholipid: Diacylglycerol acyltransferase: Enhancing fatty acid synthesis and diverting fatty acids from membrane lipids to triacylglycerol in Arabidopsis leaves. Plant Cell. 2013;25(9):3506-3518.
Crossref - Mariam I, Bettiga M, Rova U, Christakopoulos P, Matsakas L, Patel A. Ameliorating microalgal omega production using omics platforms. Trends Plant Sci. 2024;29(7):799-813.
Crossref - Giner-Robles L, Lazaro B, de la Cruz F, Moncalian G. fabH deletion increases DHA production in Escherichia coli expressing Pfa genes. Microb Cell Fact. 2018;17(1):88.
Crossref - Santos-Merino M, Garcillan-Barcia MP, de la Cruz F. Engineering the fatty acid synthesis pathway in Synechococcus elongatus PCC 7942 improves omega-3 fatty acid production. Biotechnol Biofuels. 2018;11:239.
Crossref - Han X, Zhao Z, Wen Y, Chen Z. Enhancement of docosahexaenoic acid production by overexpression of ATP-citrate lyase and acetyl-CoA carboxylase in Schizochytrium sp. Biotechnol Biofuels. 2020;13:131.
Crossref - Li Z, Meng T, Ling X, et al. Overexpression of Malonyl-CoA: ACP Transacylase in Schizochytrium sp. to Improve Polyunsaturated Fatty Acid Production. J Agric Food Chem. 2018;66(21):5382-5391.
Crossref - Wang F, Bi Y, Diao J, et al. Metabolic engineering to enhance biosynthesis of both docosahexaenoic acid and odd-chain fatty acids in Schizochytrium sp. S31. Biotechnol Biofuels. 2019;12:141.
Crossref - Liu Y, Han X, Chen Z, et al. Selectively superior production of docosahexaenoic acid in Schizochytrium sp. through engineering the fatty acid biosynthetic pathways. Biotechnol Biofuels. 2024;17(1):75.
Crossref - Amiri-Jami M, Abdelhamid AG, Hazaa M, Kakuda Y, Griffths MW. Recombinant production of ω3FA by probiotic Escherichia coli Nissle 1917. FEMS Microbiol Lett. 2015;362(20):fnv166.
Crossref - Wang S, Wang Z, Zhang H, et al. Enhancement of docosahexaenoic acid production by overexpression of ATP-citrate lyase and acetyl-CoA carboxylase in Schizochytrium sp. Biotechnol Biofuels Bioprod. 2020;13:98.
Crossref - Qin J, Liu N, Abid U, et al. Metabolic engineering of Yarrowia lipolytica for conversion of waste cooking oil into omega-3 eicosapentaenoic acid. ACS Eng Au. 2025;5(2):128-139.
Crossref - Zhu BH, Tu CC, Shi HP, et al. Overexpression of endogenous delta-6 fatty acid desaturase gene enhances eicosapentaenoic acid accumulation in Phaeodactylum tricornutum. Process Biochem. 2017;57:10-18.
Crossref - Muthu D, Kabilan C, Gummadi SN, Chadha A. Role of key enzymes in the production of docosahexaenoic acid (DHA) by Thraustochytrium sp. T01. Prep Biochem Biotechnol. 2023;53(7):807-15.
Crossref - Xie D, Miller E, Sharpe P, Jackson E, Zhu Q. Omega-3 production by fermentation of Yarrowia lipolytica: From fed-batch to continuous. Biotechnol Bioeng. 2017;114(4):798-812.
Crossref - Gemperlein K, Dietrich D, Kohlstedt M, et al. Polyunsaturated fatty acid production by Yarrowia lipolytica employing designed myxobacterial PUFA synthases. Nat Commun. 2019;10(1):4055.
Crossref - Celinska E, Borkowska M, Korpys-WoYniak P, et al. Optimization of Yarrowia lipolytica-based consolidated biocatalyst through synthetic biology approach: Transcription units and signal peptides shuffling. Appl Microbiol Biotechnol. 2020;104(13):5845-5859.
Crossref - Zhang Y, Adams IP, Ratledge C. Malic enzyme: The controlling activity for lipid production? Overexpression of malic enzyme in Mucor circinelloides leads to a 2.5-fold increase in lipid accumulation. Microbiology. 2007;153(7):2013-2021.
Crossref - Xin Y, Shen C, She Y, et al. Biosynthesis of triacylglycerol molecules with a tailored PUFA profile in industrial microalgae. Mol Plant. 2019;12(4):474-488.
Crossref - Miao C, Du M, Du H, et al. Enhanced eicosapentaenoic acid production via synthetic biological strategy in Nannochloropsis oceanica. Mar Drugs. 2024;22(12):570.
Crossref - Zhang ZX, Wu HX, Lin YC, et al. Polyketide synthase acyltransferase domain swapping for enhanced EPA recognition and efficient coproduction of EPA and DHA in Schizochytrium sp. J Agric Food Chem. 2025;73(4):2461-2470.
Crossref - Ma W, Liu M, Zhang Z, et al. Efficient co-production of EPA and DHA by Schizochytrium sp. via regulation of the polyketide synthase pathway. Commun Biol. 2022;5(1):1356.
Crossref - Metz JG, Roessler P, Facciotti D, et al. Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science. 2001;293(5528):290-293.
Crossref - Bejenari M, Spedtsberg EML, Mathiesen J, et al. First-class – biosynthesis of 6-MSA and bostrycoidin type I polyketides in Yarrowia lipolytica. Front Fungal Biol. 2024;5:1327777.
Crossref - Schmidt M, Lee N, Zhan C, et al. Maximizing heterologous expression of engineered type I polyketide synthases: Investigating codon optimization strategies. ACS Synth Biol. 2023;12(11):3366-80.
Crossref - Wang S, Lan C, Wang Z, et al. Optimizing eicosapentaenoic acid production by grafting a heterologous polyketide synthase pathway in the thraustochytrid Aurantiochytrium. J Agric Food Chem. 2020;68(40):11253-60.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.