ISSN: 0973-7510
E-ISSN: 2581-690X
This study comprehensively examined the Avicennia marina plant, a key species along the Red Sea coast of the Saudi Arabian Kingdom, focusing on its microbial, biochemical, and ecological aspects. Specimens, including leaves, seeds, and seedlings of Avicennia marina plant, were collected during the summer of 2021 from two distinct mangrove-abundant regions in Yanbu Governorate, Kingdom of Saudi Arabia. Analysis of the plant revealed significant heavy metal concentrations in its sediment, leaves, and seeds, with the sediment from region 1 recording metals such as Cr (40.202 ppm) and Se (30.522 ppm). Additionally, the plant’s extracts exhibited notable antimicrobial properties, inhibiting growth of several pathogenic bacteria, with ethyl acetate extracts from leaves being especially potent against strains like P. aeruginosa and S. aureus. Moreover, from the leaves collected in the Yanbu region, 10 unique endophytic bacterial species were identified. Utilizing genomic analysis tools, two of these bacteria were closely characterized, revealing more than 99% similarity to known bacterial species; Mixta and Cytobacillus species, emphasizing the microbial diversity within the mangrove leaves. In sum, Avicennia marina emerges as ecologically significant, with potential implications for bio-remediation and antimicrobial applications, and as a reservoir of diverse microbial species. With mounting global challenges, such as climate change, understanding these microbial-plant interactions is pivotal for both conservation and biotechnological pursuits in the future.
Avicennia Marina, Endophytic Bacteria, Heavy Metals, Yanbu, Saudi Arabia
Mangrove ecosystems, typified by halophytic shrublands and forests, play a critical role in the global environment by providing diverse ecological services ranging from carbon sequestration to coastline protection.1 Avicennia marina, commonly referred to as the grey mangrove, stands as one of the most widely distributed species within the mangrove category, stretching from the west coasts of Africa to the Indo-Pacific regions.2 Mangrove ecosystems, characterized by their saline coastal habitats, represent one of the most vital intertidal environments globally. These ecosystems are critical not only for their unique biodiversity but also for the ecosystem services they offer, including carbon sequestration, coastal stabilization, and nursery habitats for marine life.3
Saudi Arabia, with its extensive Red Sea and Arabian Gulf coastlines, harbors a significant portion of the global mangrove landscape. In Saudi Arabia, mangroves, predominantly represented by the species Avicennia marina, serve as a vital component of coastal ecosystems. These mangrove stands are particularly important in arid regions like Saudi Arabia, where they act as essential green belts, helping in reducing coastal erosion and supporting coastal fisheries.4,5 While globally mangroves have been studied for their ecological, environmental, and socio-economic significance, the mangroves of Saudi Arabia have historically received less attention. However, with increasing environmental and anthropogenic pressures, such as urban development and oil exploration, it has become imperative to study and conserve these mangrove habitats.6
The unique environmental conditions, including extreme salinity and temperatures, make the mangroves in Saudi Arabia of particular interest, potentially offering insights into the resilience and adaptability of these coastal forests.7 Its resilience to saline environments and extreme tidal fluctuations can be attributed, in part, to its symbiotic relationships with a myriad of microorganisms, including endophytic bacteria. Endophytic bacteria are microorganisms that colonize plant tissues without causing harm to the host, often benefiting the plant by enhancing its growth, nutrient uptake, or resilience to stress factors like drought or salinity.8 Over the years, studies have underscored the potential of these endophytes in biotechnological and agricultural applications due to their ability to produce bioactive compounds, promote plant growth, and protect against pathogens.9 In the context of Saudi Arabia, mangrove ecosystems, particularly those hosting Avicennia marina, also known as the grey mangrove, have largely remained unexplored in terms of their microbial diversity. Review reports by Triest et al.,10 and Triest et al.,11 underscore the limited exploration of microbial communities associated with Avicennia marina. This scarcity of available reports highlights the necessity for further investigation into the microbial diversity of this species.
As global climate changes and anthropogenic pressures mount, understanding these microbial interactions becomes increasingly important for both conservation and biotechnological pursuits.
Thus, this study seeks to identify and analyze the endophytic bacteria residing within the Avicennia marina plants of Saudi Arabia, providing a foundational exploration into the microbial richness of this critical ecosystem. The aim of this study is to explore the Avicennia marina plant from Saudi Arabia, emphasizing its microbial diversity, ecological importance, and potential in bio-remediation and antimicrobial applications, with a view to inform conservation and biotechnological strategies amidst global environmental challenges.
Field Study, Sampling and Plant Material
Sampling was conducted in various mangrove regions along the Red Sea, Yanbu Governorate in Saudi Arabia. Specific sites were chosen based on accessibility and the apparent health of the Avicennia marina population. At each site, ten healthy Avicennia marina trees were randomly selected for sampling during the months of June to August 2021. From each selected tree, young, mature leaves, small branches, and seeds were collected using sterilized scissors and immediately placed in sterile Ziploc bags. The samples were stored in a cooler with ice packs and transported to the laboratory for further processing within 24 hours.12
Heavy Metals Assay in Avicennia marina Plant Components
To accurately analyze the heavy metals in A. marina plant components (sediment, leaves, and seeds), rigorous cleaning procedures were followed to prevent contamination. Glassware and containers were initially cleaned with water and soap, followed by distilled water and an acid mixture. Subsequent rinses included a 10% HNO3 solution and a final rinse with de-ionized water before air-drying inside an incubator, away from contaminants. A. marina plant samples, specifically from 120-day-old plants, were prepped for analysis Abou Seedo et al.13
The chosen method for breaking down these samples was wet digestion, suitable for volatile metals and those that form volatile salts with acids. Each plant part (leaves, branches, and seeds) was processed separately. For each sample, weighing 1 gram, the plant material was finely cut with a scalpel and combined with 5 ml of an acid digestion mixture comprising 3 ml of HNO3 and 2 ml of HClO4 in a secure tube. After allowing the samples to stand overnight at room temperature, the tubes were heated in a 70°C water bath for 3 hours, with shaking every 30 minutes to ensure thorough digestion, as described by Zantopoulos et al.14 After cooling, the digested samples were diluted with de-ionized water and filtered. The filtrate was stored in sealed glass tubes at room temperature until analysis. Heavy metal analysis was performed using a Perkin Elmer model Atomic Absorption Spectrophotometer (AAS). Both blank and standard solutions underwent the same wet digestion method. The blank solution, made from a mix of nitric and hypochloric acids identified potential contamination from the chemicals used. Standard solutions, on the other hand, were prepared using certified metal standards for each heavy metal being investigated by Asher et al.15
Evaluation of antimicrobial effects of Avicennia marina on various pathogenic bacteria
Plant extracts from Avicennia marina leaves were prepared by macerating with ethanol or ethyl acetate and then evaporating to dryness. For the antimicrobial assessment, we employed the methodology outlined by Collins.16 The extract (0.2 g) was re-dissolved in 5 ml of sterilized distilled water and mixed thoroughly using a vortex mixer. 1 ml of this Avicennia marina solution was then incorporated into Petri dishes containing sterile, freshly poured nutrient agar. Each standardized test microorganism culture was then streaked onto this medium and kept in an incubator for 72 hours at 27°C. Control variants, which included extract-free inoculum and inoculum without extract, were concurrently set up. Wells measuring 7 mm in diameter and 5 mm in depth were carved into the agar, and each was filled with 50 µL of the designated extracts (from leaves). These plates were incubated for duration of 24 hours at 37°C. Post-incubation, the zones devoid of microbial growth surrounding the wells were quantified, with the diameter of these inhibition zones indicating the level of antimicrobial activity. Control samples for every bacterial strain (Klebsiella spp., S. aureus, B. cereus, P. aeruginosa) were also prepared, using only the solvents in lieu of the plant extracts, as described by He and Zhou.17
Endophytic Bacteria Isolation and Identification Procedures
Upon arrival at the laboratory, plant samples (leaves) were first rinsed with tap water to eliminate surface contaminants. This was followed by a systematic surface-sterilization: an initial immersion in 70% ethanol for 1 minute, then in 2.5% sodium hypochlorite for 5 minutes, and concluding with three successive rinses using sterile distilled water.18 To verify the effectiveness of the sterilization, tissues were imprinted on nutrient agar plates and incubated for 48 hours at 28°C, monitoring for microbial growth. After ensuring sterilization, the plant materials were ground using a sterile mortar and pestle, combined with sterile distilled water. This produced a suspension that was then diluted systematically and cultured on nutrient agar plates. These plates were incubated at a consistent 28°C for duration of 5-7 days. Resultant bacterial colonies were then isolated to procure pure cultures.
For primary identification, these bacterial isolates were examined based on their distinct morphological features and specific biochemical characteristics. For a more refined identification, molecular techniques were employed: bacterial genomic DNA was extracted and the 16S rRNA gene was amplified utilizing universal primers, as detailed by Weisburg et al.19 The resulting PCR products underwent sequencing, and the acquired sequences were matched against existing database records using the BLAST tool to determine bacterial species.
Analysis of statistical diversity
To assess and compare the diversity of endophytic bacterial communities from various sites, specific diversity indices were utilized. These included the Shannon-Wiener Index, which provides insights into species richness and evenness, and the Simpson’s Diversity Index, which measures the probability that two randomly selected individuals belong to different species. The computational analysis for these indices was conducted using the PAST software suite, as described by Hammer, et al.20 The outcomes from these calculations provided a clearer perspective on the biodiversity and distribution of bacterial communities across the selected sites.
Collection of Avicennia marina plant specimens for analysis
For a comprehensive understanding of the microbial and biochemical components of the Avicennia marina plant, specimens were meticulously curated. Healthy leaves, seeds, and entire seedlings of the plant were gathered from two specific sites during the summer 2021, as illustrated in Figure 1. A thorough record of these collected specimens – encapsulating the leaves, seeds, and young plants – can be accessed in Table. The locations chosen for this study were strategically positioned along the Red Sea coast, covering two distinct areas known for their mangrove abundance. These zones are notably marked by lush mangrove canopies intermingled with expanses of sandy landscapes, reflecting the unique ecological balance of the region. A location particularly noteworthy for this study is about 5 to 10 km North and South of the Yanbu City. This site stands out due to its flourishing mangroves, which display not just healthy growth but also exemplify the region’s ecological richness and the resilience of the A. marina species. Incorporating such diverse samples from varied geographical regions ensures a holistic understanding of the plant’s microbial and biochemical dynamics. This approach aids in discerning regional variations, if any, and offers insights into how local ecological factors might influence the plant’s internal microbial community and biochemical properties.
Table: The quantity of A. marina plant components (including leaves, seeds, and seedlings) gathered from four research locations was recorded
Samples/Study area |
North of the Yanbu City |
South of the Yanbu City |
|---|---|---|
Location code |
Region 1 |
Region 2 |
Seeds |
25 |
24 |
Sediment |
10 |
11 |
Whole plant (Seedlings) |
4 |
3 |
Leaves |
45 |
55 |
Figure 1. Various samples of A. marina plant from distinct locations. a: Entire plant (seedlings), b: Leaves, c, d: Seeds
Assay of Heavy metals for Avicennia marina plant
An analysis of heavy metals was performed on different parts of the A. marina plant, encompassing its sediment, leaves, and seeds. Mangroves play a pivotal role in metal absorption within tropical and subtropical marine ecosystems. To understand the absorption and environmental impact of these metals, sediment samples and parts of A. marina were collected from two sites. The sediment samples from region (1) showed the average metal concentrations in the order: Cr (40.202 ppm), Se (30.522 ppm), Ni (3.161 ppm), Zn (3.05 ppm), Cu (0.879 ppm), Au (0.296 ppm), Pb (0.29 ppm), Cd (0.169 ppm), and Al (0.165 ppm). These results are visualized in Figure 2. In addition, the results revealed that among the ten sediment samples from region 2, the mean of heavy metal concentrations were as follows: Cr (10.145 ppm), Se (9.319 ppm), Zn (5.144 ppm), Au (2.43 ppm), Ni (2.099 ppm), Pb (1.678 ppm), Cu (1.505 ppm), Cd (0.373 ppm), and Al (0.168 ppm). This information can be visualized in Figure 2.
Figure 2. Average concentrations of heavy metals (ppm) of Avicennia marina pneumatophores of studied regions
Antimicrobial efficacy of Avicennia marina extracts
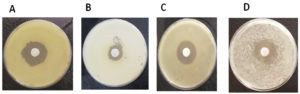
A comprehensive study was conducted to investigate the antimicrobial properties of Avicennia marina plant extracts against a variety of pathogenic bacteria. The test bacteria were introduced onto nutrient agar plates. Wells with a 7 mm diameter and 5 mm depth were created in these plates, into which 50 µL of either leaf or seed extracts were added. Post an incubation period of 24 hours at 37°C, the zones of inhibition were examined. At a concentration of 100 μg/ml, both leaf and seed extracts of Avicennia marina demonstrated inhibition of bacterial growth. The degree of this inhibition was quantified by the diameter of the inhibition zones, as illustrated in Figure 3. Controls, wherein only solvents were applied, were also maintained for comparison purposes. Ethyl acetate extracts from A. marina leaves inhibited P. aeruginosa (3.7 cm, Figure 3A), Bacillus cereus (3.2 cm, Figure 3B), S. aureus (4.6 cm, Figure 3C) and K. pneumonia (3.5 cm, Figure 3D).
Figure 3. Antimicrobial activity of extractions of Avicennia marina leaves by ethyl acetate against some pathogenic bacteria; (A): Pseudomonas aeruginosa. (B): Bacillus cereus (C): Staphylococcus aureus (D): Klebsiella pneumonia
Detailed Analysis of Endophytic Bacteria Isolated from Mangrove Leaves
From the mangrove leaves of Avicennia marina collected in the Yanbu region of Saudi Arabia, endophytic bacteria, which are microbes that reside within plant tissues without causing any harm, were isolated and studied. Upon comprehensive analysis of the bacterial species within these specific mangrove leaves, a total of 10 distinct endophytic bacteria were recognized. To classify and better understand these bacteria, a genomic analysis tool known as BLASTN was utilized, focusing on the 16S rRNA gene fragments – a pivotal molecular marker in microbial genomics. Of the 10 identified endophytic bacteria, two were meticulously characterized through their amplified 16S rRNA gene fragments.
This characterization grouped these bacteria under two distinct genera: Cytobacillus and Mixta. When the analyzed 16S rRNA gene sequences were matched against a comprehensive microbial database, there was an approximate 99 % similarity. This high degree of similarity indicates a close relationship with known bacterial species in the database. The first isolate has similarity 99.85% with Mixta calida, so it was identified as Mixta calida strain “Yanbu7” and has been assigned the accession number (OR762485) in the database. This strain was grouped with the different Mixta calida strains in the same clade as shown in the phylogenetic tree Figure 4.
Figure 4. Phylogenetic tree based on nucleotide sequencing of 16S rRNA gene of different strains of Mixta species; showing relationship between our isolate and other strains obtained from Gene Bank using Neighbor-Joining method
The second isolate has 99.11 % similarity with Cytobacillus oceanisediminis strains in the data base, so it was termed as Cytobacillus oceanisediminis strain “Yanbu2”, and its database accession number is (OR762486). This strain was grouped with the different Cytobacillus oceanisediminis strains in the same clade as shown in the phylogenetic tree Figure 5.
Figure 5. Phylogenetic tree based on nucleotide sequencing of 16S rRNA gene of different strains of Cytobacillus species; showing relationship between our isolate and other strains obtained from Gene Bank using Neighbor-Joining method
In essence, the precise analysis and categorization of these endophytic bacteria provide crucial insights into the microbial diversity present within the mangrove leaves of Avicennia marina in the Yanbu region.
The in-depth examination of Avicennia marina from specific regions along the Saudi Arabia furnishes intriguing insights into its intricate ecological role and interactions, both biochemical and microbial. Mangroves such as Avicennia marina serve as ecological keystones, particularly in regions characterized by their unique blend of lush mangrove expanses and sandy terrains. The inclusion of sites like Yanbu Governorate, known for its flourishing mangrove growth, underscores the importance of geographical factors in determining plant health, resilience, and its internal microbial community. Studies have shown that regional variations can substantially affect the microbial diversity within plant tissues, shaping their adaptability and response to environmental stressors.21
The extensive assay of heavy metals across different parts of A. marina underscores mangroves’ crucial role as natural bio-filters in marine ecosystems. Elevated levels of metals like Chromium (Cr) and Selenium (Se) suggest potential anthropogenic influences or unique soil compositions in the collection areas. Prior research has emphasized that mangroves can act as bio-indicators of marine pollution, accumulating heavy metals from the surrounding environment.21,22 Such accumulation can have both positive implications acting as bio-filters and negative ones, impacting the plant’s health.
The evident antimicrobial properties of Avicennia marina extracts, particularly from the leaves, align with the growing body of evidence highlighting the antimicrobial potentials of mangrove-derived compounds. The distinct zones of inhibition observed against pathogenic bacteria, such as P. aeruginosa and S. aureus, underscore A. marina‘s promise as a source of potent antimicrobial agents. Our results are consistent with Okla et al.23 Such findings mirror prior studies which identified mangroves as a rich source of bioactive compounds, with potential pharmaceutical applications.24
The presence of a diverse endophytic bacterial community within the mangrove leaves, especially from the Yanbu region, further cements the idea of mangroves being reservoirs of microbial diversity. The use of modern genomic tools like BLASTN has allowed for precise categorization and understanding of these bacterial communities, with genera like Mixta and Cytobacillus emerging prominently.
Previous research has indicated the multifaceted roles these endophytes play, from promoting plant growth to bolstering resistance against environmental stressors.9 As climatic challenges mount, understanding the inherent resilience mechanisms within plants like Avicennia marina becomes paramount. Their unique microbial communities might provide clues for innovative solutions, from bio-remediation to the development of novel antimicrobial agents. Diversity within the microbial community ensures functional redundancy, where if one species is absent or non-functional, others can take over its role, ensuring the host plant’s health and resilience.25
Understanding the microbial interactions within mangroves, as done in this study, holds substantial importance, especially in today’s context where these ecosystems are under threat due to climate change and anthropogenic activities. The symbiotic relationships these plants share with their endophytic communities might hold keys to their resilience. Moreover, as global challenges like salinity in agricultural lands intensify, the endophytes from such environments could offer solutions to enhance crop tolerance to these challenges.26
This study, by shedding light on the endophytic communities of Avicennia marina in Saudi Arabia, has paved the way for future research endeavors, focusing on not just conservation but also harnessing these microbial communities for societal benefits. In summation, the detailed exploration into Avicennia marina‘s ecological, microbial, and biochemical profiles provides not just a snapshot of its present state but also paves the way for future research avenues. It emphasizes the mangrove’s significance as an ecological sentinel, a reservoir of bioactive compounds, and a potential beacon for sustainable solutions in a rapidly changing world.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the approval and the support of this research study by grant no. SCAR-2022-11-1627 from the Deanship of Scientific Research at Northern Border University, Arar, K.S.A.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Both authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was supported by the Deanship of Scientific Research at Northern Border University, Arar, Saudi Arabia, with grant no. SCAR-2022-11-1627.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Alongi DM. Present state and future of the world’s mangrove forests. Environmental Conservation. 2002;29(3):331-349.
Crossref - Duke NC. Mangrove Floristics and Biogeography. Tropical Mangrove Ecosystems. 1992;41:63-100.
Crossref - Kathiresan K, Bingham BL. Biology of mangroves and mangrove Ecosystems. Advances in Marine Biology. 2001;40:81-251.
Crossref - Sohaib M, Al-Barakah FNI, Migdadi HM, Alyousif M, Ahmed I. Ecological assessment of physico-chemical properties in mangrove environments along the Arabian Gulf and the Red Sea coasts of Saudi Arabia. Egypt J Aquat Res. 2023;49(1):9-16.
Crossref - Aljahdali MO, Alhassan AB. Rare Earth Elements and Bioavailability in Northern and Southern Central Red Sea Mangroves, Saudi Arabia. Molecules. 2022;27(14):4335.
Crossref - Almahasheer H, Aljowair A, Duarte CM, Irigoien X. Decadal Stability of Red Sea Mangroves. 2015. Accessed November 13, 2023. http://hdl.handle.net/10754/584005
- Almahasheer H, Serrano O, Duarte CM, Arias-Ortiz A, Masque P, Irigoien X. Low Carbon sink capacity of Red Sea mangroves. Sci Rep. 2017;7:9700.
Crossref - Hallmann J, Quadt-Hallmann A, Mahaffee WF, Kloepper JW. Bacterial endophytes in agricultural crops. Can J Microbiol. 1997;43(10):895-914.
Crossref - Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN. Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett. 2008;278(1):1-9.
Crossref - Triest L, Hasan S, Mitro P, De Ryck D, Van der Stocken T. Geographical Distance and Large Rivers Shape Genetic Structure of Avicennia officinalis in the Highly Dynamic Sundarbans Mangrove Forest and Ganges Delta Region. Estuaries and Coasts. 2017;41:908-920.
Crossref - Triest L, Socorro A, Gado VJ, Mazo A, Sierens T. Avicennia Genetic Diversity and Fine-Scaled Structure Influenced by Coastal Proximity of Mangrove Fragments. Front Mar Sci. 2021;8:643982.
Crossref - Hardoim PR, Hardoim CCP, van Overbeek LS, van Elsas JD. Dynamics of seed-borne rice endophytes on early plant growth stages. PLoS One. 2012;7(2):e30438.
Crossref - Abou Seedo K, Abido MS, Salih A, Abahussain A. Structure and Composition of Mangrove Associations in Tubli Bay of Bahrain as Affected by Municipal Wastewater Discharge and Anthropogenic Sedimentation. International Journal of Biodiversity. 2017;e2084256.
Crossref - Zantopoulos N, Antoniou V, Petsaga V, Zdragas A. Copper concentrations in sheep liver and kidney in Greece. Vet Hum Toxicol. 1996;38(3):184-185.
- Asher AS, Samuel KE, Mary DS. Analytical Method for Comparison of Suitable Wet Digestion Methods for Heavy Metal Analysis in Soil around a Cement Industry. International Journal of Research and Scientific Innovation (IJRSI). 2020;7(6):41-47.
- Collins CH, Lyne PM, Grange JM. Collins and Lyne’s Microbiological Methods. 8th Edition, Butterworth-Heinemann, London. – References – Scientific Research Publishing. 2004. https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje))/reference/referencespapers.aspx?referenceid=2686584. Accessed November 13, 2023.
- He F, Zhou J. A new antimicrobial susceptibility testing method of Escherichia coli against ampicillin by MSPQC. J Microbiol Methods. 2007;68(3):563-567.
Crossref - Strobel G, Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev. 2003;67(4):491-502.
Crossref - Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173(2):697-703.
Crossref - Hammer O, Harper D, Ryan P. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica. 2001;4:1-9.
- Menezes S, Baird DJ, Soares AMVM. Beyond taxonomy: a review of macroinvertebrate trait-based community descriptors as tools for freshwater biomonitoring. J Appl Ecol. 2010;47(4):711-719.
Crossref - Thomson T, Fusi M, Bennett-Smith MF, et al. Contrasting Effects of Local Environmental and Biogeographic Factors on the Composition and Structure of Bacterial Communities in Arid Monospecific Mangrove Soils. Microbiol Spectr. 2022;10(1):e0090321.
Crossref - Okla MK, Alatar AA, Al-amri SS, Soufan WH, Ahmad A, Abdel-Maksoud MA. Antibacterial and Antifungal Activity of the Extracts of Different Parts of Avicennia marina (Forssk.) Vierh. Plants (Basel). 2021;10(2):252.
Crossref - Simlai A, Roy A. Biological activities and chemical constituents of some mangrove species from Sundarban estuary: An overview. Pharmacogn Rev. 2013;7(14):170-178.
Crossref - He S, Ivanova N, Kirton E, et al. Comparative Metagenomic and Metatranscriptomic Analysis of Hindgut Paunch Microbiota in Wood- and Dung-Feeding Higher Termites. PLoS One. 2013;8(4):e61126.
Crossref - Egamberdieva D, Wirth SJ, Alqarawi AA, Abd_Allah EF, Hashem A. Phytohormones and Beneficial Microbes: Essential Components for Plants to Balance Stress and Fitness. Front Microbiol. 2017;8:2104.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.