ISSN: 0973-7510
E-ISSN: 2581-690X
Emerging antibiotic resistance among mycoplasma microorganisms is of major concern in present times as they cause various diseases in both animals and humans. Mycoplasmoses, infections caused by mycoplasma microorganisms have become common in recent past and have gained importance both due to inability to diagnose and difficulty to treat. Respiratory tract infection, mastitis, arthritis, and septicemia caused by Mycoplasma in livestock are responsible for causing heavy economic losses. These diseases are frequently reported from countries of Africa and Asia, including India. Antimycoplasma antibiotics are frequently being used as therapeutic agents for the treatment of mycoplasmoses infection in livestock. They include macrolides, tetracyclines, fluoroquinolones, and aminoglycosides which are the main antibiotic classes commonly used against mycoplasma globally. Oxytetracyclines are the commonest antibiotics used for decades followed by enrofloxacin, tylosin, and streptomycin. Danofloxacin, lincomycin, spiramycin, erythromycin, gamithromycin, azithromycin, clarithromycin, gentamicin, doxycycline, and tulathromycin are also used occasionally. Continuous and unregulated use of these antibiotics over prolonged period can lead to menace of antibiotic resistance which is aided by inappropriate doses and uncontrolled use. Resistance to some antibiotics is already emerging. Mycoplasmas have devised different resistance mechanisms for combating antimicrobial action of these drugs. Common mechanisms noted are acquisitions of proteins affecting ribosomal subunits, inhibition of antibiotic efflux, structural changes in the ribosomal subunit, target mutations, expression or production of enzymes. Additional novel mechanisms of resistance still need to be investigated. Strategies for prevention and encountering of this antibiotic resistance are being devised by alternating antibiotics in application, using antimycoplasma antibiotic sensitivity tests, along with evaluation of specific doses and exploration of novel mycoplasma specific class of antibiotics. Novel targets based on various cell structures including cell membrane, organelles, proteins, enzymes or metabolites are being explored for antimycoplasma therapy. These all will help in effective therapeutic management of mycoplasmoses with minimal side effects.
Antibiotics, Mycoplasma, Novel drug Targets, antibiotic resistance.
Mycoplasmoses, infections caused by mycoplasma (smallest prokaryotes lacking cell wall) microorganisms have become common in both livestock and humans in recent past which might be due to emergence of these pathogens and secondly due to resistance to antibiotics1,2,3,4,5. For farmers it has vast economic constraints. Mycoplasmoses has gained importance both due to occurrence of frequent outbreaks, inability to diagnose and difficulty to treat4,5,6,7,8. Utilization of inappropriate doses of mycoplasma non specific antibiotics is the routine practice in both medical and veterinary practice4,9,10. Continuous use of antibiotics against other microbial pathogens also flares up mycoplasma growth and hence frequent emergence of outbreaks9,10,11,12. Lack of prophylactic measures adds to the problem4,8. Fastidious nature and special requirements of costly media for mycoplasma along with lack of laboratory facilities renders diagnosis difficult hence non utilization of mycoplasma specific therapies or control measures for mycoplasma infections usually get unnoticed or ignored or remain undiagnosed4,8,13,14. Prolonged usage of antibiotics against microbial infections in inappropriate doses leads to development of antibiotic resistance which is recent concern of importance15,16.
Even though Mycoplasma lack cell wall but many other cellular systems are similar to bacteria. They are cellular parasites and derive their requirements from host17,18 and have limited synthetic ability due to lack of machinery and hence parasitise host cells in vivo and require specialized media in vitro17,19,20,21. These wall less organisms contain capsule, lipoprotein plasma membrane, genetic material (DNA), ribosomes, soluble proteins, metabolites and enzymes18,20. Of these capsule or plasma membrane is believed to be of importance for antigenicity. Despite such limited capability and specific requirements mycoplasma have enormous reproducibility and cause serious infections and a range of diseases in farm animals and humans18,22. These smallest fastidious bacteria affect respiratory tract, udder, eyes, ear, joints, and reproductive system and cause various diseases in animals as well as in humans.
There are around 32 species of mycoplasma of veterinary importance23 and around 7 species of mycoplasma that are known to cause disease in humans24,25. Of the important species of mycoplasma, Mycoplasma bovis, M. mycoides, M. alkalescens, M. ovipneumonia, M. capripneumonia, M. capricolum, M. capri, M. gallisepticum, M. gallinarium, M. agalactiae, M. canadense, M. californicum, M. bovigenitalium, M. putrefaciens, M. synoviae, M. agalactiae, M. putrefaciens, M. conjunctiva, M. arginine are important causative agents of various diseases affecting respiratory tract, udder, joints, eyes and ears, reproductive tract etc17,19,20,21,26. M. alkalescens, M. ovipneumonia, M. capri-pneumonia, M. capricolum, M. capri, M. galli-septicum, M. gallinarium), mastitis (Mycoplasma bovis, M. agalactiae, M. canadense, M. californicum, M. alkalescens, M. bovigenitalium, M. putrefaciens), arthritis (M. canadense, M. synoviae, M. agalactiae, M. capricolum, M. putrefaciens), abortion (M. bovigenitalium), eye and ear infections (M. conjunctiva, M. arginini), other conditions like lymphadenitis, peritonitis, pericarditis, septicemia, hepatopathy, air sac disease, decrease fertility, hatchability and repeat breeding17,19,20,21,27,28,29,30. These range from mild to severe peracute and highly fatal infections. Pneumonia, mastitis, and arthritis are the common diseases of mycoplasma whereas meningitis, otitis, kerato-conjunctivitis, decubital abscesses, vaginitis and/or abortionare occasional findings31,32. In small ruminants, they commonly cause respiratory diseases, arthritis, eye lesions, genital disease and mastitis20,33. Contagious bovine pleuropneumonia (CBPP), contagious caprinepleuropneumonia (CCPP), contagious agalactia, chronic respiratory disease (CRD), mastitis, arthritis, kerato-conjunctivitis (pink eye), infectious sinusitis are some of the important diseases caused by mycoplasma in livestock and poultry4,5,34,35,36. In humans they cause pneumonia (M. pneumonia), inflammatory diseases (M. genitalium), infections of the genitourinary tract, infertility, septic abortion and amnionitis (M. pneumonia, M. hominis), disorders of the hematopoietic, cardiovascular, central nervous, musculoskeletal, cutaneous and gastrointestinal systems (M. pneumonia, M. hominis)24,25,37,38,39.
Among the mycoplasmal diseases of livestock, respiratory tract infection, mastitis, arthritis, and septicemia are considered to cause heavy economic losses and are often reported from countries of Africa and Asia, including India4,35,36,40,41,. These usually terminate in chronic complications, leading to huge economic losses in the form of diminished production, treatment cost, high mortality and decreased export, and are causing threat to production, creating carriers and constant risk for disease outbreak. Due to lack of vaccines, preventive measures are non specific and usually rarely adopted against mycoplasma. Diagnostic facilities are not well equipped for culture, isolation or detection of mycoplasma. Moreover there is also difficulty in controlling the diseases because of lack in consistency of expression of disease30. Hence the only possible option and current practice of dealing with mycoplasma is utilizing antibiotics for therapeutic purposes. They are usually used for treating mycoplasma affected animals and now a days few have been used for prophylactic purposes for preventing mycoplasma infections in risk herds e.g. oxytetracycline. Treatment of affected flocks with broad acting antibiotics is practiced to control the spread of the infection. These treatments and the antibiotics used however are having fewer efficacies, thus there is a doubt on the efficacy of antibiotic therapy42.A very significant instance is the disease contagious caprinepleuropneumonia (CCPP) in India wherein antibiotic therapy (usual) is proven to be a failure ultimately resulting in quick disease spread43. Since Mollicutes lack cell wall, hence the cell wall acting antibiotics, such as betalactam antibiotics, glycopeptides, and fosfomycin, do not affect them and also the biological characteristic features of mycoplasmas results in the ineffectiveness of a number of other antimicrobials (sulfonamides, trimethoprim, rifampin, polymyxin, nalidixic acid, linezolid, and some others)44. In addition to this use of inappropriate doses of antibiotics and use at large scale in field conditions endangers to the risk of antibiotic resistance.
Mycoplasmas have devised a number of resistance mechanisms for combating the antibiotics. Due to lack of cell wall mycoplasma have intrinsic resistance to antibiotic classes beta-lactams and to all antimicrobials which target the cell wall45. Target alterations and efflux mechanisms are novel acquired resistance mechanisms of mycoplasma developed through genetic mutation or transfer of new genes44. Target alterations, in ribosome and in topoisomerase II genes have been found as resistance mechanisms to macrolide and tetracycline and fluoro-quinolone class of antibiotics, respectively44,45. However other mechanisms of antibiotic resistance in mycoplasma need to be explored46.
As there is rise of antibiotic resistance against mycoplasma, ineffectiveness of commonly available and used antibiotics adds to problem hence there is dire need for exploring novel strategies and novel targets for antimycoplasma therapy47,48,49. These include the follow ups for antibiotic sensitivity testing, employing alternativeantibiotics, and alternate therapies. Novel targets are based on proteomics46,50, genomics, enzymes52 or metabolism53, besides there are numerous other novel targets which are under investigation44,47, 48,49 and further exploration can help in identifying specific and effective therapeutic and drug targets in future. All these measures will help in preventing antibiotic resistance in mycoplasma, emergence of resistant species, exploring novel targets, safe and effective therapy of mycoplasma diseases in future.
The present review highlights the situation of emerging antibiotic resistance in Mycoplasma microorganisms, and designing effective and novel drugs / therapeutic targets to counter these pathogens for safeguarding health of animals and humans.
Antimycoplasma therapy
Commonly used therapeutics in antimycoplasma therapy include antibiotics and rarely anti-inflammatory, antipyretic, analgesic and antiallergic agents. The antibiotics used against mycoplasma are usually of class tetracycline, flouroquonolones, macrolides, aminoglycosides, and cephalosporins. Among these oxytetracycline is commonly used followed by enrofloxacin, tylosin5,8,54, streptomycin, sometimes danofloxacin, tiamulin, and azithromycin55,56. In humans, commonly used antibiotics for mycoplasma belong to tetracyclines, fluoroquinolones, and macrolides besides commonly used ones include lincosamides, streptogramines, and ketolides57. The antimycoplasmal activity of triclosan against species of the entity (Mycoplasma) which are both sensitive as well as resistant to fluroquinolones has been well documented58. Spectinomycin therapy has been documented against urethritis caused by Mycoplasma genitalium (macrolide resistant) in human59. A list of antibiotics used against various species of mycoplasma is given in Table 1. Prolonged use of these antibiotics in inappropriate doses in common herds may lead to adverse effects including abortion and teratogenicity (tetracyclines), tooth decay and bone weakness (flouroquonolones), ototoxicity (amino-glycosides) besides predisposing to menace of antibiotic resistance5,8.
Table (1):
Antimycoplasma antibiotics used in various mycoplasma diseases
Name of antibiotic |
Class |
Dose used/ Recommended |
Used against Mycoplasma / mycoplasma disease |
Reference |
|---|---|---|---|---|
Tylosin |
Macrolide |
10 mg/kg/day |
Mycoplasma capricolum capripneumoniae, M. agalactiae, Mycoplasma californicum |
34,60,61,62,63,64,65,66 |
Oxytetracycline |
Tetracycline |
14-15 mg/kg |
Mycoplasma capricolum capripneumoniae, M. agalactiae, Mycoplasma californicum |
34,60,61,62,63,66,67
|
Lincomycin |
Lincosamide |
5 mg/kg IM |
Mycoplasma ovipneumoniae |
34,68 |
Oxytetracycline |
Tetracycline |
20 mg/kg |
Mycoplasma ovipneumoniae |
34,68 |
Spiramycin |
Macrolide |
100 mg or 200 mg/dose |
Mycoplasma gallisepticum |
55 |
Enrofloxacin |
Fluoroquinolone |
15 mg/kg |
Mycoplasma gallisepticum |
69 |
Erythromycin |
Macrolide |
25 mg/kg |
M. agalactiae, |
64,65 |
Gamithromycin |
Macrolide |
6 mg/kg |
mycoplasma bovis |
70 |
Azithromycin |
Macrolide |
500mg/dose |
M. pneumonia |
6 |
Clarithromycin |
Macrolide |
15mg/kg/day |
M. pneumonia |
6 |
Gentamicin |
Aminiglycoside |
Topical application of 0.5 % gentamycin (100 mg/ml) |
Mycoplasma conjunctivae |
41 |
Doxycycline |
Tetracycline |
10 mg/kg/day (oral) |
Mycoplasma haemofelis |
71 |
Enrofloxacin |
fluoroquinolones |
5 mg/kg |
Mycoplasma haemofelis |
71 |
Spiramycin |
Macrolide |
100mg/dose |
Mycoplasma gallisepticum |
55 |
Tulathromycin |
Macrolide |
2.5/mg/kg |
Mycoplasma ovipneumoniae |
72 |
Doxycycline |
Tetracycline |
200mg |
Mycoplasma genitalium |
73 |
Doxycycline |
Tetracycline |
0.03125-16 mg/L |
Mycoplasma gallisepticum |
74 |
Tulathromycin |
Macrolide |
2.5 mg/kg SC as single dose |
Mycoplasma mycoides |
34 |
Florfenicol |
chloramphenicol |
20 mg/kg q48 IM |
Mycoplasma mycoides |
34 |
Tilmicosin
|
Macrolide |
10 mg/kg SC as single dose |
Mycoplasma mycoides
|
34 |
Gamithromycin
|
Macrolide |
6 mg/kg SC as single dose |
Mycoplasma mycoides |
34 |
Danofloxacin |
Fluoroquinolone |
2.5 mg/kg q24h SC |
Mycoplasma mycoides |
34 |
Oxytetracycline |
Tetracycline |
10 mg/kg IM q24 for at least 4 days |
Mycoplasma mycoides |
34 |
Danofloxacin |
Fluoroquinolone |
danofloxacin (6 mg/kg subcutaneously |
Mycoplasma capricolum subsp. capripneumoniae |
61 |
Marbofloxacin
|
Fluoroquinolone |
2 mg/kg BW for 3 days |
Mycoplasma capricolum subsp. capripneumoniae
Mycoplasma haemofelis |
63,75 |
Tilmicosin |
Macrolide |
Mycoplasma capricolum subsp. capripneumoniae |
56,76 |
|
Tylosin |
Macrolide |
Mycoplasma gallisepticum
Mycoplasma synoviae |
77 |
|
Gamithromycin |
Macrolide |
6mg/ kg subcutaneously |
Mycoplasma bovis |
70,78 |
Spectinomycin |
It falls in aminocyclitol class; has close relation with aminoglycosides |
20 mg/kg i/m in three repeated doses. |
Mycoplasma bovis |
79 |
Minocycline |
Tetracycline |
Macrolide resistant Mycoplasma pneumoniae (MRMP) |
80,81,82,83,84 |
|
Tosufloxacin |
Fluroquinolone |
Macrolide resistant Mycoplasma pneumoniae (MRMP) |
81 |
Novel, specific and effective antimycoplasma antibiotics need to be explored for better therapeutic results. In vitro testing54, alternating antibiotics81,84,85, long acting antibiotics86, novel macrolide, tetracyclines, aminoglycosides or quinolones81,84, pleuro-mutilin antibiotics like valnemulin and tiamulin87,88 and advanced antimicrobials89,90 are the future prospects with possible promising results for making antibiotic therapy of mycoplasma safe and effective and combating antibiotic resistance44.
Antimycoplasma antibiotic resistance
Antimycoplasma antibiotic resistance is the current issue of emergence and requires timely intervention for proper resolution. Since decades mycoplasmosis is being treated mainly with antimicrobials with varying degree of success, as vaccination is either lacking or has not been effective49,91. Antimicrobials like tetracyclines, marcolides,aminoglycosides, fluoroquinolones, and cephalosporins are common agents used in animals and humans against mycoplasmosis34,57,92,93,94. Other antibiotics used are lincosamides, streptogramines, and ketolides57. Since mycoplasmas are among the fastest evolving bacteria, with high mutation rates hence resistance to antibiotics has developed44. Also due to indiscriminate use of antimicrobials that too in inappropriate doses drug resistance has developed91,95. It has been also observed that in Mycoplamas there is development of intrinsic resistance to b-lactams and other antimicrobials targeting the bacterial cell wall96.
It is interesting to note that the antibiotics to which the entities are usually sensitive may prove to be ineffective due to development of resistance97. Recently a number of studies have been carried out for evaluating antibiotic sensitivity of mycoplasma species against antimycoplasma antibiotics98,99,100,101,102. The antibiotics under focus included enro-floxacin, marbofloxacin, melittin, gramicidin D, spiramycin, tulathromycin, erythromycin , tylosin, florfenicol, oxytetra-cycline, doxycycline, tilmicosin, lincomycin, tiamulin, gamithromycin, tildipirosin and valnemulin98,99,100,101,102. These studies predict decrease of sensitivity and rise of resistance to antibiotics. Increased resistance of Mycoplasma bovis to antibiotics like spectinomycin and tilmicosin is also quite noteworthy103.
Antibiotic resistance in microbes is an emergent issue, which poses a serious concern throughout the world3,104,105. In the initial stages of discovery, most pathogenic organisms were susceptible to antibiotics and hence were cured successfully but with the passage of time resistance developed and effectiveness of antibiotics decreased106. In the present times commonly tetracyclines, macrolides, flouro-quinolones and aminoglycosides are being used against mycoplasma. Generally, marcolides such as tylosin is considered as the drug of choice and is extensively used in practice for the treatment of caprine, bovine and avian mycoplasmosis. Enrofloxacin was considered as the most efficacious among the antimicrobial agents against mycoplasma infection93,107,108. In many European countries, antimicrobial resistance of Mycoplasma spp. has been reported against tylosin, oxytetracycline and spectinomycin109. Resistance to tetracyclines, quinolones, and macrolides in mycoplasma develops in a similar manner as observed in other bacteria. However in some mycoplasmas such mechanisms are yet to be known57 indicating unexplored mechanisms of resistance or adaptations in mycoplasma44. Resistance to oxytetracycline is suspected in Mccp5,8.
Of the various antimicrobials used against mycoplasma, tetracyclines are the most commonly and widely in humans and farm animals57,110,111. Their mechanisms of action involves inhibition of protein synthesis by affecting 30S subunit of ribosome through blocking the attachment of charged aminoacyl-tRNA to the A site on the 30S subunit ribosome112. Thus, it prevents introduction of new amino acids to the nascent peptide chain113. Because of prolonged and continuous use of tetracyclines against microbial infections the problem of antibiotic resistance has developed. The different mechanisms involved in resistance are active cellular efflux, expression of protective proteins, antibiotic degradation with enzymes, interfering drug entry, and target modification112,114. These are considered to be the main mechanisms of tetracyclines resistance in classic bacteria. The development of tetracycline resistance in mycoplasmas in some cases is associated with the acquisition of tet(M) determinants located at the Tn916 transposon115. The transposon encodes the TetM protein, protecting ribosomes from the effects of tetracyclines. This protein is homologous to the eF-Tu and eF-G elongation factors. It can cause conformational changes in the 30S ribosomal subunit, preventing it from binding to tetracyclines. TetM determinant when present is found to be associated with a greater degree of tetracycline resistance. This is actually responsible for mycoplasmal cross resistance to other members of tetracycline group of antibiotics44,57. Also, resistance mechanisms in mycoplasma are also believed to be due to mutations in the tetracycline-binding unit of 16S rRNA116,117. Preliminary studies suggest that Mccp may have developed resistance to oxytetracycle5,8.
Macrolides are recommended as drug of choice against mycoplasma and are also being applied in few areas. The mechanism of action of macrolides is by inhibition of bacterial protein biosynthesis, by preventing peptidyltransferase from adding the growing peptide attached to tRNA to the next amino acid118. Resistance to macrolides is noted in both classical bacteria and mycoplasma (e.g. Mycoplasma pneumoniae)119, 120,121. Target modification, alteration in drug efflux, and degradation of antibiotics by enzyme are the three mechanisms of macrolide resistance122,123. Development of resistance to macrolides in mycoplasma is on rise and is linked to inhibition of efflux of antibiotic and changes in structure of 50S ribosomal sub unit57. The alteration in the central loop of domain V of 23S rRNA can also cause resistance in mycoplasmas57. Mutation can also lead to resistance to macrolides in mycoplasma44. It has been observed that due to point mutations at various sites in the 23S rRNA domain V there may be development of resistance to macrolides124,125, 126,127. It is to be noted that resistance of greater intensity to macrolides (14- as well as 15-membered) is associated with mutation A2058G128. In vivo mutation has been reported in certain instances in the L4 as well as L22 proteins (ribosomal)12,120,129. Rise of new mutants can also infer resistance98.
Tylosin is considered as the drug of choice and is widely used in practice for the treatment of caprine, bovine and avian mycoplasmosis130. But 131 Shahet al. has reported that tylosin showed resistance in clinical cases of CCPP and attributed it to indiscriminate use of the antimicrobial drug.
Fluoroquinolones though constitute the most popular group of drugs in human medicine against mycoplasma57,132,133 but are occasionally used in animal mycoplasma diseases134,135. These antimicrobials act by interfering DNA synthesis targeting DNA gyrase and topoisomerase IV136. The resistance mechanisms involve either alterations in the drug target or alterations in permeability135, 134,136,137. Quinolone-modifying or degrading enzymes have not been identified yet for resistance mechanism in bacteria but in some fungi138. There may be development of mutations in the genes coding for particular enzyme significantly and that depends on the antibiotic. For instance resistance to ofloxacin, trova-floxacin and ciprofloxacin has been observed in Mycoplasma hominis. This is due to topo-isomerase IV gene mutation. Due to mutation of the gene coding for DNA gyrase, sparfloxacin resistance is observed44,139.
Main mechanisms of fluoroquinolone resistance in bacteria include target modifications due to mutations in the quinolone resistance-determining region (QRDR) of the target genes gyrA (DNA gyrase subunit A), gyrB (DNA gyrase subunit B), parC (topoisomerase IVsubunit A), parE (topoisomerase IV subunit B), reduced drug assimilation by inhibiting influx and increasing efflux and acquiring resistance through genes140. In case of mycoplasmas, development of drug resistance is most probably associated with mutations in the QRDR region of the target genes (DNA gyrase and topoisomerase IV)57. Fluoroquinolones show cross-resistance among members of this group which is related to mutations and their location57,137,141.
Aminoglycosides are the other mainly used antibiotic class against mycoplasma. They inhibit protein synthesis by interfering with 30S subunit of ribosome and contain an amino-modified glycoside (sugar)142. Due to prolonged and large scale use, emergence of resistant strains decreased the therapeutic potential of aminoglycosides143. Resistance mechanisms include alteration of the ribosomal binding sites in case of streptomycin, reduced uptake of drug and expression of drug inactivating enzymes144, 145,146.
In case of mycoplasma species, mutational resistance has been recorded to aminoglycosides147. In case of Gram-negative and Gram-positive bacteria three types of antibiotic modifying enzymes are found viz., phospho-transferases, acetyltransferases and nucleo-transferases148 but none of these enzymes have been revealed in mycoplasma. Experimentally, one transposon encoding for gentamicin resistance was introduced by transformation into mycoplasma strains, which expressed them149,150.
The other resistance mechanisms to these classes of drugs which have been noted in other classical microbes are yet to be deciphered in mycoplasma. The resistance mechanisms to other occasionally used antibiotics are also under investigation. Further resistance mechanisms in various species and subspecies of mycoplasma causing severe diseases in humans and animals are also not known. Proper evaluation of antibiotic resistance mechanisms in mycoplasma will help in devising better future therapeutics.
Combating antibiotic resistance
The regulation of antimycoplasma antibiotic therapy is an important aspect of prevention of resistance to antibiotics besides making antibiotic therapy effective. Antibiotic usage needs to be routinely checked in livestock rearing areas. Farmers should be made aware about disadvantages and side effects of prolonged usage of single type of antibiotics in animals. Proper dose of antibiotic is essential as subnormal doses can lead to ineffectiveness and hence resistance. Alternating two or more antibiotics periodically can help in preventing antibiotic resistance. Though novel, specific and effective antimycoplasma antibiotics are need of the hour but better is to develop prophylactic measures as need for the use of antibiotics should not arise. Better managemental practices along with prophylactics can help in preventing mycoplasma diseases and hence limiting use of antibiotics.
Antibiotic sensitivity testing (AST) is one of the most commonly used and convenient method for evaluating sensitivity of mycoplasma to antibiotics99,100. It can predict decrease of sensitivity and rise of resistance99. Regulation of antibiotic application in mycoplasmosis based on AST will be helpful in preventing antibiotic resistance. The antibiotics showing better sensitivity can be employed in mycoplasmosis. Recently a number of studies have been carried out for evaluating antibiotic sensitivity of mycoplasma species against antimycoplasma antibiotics99,100,101,102. The antibiotics under focus included enrofloxacin, marbofloxacin, spiramycin, tulathromycin, erythromycin, tylosin, florfenicol, oxytetracycline, doxycycline, tilmicosin, lincomycin, tiamulin, gamithromycin, tildipirosin and valnemulin. These studies predict decrease of sensitivity and rise of resistance to antibiotics. Novel methods of AST are being employed for sensitivity testing151.
In vitro testing will help in determining sensitivity and standardizing appropriate dose, long acting antibiotics will help in minimizing frequent use of antibiotics, novel members of commonly used classes of antibiotics, and advanced antimicrobials like CRISPER designed antibiotics will be resistance free. Antibiotic alternatives are also being evaluated for effective therapy and prevention of rise of antibiotic resistance152. The use of antibiotic alternatives along with immunomodulators (systemic) viz., corticosteroids or immunoglobulins intra-venously are found to be efficacious to treat infections due to macrolide-resistant Mycoplasma pneumoniae (MRMP)153,154,155. Identifying the novel drug targets for designing effective anti-mycoplasma antibiotics has future prospects for exploration.
Novel targets for antimycoplasma therapy
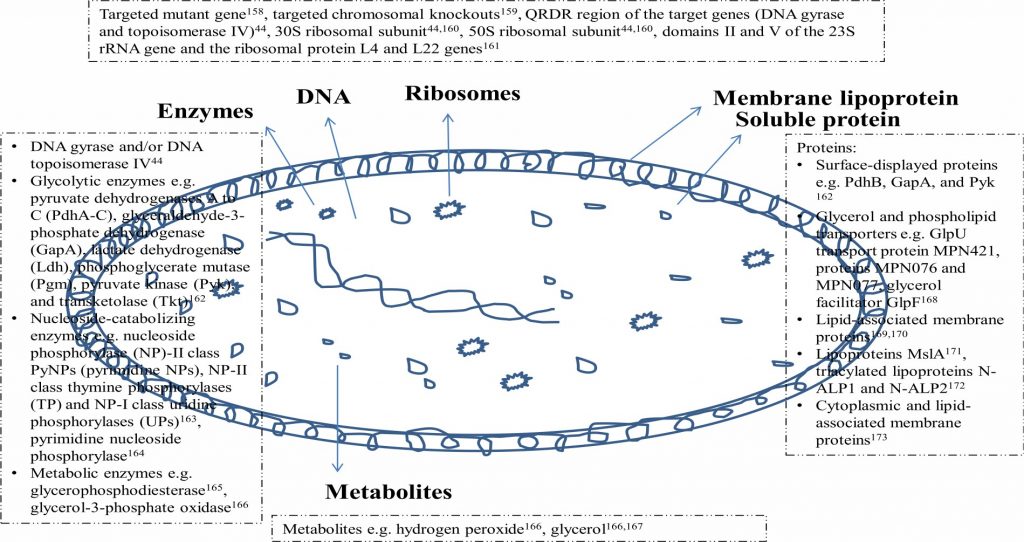
The rise of resistance against commonly available and used antimycoplasma antibiotics ensues exploration of novel targets of mycoplasma for therapeutic purposes. This can also be helpful in designing effective prophylactic measures. As the mycoplasma consists of cell membranes and genetic material hence molecular structures of these organelles can be targeted besides the mechanisms in which these are involved like adhesion, invasion, pathogenesis, transcription and translation (DNA and protein synthesis)48,49. Capsular lipopoly-saccharides (LPS) of mycoplasma have been investigated156. The main components of mycoplasma cell membranes are lipoproteins and immediate targets can be lipids or proteins of membrane157. Proper exploration of biological membranes of mycoplasma for various molecular components can enable better evaluation of molecular targets. Such studies are lacking. Other possible targets are enzymes, proteins, metabolites, metabolic or cycle pathways47,48,49. Besides therapeutics based on these various targets,having different mechanisms of action can also be helpful in preventing antibiotic resistance as mutations of molecular targets can be overpowered. Exploration of novel antigenic structures can be exploited for raising antibodies or serum for therapeutic use or developing vaccines for prophylactic use. For all these novel approaches, exploration of molecular structures of mycoplasma is must. Common structures of mycoplasma include DNA, ribosome, membrane lipoproteins, soluble proteins, enzymes and metabolites. Fig. 1 depicts the common structural components of mycoplasma cell. Among these plasma membrane157, proteins174, toxic molecules, metabolic pathways, and attachment and motility49, peptidoglycan synthesis or ribosomal activity48, glycolipids175, genomics, proteomics, and metabolic pathways47are being evaluated as novel targets of antimycoplasma therapy.
Rise of antimycoplasma antibiotic resistance and continuous use of commonly available antibiotics which are usually in effective against mycoplasma further aggravates resistance situation. Hence exploration of novel targets for antimycoplasma antibiotics is essential47,48,49. These novel targets are being investigated and are usually based on protein46,50, gene47,51, enzymes52or metabolism53. In addition a number of novel targets are being elucidated44,47,48,49 and future research will help in identifying specific and effective therapeutic targets in future.
Targetable metabolic pathways include ABC transport pathway, phosphotransferase system, secretary pathway system, fatty acid and glycerophospholipid metabolism, pentose phosphate pathway, glycolysis, carbohydrate metabolism, amino acid metabolism, terpenoid backbone biosynthesis, carotenoid biosynthesis, purine and pyrimidine metabolism44,47. Targetable genetic pathways include recombination and reparation, replication, transcription, trans-lation, RNA degradation, chaperons and folding44,47.
Some of the targetable proteins and enzymes are 50S ribosomal protein L10, phenylalanyl-trnasynthetase subunit beta, cytidylate kinase, 50S ribosomal protein L32, putative nicotinate-nucleotide adenylyl transferase, DNA polymerase III subunit beta, DNA polymerase I, thymidylate kinase, DNA-directed RNA polymerase subunit alpha, fructose-bisphosphatealdolase, putative 2, 3-bisphos-phoglycerate-independent phospho-glycerate-mutase, ribose-5-phosphate isomerase, ATP synthase subunit C, acetate kinase, purine-nucleoside phosphorylase, and putative nucleoside phosphorylase44,47.
Some of the novel therapeutics approved and/or under experimental trials that target these pathways, enzymes or proteins have been highlighted previously. They target glycolysis/gluconeogenesis, pentose phosphate pathway, fructose and mannose metabolism (phosphogly-colohydroxamic acid), cysteine and methionine metabolism, biosynthesis of amino acids (adenine), pyrimidine metabolism (P1-(52 -AdeNosyl)P5-(52 -Thymidyl) Pentaphosphate), nicotinateand nicotinamide metabolism (deamido-nad+, citric acid), pyrimidine metabolism (cytosine arabinose-5′-Phosphate, 2′,3′-Dideoxycytidine-5′-Monophosphate Cytidine-5′-Monophosphate, Cytidine-5′-diphosphate), purine metabolism, RNA polymerase (rifabutin, Myxopyronin B, Methyl carbamate), oxidative phosphorylation (N-Formylmethionine, Nonan-1-Ol), biosynthesis of secondary metabolites (guanosine, cyclouridine, cysteinesulfonic acid, 4-phospho-D-erythronate), glycolysis/gluconeogenesis, glycine, serine and threonine metabolism, methane metabolism (2-Phosphoglyceric Acid, 3-Phosphoglyceric Acid, formic acid), pyruvate metabolism, propanoate metabolism, pentose phosphate pathway, nucleotide excision repair, homologous recombination (aAzelaic Acid, B-2-octylglucoside), aminoacyl-tRNA biosynthesis(1-{3-[(4-pyridin-2-ylpiperazin-1-14yl) sulfonyl]phenyl}-3-(1,3-thiazol-2-yl)urea), DNA replication, mismatch repair, homologous recombination [(5R)-5-(2,3-dibromo-5-ethoxy-4-hydroxybenzyl)-4-oxo-2-thioxo-1,3-thiazolidin-3-yl]acetic acid)], ribosome (roxithromycin, clindamycin, clarithromycin, quinupristin, lincomycin, troleandomycin)44,47.
Recently glycolytic enzymes e.g. pyruvate dehydrogenases A to C (PdhA-C), glyceraldehyde-3-phosphate dehydrogenase (GapA), lactate dehydrogenase (Ldh), phosphoglyceratemutase (Pgm), pyruvate kinase (Pyk), and transketolase (Tkt)162, nucleoside-catabolizing enzymes e.g. nucleoside phosphorylase (NP)-II class PyNPs (pyrimidine NPs), NP-II class thymine phosphorylases (TP) and NP-I class uridinephosphorylases (UPs)163, pyrimidine nucleoside phosphorylase164, metabolic enzymes e.g. glycerophospho-diesterase165, glycerol-3-phosphate oxidase166, metabolites e.g. hydrogen peroxide166, glycerol166,167, surface-displayed proteins e.g. PdhB, GapA, and Pyk162, glycerol and phospholipid transporters e.g. GlpU transport protein MPN421, proteins MPN076 and MPN077, glycerol facilitator GlpF168, lipid-associated membrane proteins169,170,173, lipoproteins MslA171, triacylated lipoproteins N-ALP1 and N-ALP2172, and cytoplasmic proteins173, targeted mutant gene158, targeted chromosomal knockouts159, QRDR region of the target genes (DNA gyrase and topoisomerase IV)44, components of 30S ribosomal subunit44 and 50S ribosomal subunit44,160, domains II and V of the 23S rRNA gene and the ribosomal protein L4 and L22 genes173of mycoplasma have been explored for therapeutic and/or prophylactic possibilities.
It has also been observed that for the purpose of designing therapeutics in order to treat infections caused by Mycoplasma pneumonia, community-acquired respiratory distress syndrome (CARDS) toxin acts as a target (promising) as well as a significant candidate. The NLRP3 inflammasome is activated by the toxin intracellularly which leads to vigorous inflammation in association with infection due to M. pneumoniae49,176. CARDS is a significant virulent factor generating antibody titers at high level177,178,179.
Novel drugs/therapies against mycoplasma infections
Considering the rise of resistance against main antimycoplasma antibiotics like tetracyclines, macrolides or fluoroquinolones, novel drugs or therapies are being explored. Josamycin, pristinamycin, marbofloxacin, solithromycin, lefamulin, sitafloxacin, zoliflodacin, tigecycline, spectinomycin and moxifloxacin are newer antimycoplasma antibiotics that have shown promising results in antimycoplasma therapy181,182. Herbal medicines182,183, traditional medicine184, or their combination185 have been elucidated for mycoplasma infections. Novel technologies for safeguarding health of humans and animals are being evaluated186. Phage therapy187,188 has proven effective in some antimycoplasma therapies. Immunotherapy189, cytokine therapy190,191 and immunomodulation192 have potent biomedical applications and are being applied in mycoplasma infections also189,190. Immuno-modulators which help in boosting immunity193 can be helpful in mycoplasma therapy. Nano therapeutics194 involving nanomedicines195 are advanced therapeutics in biomedicine and pharmacotherapy and can help in effective targeting and accurate drug delivery196. These novel and emerging therapies can overcome resistance problems in mycoplasma infections. Still many aspects of these drugs or therapies are yet to be explored however they hold a great promise for future of antimycoplasma therapies.
Antibiotics are frequently being used as antimicrobial agents against mycoplasma and as first line of treatment for the therapeutic management of mycoplasmoses infection. Commonly macrolides, tetracyclines, fluoro-quinolones, and aminoglycosides are the main classes of antibiotics being employed worldover. Among these, oxytetracycline has been the most commonly used antibiotic for a prolonged period followed by enrofloxacin, tylosin, streptomycin and rarely danofloxacin, lincomycin, spiramycin, erythromycin, gamithromycin, azithromycin, clarithromycin, gentamicin, doxycycline, tulathromycin have been employed. Due to emergence of drug resistant strains of Myco-plasmathe effectiveness of these antibiotics isdramaticallyreduced. Frequent prolonged usage of single type of antibiotics and inappropriate doses can lead to menace of antibiotic resistance. Different microbial mechanisms have been developed by mycoplasma for resistance to antibiotics. This usually include acquisition of proteins affecting ribosomal subunits, inhibition of antibiotic efflux, structural changes in the ribosomal subunit, target mutations, expression or production of enzymes. Still the exploration of several novel mechanisms to counter Mycoplasma infections isunder evaluation. Besides alternating anti-biotics, antimycoplasma antibiotic sensitivity evaluation should be undertaken on routine basis for checking the emerging drug resistance. Evaluation of specific doses and novel mycoplasma specific class of antibiotics need to be explored. Exploring novel therapeutic and drug targets in mycoplasma for effective antibiotic therapy is the recent area of research with promising results.
The rise of antibiotic resistance and ineffectiveness of conventional antibiotic therapy in mycoplasmosis has led to devising strategies and exploring possibilities for combating antimycoplasma resistance and treating mycoplasmosis effectively. It cannot be denied that changes in genomic, transcriptomic, secretomic as well as proteomic profiles of the microbes (including Mycoplasma) have influenced the phenomenon of antimicrobial resistance significantly. Regulating antibiotic use, alternating therapies along with appropriate doses of antibiotics, antibiotic sensitivity testing and exploring novel antimycoplasma antibiotics could solve the problem. Novel therapeutic targets are being explored in mycoplasma for antibiotic action which can help in safe and effective treatment of mycoplasmosis. These targets may be toxins, toxic metabolites, enzymes, proteins, metabolic pathways, metabolites, cell division, nucleotides, or cellular structural parts of mycoplasma. However to be successful, these measures need to be explored after proper experimental evaluation followed by application in clinical cases. Future of antibiotic therapy in mycoplasmosis relies solely on devising safe and effective antibiotics with mycoplasma specific targets or mechanism of action and less prone to development of resistance.
The financial support by Science and Engineering Research Board, Department of Science and Technology, Government of India to the first author through SERB-DST Project (EMR/2016/004929) for this study is highly acknowledged.
The authors declare that there is no conflict of interest for the publication of this article.
- Waites, K.B., Talkington, D.F. Mycoplasma pneumoniae and its role as a human pathogen. Clinical microbiology reviews, 2004; 17(4): 697-728.
- Kumar, P., Roy, A., Bhanderi, B.B., Pal, B.C. Isolation, identification and molecular characterization of Mycoplasma isolates from goats of Gujarat State, India. Veterinarski Arhiv, 2011; 81(4): 443-458.
- Tiwari, R., Chakraborty, S., Dhama, K., Rajagunalan, S., Singh, S.V. Antibiotic resistance-an emerging health problem: Causes, worries, challenges and solutions: A review. International Journal of Current Research, 2013; 5(7): 1880-1892.
- Yatoo, M.I., Parray, O.R., Mir, M.S., Qureshi, S., Amin, Z., Kashoo, M.N., Fazili, M.U.R., Tufani, N.A., Singh, M., Kanwar, S.C., Dhama, K. Mycoplasmosis in small ruminants in India: a review. Journal of Experimental Biology, 2018; 6: 2.
- Yatoo, M.I, Parray, O.R, Bashir, S.T, Muheet, Bhat, R.A., Gopalakrishnan, A., Karthik, K., Dhama, K., Singh, S.V. Contagious caprine pleuropneumonia – A Comprehensive review. Veterinary Quarterly. In Press, 2019.
- Kashyap, S., Sarkar, M. Mycoplasma pneumonia: clinical features and management. Lung India: Official Organ of Indian Chest Society, 2010; 27(2): 75.
- Chakraborty, S., Kumar, A., Tiwari, R., Rahal, A., Malik, Y., Dhama, K., Pal, A., Prasad, M. Advances in diagnosis of respiratory diseases of small ruminants. Veterinary medicine international, 2014; 2014: 508304.
- Parray, O.R., Yatoo, M.I., Bhat, R.A., Malik, H.U., Bashir, S.T., Magray, S.N. Seroepidemiology and risk factor analysis of contagious caprine pleuropneumonia in Himalayan Pashmina Goats. Small Ruminant Research, 2019; 171: 23-36.
- Ho, P.L., Law, P.Y., Chan, B.W., Wong, C.W., To, K.K., Chiu, S.S., Cheng, V.C., Yam, W.C. Emergence of macrolide-resistant Mycoplasma pneumoniae in Hong Kong is linked to increasing macrolide resistance in multilocus variable-number tandem-repeat analysis type 4-5-7-2. Journal of clinical microbiology, 2015; 53(11): 3560-3564.
- Phuong, N.T., Hoang, T.T., Van, P.H., Tu, L., Graham, S.M., Marais, B.J. Encouraging rational antibiotic use in childhood pneumonia: a focus on Vietnam and the Western Pacific Region. Pneumonia, 2017; 9(1): 7.
- Matsuda, K., Narita, M., Sera, N., Maeda, E., Yoshitomi, H., Ohya, H., Araki, Y., Kakuma, T., Fukuoh, A., Matsumoto, K. Gene and cytokine profile analysis of macrolide-resistant Mycoplasma pneumoniae infection in Fukuoka, Japan. BMC infectious diseases, 2013; 13(1): 591.
- Pereyre, S., Goret, J., Bיbיar, C. Mycoplasma pneumoniae: current knowledge on macrolide resistance and treatment. Frontiers in microbiology, 2016; 7: 974.
- Waites, K.B., Katz, B., Schelonka, R.L. Mycoplasmas and ureaplasmas as neonatal pathogens. Clinical microbiology reviews, 2005; 18(4): 757-789.
- Fair, R.J., Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspectives in medicinal chemistry, 2014; 6: PMC-S14459.
- Llor, C., Bjerrum, L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Therapeutic advances in drug safety, 2014; 5(6): 229-241.
- Ventola, C.L. The antibiotic resistance crisis: part 1: causes and threats. Pharmacy and therapeutics, 2015; 40(4): 277.
- Thiaucourt, F., Boelske, G. Contagious caprine pleuropneumonia and other pulmonary mycoplasmoses of sheep and goats [Mycoides cluster, Mycoplasma capricolum subsp. capripneumoniae, Mycoplasma agalactiae, Mycoplasma putrefasciens]. Revue Scientifique et Technique, 1996; 15(4):1397-1414.
- Razin, S., Yogev, D., Naot, Y. Molecular biology and pathogenicity of mycoplasmas. Microbiology and Molecular Biology Reviews, 1998; 62(4): 1094-1156.
- DaMassa, A.J., Wakenell, P.S., Brooks, D.L. Mycoplasmas of goats and sheep. Journal of Veterinary Diagnostic Investigation, 1992; 4: 101-113.
- Nicholas, R.A.J. Improvements in the diagnosis and control of diseases of small ruminants caused by mycoplasmas. Small Ruminant Research, 2002; 45(2): 145-149.
- Nicholas, R., Ayling, R., McAuliffe, L. Mycoplasma diseases of ruminants, Disease, Diagnosis and Control, 2009; pp 256.
- Rosengarten, R., Citti, C., Glew, M., Lischewski, A., Droe e, M., Much, P., Winner, F., Brank, M., Spergser, J. Host-pathogen interactions in mycoplasma pathogenesis: virulence and survival strategies of minimalist prokaryotes. International journal of medical microbiology, 2000; 290(1): 15-25.
- McAuliffe, L., Ellis, R.J., Ayling, R.D., Nicholas, R.A. Differentiation of Mycoplasma species by 16S ribosomal DNA PCR and denaturing gradient gel electrophoresis fingerprinting. Journal of Clinical Microbiology, 2003; 41(10): 4844-4847.
- Harwick, H.J., Kalmanson, G.M., Guze, L.B. Human diseases associated with mycoplasmas—with an appendix on simple culture techniques. California medicine, 1972; 116(5): 1.
- Embree, J.E., Embil, J.A. Mycoplasmas in diseases of humans. Canadian Medical Association Journal, 1980; 123(2): 105.
- Kumar, A., Rahal, A., Chakraborty, S., Verma, A.K., Dhama, K. Mycoplasma agalactiae, an etiological agent of contagious agalactia in small ruminants: a review. Veterinary medicine international, 2014; 2014: e286752.
- Gourlay, R.N. Mycoplasma-induced arthritis in farm animals. Israel journal of medical sciences, 1981; 17(7): 626-627.
- Gonzבlez, R.N., Wilson, D.J. Mycoplasmal mastitis in dairy herds. Veterinary Clinics: Food Animal Practice, 2003; 19(1): 199-221.
- OIE. Avian Mycoplasmosis (Mycoplasma gallisepticum and M. synoviae). OIE Terrestrial Manual, 2008; pp 1-16.
- Maunsell, F.P., Woolums, A.R., Francoz, D., Rosenbusch, R.F., Step, D.L., Wilson, D.J., Janzen, E.D. Mycoplasma bovis infections in cattle. Journal of Veterinary Internal Medicine, 2011; 25(4): 772-783.
- Nicholas, R., Baker, S., Ayling, R., Stipkovits, L. Mycoplasma infections in growing cattle. Cattle Practice, 2000; 8(2): 115-118.
- Ruffin, D.C. Mycoplasma infections in small ruminants. Veterinary Clinics of North America: Food Animal Practice, 2001; 17(2): 315-32.
- Sharif, A., Muhammad, G. Mastitis control in dairy animals. Pakistan Veterinary Journal, 2009; 29(3): 145-148.
- Constable, P.D., Hinchcliff, K.W., Done, S.H., Gruenberg, W. Diseases of the Ovine and Caprine Respiratory Tract. A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs, and Goats. Elsevier, Riverport Lane Ed.11th, St. Louis, Missouri, USA, 2017; pp 969-973.
- Nicholas, R., Ayling, R., McAuliffe, L. Mycoplasma diseases of ruminants. CABI, 2008.
- OIE. OIE-listed Diseases, Infections and Infestations in Force in 2017. Office International Des Epizootics, World Organization for Animal Health, Paris, France, 2017.
- Waites, K.B., Schelonka, R.L., Xiao, L., Grigsby, P.L., Novy, M.J. August. Congenital and opportunistic infections: Ureaplasma spp and Mycoplasma hominis. Seminars in Fetal and Neonatal Medicine, 2009; 14(4): 190-199.
- Lis, R., Rowhani-Rahbar, A., Manhart, L.E. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clinical Infectious Diseases, 2015; 61(3): 418-426.
- Prince, O.A., Krunkosky, T.M., Sheppard, E.S., Krause, D.C. Modelling persistent Mycoplasma pneumoniae infection of human airway epithelium. Cellular microbiology, 2018; 20(3): e12810.
- Sadique, U., Chaudhry, Z.I., Rana, M.Y., Anjum, A.A., Sajid, A., Mushtaq, M. Pathogenesis and Immunohistochemical Studies of Caprine Pleuropneumonia in Experimentally Infected Goats. Pakistan Veterinary Journal, 2012; 32(3): 427–731.
- Shahzad, W., Munir, R., Rana, M.Y., Ahmad, R., Khan, M.S., Akbar, G., Ijaz, M., Mehmood, F. Prevalence, molecular diagnosis and treatment of Mycoplasma conjunctivae isolated from infectious keratoconjunctivitis affected Lohi sheep maintained at Livestock Experiment Station, Bahadurnagar, Okara, Pakistan. Tropical animal health and production, 2013; 45(3): 737-742.
- Zekarias, B., Roger, F., Yigezu, L.M. Effects of antibiotic treatments against contagious caprine pleuropneumonia. In: 10th International conference of the Association of institutions for Tropical veterinary medicine, “Livestock, community and environment”, Copenhagen, Denmark, 2001.
- Abraham, S.S., Asha, T.T., Julie, B., Prathiush, P.R., Nandakumar, S., Prasad, P.M. Pathological and molecular characterization of contagious caprine pleuropneumonia (CCPP) outbreak in Kerala. Indian Journal of Veterinary Pathology, 2015; 39(2): 121-124.
- Chernova, O.A., Medvedeva, E.S., Mouzykantov, A.A., Baranova, N.B., Chernov, V.M. Mycoplasmas and their antibiotic resistance: The problems and prospects in controlling infections. Acta Naturae, 2016; 8(2 (29)).
- Bיbיar, C.M., Pereyre, S. Mechanisms of drug resistance in Mycoplasma pneumoniae. Current Drug Targets – Infectious Disorders, 2005; 5(3): 263-71.
- Chernov, V.M., Chernova, O.A., Baranova, N.B., Gorshkov, O.V., Medvedeva, E.S., Sha-mardanova, G.F. The adaptation of mycoplasmas to stress conditions: features of proteome shift in Mycoplasma hominis PG37 under starvation and low temperature. Molekuliarnaia biologiia, 2011; 45(5): 914-923.
- Parvege, M.M., Rahman, M., Hossain, M.S. Genome-wide Analysis of Mycoplasma hominis for the Identification of Putative Therapeutic Targets. Drug target insights, 2014; 8: DTI-S19728.
- Maddocks, S.E. Novel Targets of Antimicrobial Therapies. Microbiology spectrum, 2016; 4(2): VMBF-0018-2015.
- Balish, M.F., Distelhorst, S.L. Potential molecular targets for narrow-spectrum agents to combat Mycoplasma pneumoniae infection and disease. Frontiers in microbiology, 2016; 7: 205.
- Reolon, L.A., Martello, C.L., Schrank, I.S., Ferreira, H.B. Survey of surface proteins from the pathogenic Mycoplasma hyopneumoniae strain 7448 using a biotin cell surface labeling approach. PloS one, 2014; 9(11): e112596.
- Masukagami, Y., Tivendale, K.A., Mardani, K., Ben-Barak, I., Markham, P.F., Browning, G.F. The Mycoplasma gallisepticum virulence factor lipoprotein MslA is a novel polynucleotide binding protein. Infection and immunity, 2013; 81(9): 3220-3226.
- Elkhal, C.K., Kean, K.M., Parsonage, D., Maenpuen, S., Chaiyen, P., Claiborne, A., Karplus, P.A. Structure and proposed mechanism of l ב glycerophosphate oxidase from Mycoplasma pneumoniae. The FEBS journal, 2015; 282(16): 3030-3042.
- Sippel, K.H., Venkatakrishnan, B., Boehlein, S.K., Sankaran, B., Quirit, J.G., Govindasamy, L., Agbandje McKenna, M., Goodison, S., Rosser, C.J., McKenna, R. Insights into Mycoplasma genitalium metabolism revealed by the structure of MG289, an extracytoplasmic thiamine binding lipoprotein. Proteins: Structure, Function, and Bioinformatics, 2011; 79(2): 528-536.
- Cooper, A.C., Fuller, J.R., Fuller, M.K., Whittlestone, P., Wise, D.R. In vitro activity of danofloxacin, tylosin and oxytetracycline against mycoplasmas of veterinary importance. Research in veterinary science, 1993; 54(3): 329-334.
- Arzey, G.G., Arzey, K.E. Successful treatment of mycoplasmosis in layer chickens with single dose therapy. Australian veterinary journal, 1992; 69(6): 126-128.
- Ayling, R.D., Baker, S.E., Nicholas, R.A., Peek, M.L., Simon, A.J. Comparison of in vitro activity of danofloxacin, florlenicol, oxytetracycline, spectinomycin and tilmicosin against Mycoplasma mycoides subspecies mycoides small colony type. Veterinary Record, 2000; 146(9): 243-246.
- Waites, K.B., Lysnyansky, I., Bיbיar, C.M. Emerging antimicrobial resistance in mycoplasmas of humans and animals. In Mollicutes: molecular biology and pathogenesis. Caister Academic Press, Norfolk, 2014; pp 289-322.
- Li, L., Shen, W., Zhang, K., Tang, X., Guo, N., Shen, F., Xing, M., Liua, L., Yuan, P., Shi, Q., Liang, J. In-vitro Antimycoplasmal Activity of Triclosan in Combination with Fluoroquinolones against Five mycoplasma Species. Iranian journal of pharma-ceutical research: IJPR, 2012; 11(4): 1111.
- Falk, L., Jensen, J.S. Successful outcome of macrolide-resistant Mycoplasma genitalium urethritis after spectinomycin treatment: a case report. Journal of Antimicrobial Chemotherapy, 2016; 72(2): 624-625.
- Kahn, C.M. Respiratory diseases of sheep and goats. The Merck Veterinary Manual. Merck Co., Inc. Whitehouse Station, N.J., U.S.A. (Ed.10th), 2010; pp 1357-1363.
- Ozdemir, U., Loria, G.R., Godinho, K.S., Samson, R., Rowan, T.G., Churchward, C., Ayling, R.D., Nicholas, R.A.J. Effect of danofloxacin (Advocin A180) on goats affected with contagious caprine pleuropneumonia. Tropical animal health and production, 2006; 38(7-8): 533-540.
- ײzdemir, ײ.M., Ergin, H., Yenisey, ַ., T rk, N.×., ×im÷ek, N.G. Protective effects of clarithromycin in rats with hypoxia/reoxygenation-induced intestinal injury. Journal of pediatric surgery, 2010; 45(11): 2169-2174.
- Balikci, E., Kizil, O., Karapinar, T., Karahan, M., Ozdemir, H., Dabak, M. Efficacy of marbofloxacin for naturally occurring contagious caprine pleuro-pneumonia. Small Ruminant Research, 2008; 77(1): 75-79.
- Nicolas, J.A., Chauchef, S., Parbelle, M., Ferial, M.L. Mycoplasmoses caprines vues par un laboratoire de diagnostic. Revue de medecine veterinaire. Revue de Mיdecine Vיtיrinaire (Toulouse), 1982; 133: 423-426.
- Kinde, H., DaMassa, A.J., Wakenell, P.S., Petty, R. Mycoplasma infection in a commercial goat dairy caused by Mycoplasma agalactiae and Myco-plasma mycoides subsp. mycoides (caprine biotype). Journal of Veterinary Diagnostic Investigation, 1994; 6(4): 423-427.
- Ball, H.J., Logan, E.F., Campbell, J.N. Mycoplasma californicum mastitis in ewes as an experimental model for antibiotic treatment. Epidemiology & Infection, 1987; 98(3): 369-378.
- Nicolet, J. Mycoplasma infections in cattle, sheep and goats: methods for diagnosis and prophylaxis. Mycoplasma infections in cattle, sheep and goats: methods for diagnosis and prophylaxis, 1994; pp 31-66.
- Skoufos, J., Mavrogianni, V.S., Tzora, A., Mavrommatis, I., Alexopoulos, C., Fthenakis, G.C. Use of lincomycin to control respiratory infections in lambs: Effects on health and production. Small ruminant research, 2006; 66(1-3): 214-221.
- Wellehan, J.F., Zens, M.S., Calsamiglia, M., Fusco, P.J., Amonsin, A., Kapur, V. Diagnosis and treatment of conjunctivitis in house finches associated with mycoplasmosis in Minnesota. Journal of wildlife diseases, 2001; 37(2): 245-251.
- Lechtenberg, K., Tessman, R.K., Theodore Chester, S. Efficacy of gamithromycin injectable solution for the treatment of Mycoplasma bovis induced pneumonia in cattle. International Journal of Applied Research in Veterinary Medicine, 2011; 9(3): 233.
- Tasker, S., Helps, C.R., Day, M.J., Harbour, D.A., Gruffydd-Jones, T.J., Lappin, M.R. Use of a Taqman PCR to determine the response of Mycoplasma haemofelis infection to antibiotic treatment. Journal of microbiological methods, 2004; 56(1): 63-71.
- Naccari, V., Giofre, F., Naccari, F. Tulathromycin in the Treatment of Respiratory Infections in Sheep. International Journal of Animal and Veterinary Advances, 2015; 7(2): 34-39.
- Horner, P., Blee, K. and Adams, E. Time to manage Mycoplasma genitalium as an STI: but not with azithromycin 1 g!. Current opinion in infectious diseases, 2014; 27(1): 68-74.
- Zhang, N., Gu, X., Ye, X., Wu, X., Zhang, B., Zhang, L., Shen, X., Jiang, H., Ding, H. The PK/PD interactions of doxycycline against Mycoplasma gallisepticum. Frontiers in microbiology, 2016; 7: 653.
- Ishak, A.M., Dowers, K.L., Cavanaugh, M.T., Powell, C.C., Hawley, J.R., Radecki, S.V., Lappin, M.R. Marbofloxacin for the treatment of experimentally induced Mycoplasma haemofelis infection in cats. Journal of veterinary internal medicine, 2008; 22(2): 288-292.
- Frantzen, S., March, J.B., Godinho, K., Nicholas, R.A.J. Assessing the in vitro effectiveness of antimicrobials against Mycoplasma mycoides subsp. mycoides small-colony type to reduce contagious bovine pleuropneumonia infection. Antimicrobial Agents Chemotherapy, 2005; 49(12): 5162-5165.
- Amer, M.M., Hanafei, A.H.A., El-Bayomi, K.M., Zohair, G.A. Comparative Study on the Efficacy of Some Antimycoplasma Drugs on the Performance of Commercial Broiler Flocks from Infected Breeders. Global Veterinaria, 2009; 3(2): 69-74.
- Baggott, D., Casartelli, A., Fraisse, F., Manavella, C., Marteau, R., Rehbein, S., Wiedemann, M., Yoon, S. Demonstration of the metaphylactic use of gamithromycin against bacterial pathogens associated with bovine respiratory disease in a multicentre farm trial. The Veterinary Record, 2011; 168(9): 241.
- Poumarat, F., Le Grand, D., Philippe, S., Calavas, D., Schelcher, F., Cabaniי, P., Tessier, P., Navetat, H. Efficacy of spectinomycin against Mycoplasma bovis induced pneumonia in conventionally reared calves. Veterinary microbiology, 2001; 80(1): 23-35.
- Okada, T., Morozumi, M., Tajima, T., Hasegawa, M., Sakata, H., Ohnari, S., Chiba, N., Iwata, S., Ubukata, K. Rapid effectiveness of minocycline or doxycycline against macrolide-resistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children. Clinical infectious diseases, 2012; 55(12): 1642-1649.
- Kawai, Y., Miyashita, N., Kubo, M., Akaike, H., Kato, A., Nishizawa, Y., Saito, A., Kondo, E., Teranishi, H., Ogita, S., Tanaka, T. Therapeutic efficacy of macrolides, minocycline, and tosufloxacin against macrolide-resistant Mycoplasma pneumoniae pneumonia in pediatric patients. Antimicrobial agents and chemotherapy, 2013; 57(5): 2252-2258.
- Komatsu, H., Tsunoda, T., Inui, A., Sogo, T., Fujisawa, T. Characteristics of hospitalized children infected with macrolide-resistant Mycoplasma pneumoniae. Brazilian Journal of Infectious Diseases, 2014; 18(3): 294-299.
- Miyashita, N., Akaike, H., Teranishi, H., Ouchi, K., Okimoto, N. Macrolide-resistant Mycoplasma pneumoniae pneumonia in adolescents and adults: clinical findings, drug susceptibility, and therapeutic efficacy. Antimicrobial agents and chemotherapy, 2013; 57(10): 5181-5185.
- Ishiguro, N., Koseki, N., Kaiho, M., Ariga, T., Kikuta, H., Togashi, T., Oba, K., Morita, K., Nagano, N., Nakanishi, M., Hara, K. Therapeutic efficacy of azithromycin, clarithromycin, minocycline and tosufloxacin against macrolide-resistant and macrolide-sensitive Mycoplasma pneumoniae pneumonia in pediatric patients. PloS one, 2017; 12(3): e0173635.
- Lee, H., Yun, K.W., Lee, H.J., Choi, E.H. Antimicrobial therapy of macrolide-resistant Mycoplasma pneumoniae pneumonia in children. Expert review of anti-infective therapy, 2018; 16(1): 23-34.
- Babu, K.S., Kastelik, J., Morjaria, J.B. Role of long term antibiotics in chronic respiratory diseases. Respiratory medicine, 2013; 107(6): 800-815.
- Grףzner, D., Kreizinger, Z., Sulyok, K.M., Rףnai, Z., Hrivnבk, V., Turcsבnyi, I., Jבnosi, S., Gyuranecz, M. Antibiotic susceptibility profiles of Mycoplasma sp. 1220 strains isolated from geese in Hungary. BMC veterinary research, 2016; 12(1): 170.
- Garmyn, A., Vereecken, M., Degussem, K., Depondt, W., Haesebrouck, F., Martel, A. Efficacy of tiamulin alone or in combination with chlortetracycline against experimental Mycoplasma gallisepticum infection in chickens. Poultry science, 2017; 96(9): 3367-3374.
- Beisel, C.L., Gomaa, A.A., Barrangou, R. A CRISPR design for next-generation antimicrobials. Genome biology, 2014; 15(11): 516.
- Greene, A.C. CRISPR-based antibacterials: Transforming bacterial defense into offense. Trends in biotechnology, 2018; 36(2): 127-130.
- Citti, C., Blanchard, A. Mycoplasmas and their host: emerging and re-emerging minimal pathogens. Trends in microbiology, 2013; 21(4): 196-203.
- Ogrendik, M. Antibiotics for the treatment of rheumatoid arthritis. International journal of general medicine, 2014; 7: 43.
- Antunes, N.T., Tavםo, M.M., Assunחדo, P., Rosales, R.S., Aquili, V., De la Fe, C., Poveda, J.B. In vitro susceptibilities of field isolates of Mycoplasma mycoides subsp. mycoides large colony type to 15 antimicrobials. Veterinary microbiology, 2007; 119(1): 72-75.
- Yang, H.J., Song, D.J., Shim, J.Y. Mechanism of resistance acquisition and treatment of macrolide-resistant Mycoplasma pneumoniae pneumonia in children. Korean journal of pediatrics, 2017; 60(6): 167.
- Scott, L.C., Menzies, P.I. Antimicrobial resistance and small ruminant veterinary practice. Veterinary Clinics: Food Animal Practice, 2011; 27(1): 23-32.
- Bיbיar, C., Pereyre, S., Peuchant, O. Mycoplasma pneumoniae: susceptibility and resistance to antibiotics. Future microbiology, 2011; 6(4): 423-431.
- Kenny, G.E., Frank, F.D. Cartwright. “Suscep-tibilities of Mycoplasma hominis, M. pneumoniae, and Ureaplasma urealyticum to GAR-936, dalfopristin, dirithromycin, evernimicin, gati-floxacin, linezolid, moxifloxacin, quinupristin-dalfopristin, and telithromycin compared to their susceptibilities to reference macrolides, tetra-cyclines, and quinolones.” Antimicrobial agents and chemotherapy, 2001; 45, 9(2001): 2604-2608.
- Fehri, L.F., Sirand-Pugnet, P., Gourgues, G., Jan, G., Wrףblewski, H., Blanchard, A. Resistance to antimicrobial peptides and stress response in Mycoplasma pulmonis. Antimicrobial agents and chemotherapy, 2005; 49(10): 4154-4165.
- Gautier-Bouchardon, A.V., Ferrי, S., Le Grand, D., Paoli, A., Gay, E., Poumarat, F. Overall decrease in the susceptibility of Mycoplasma bovis to antimicrobials over the past 30 years in France. PLoS One, 2014; 9(2): e87672.
- Sulyok, K.M., Kreizinger, Z., Fekete, L., Hrivnבk, V., Magyar, T., Jבnosi, S., Schweitzer, N., Turcsבnyi, I., Makrai, L., Erdיlyi, K., Gyuranecz, M. Antibiotic susceptibility profiles of Mycoplasma bovis strains isolated from cattle in Hungary, Central Europe. BMC veterinary research, 2014; 10(1): 256.
- Barberio, A., Flaminio, B., De Vliegher, S., Suprי, K., Kromker, V., Garbarino, C., Arrigoni, N., Zanardi, G., Bertocchi, L., Gobbo, F., Catania, S. In vitro antimicrobial susceptibility of Mycoplasma bovis isolates identified in milk from dairy cattle in Belgium, Germany, and Italy. Journal of dairy science, 2016; 99(8): 6578-6584.
- Klein, U., de Jong, A., Moyaert, H., El Garch, F., Leon, R., Richard-Mazet, A., Rose, M., Maes, D., Pridmore, A., Thomson, J.R., Ayling, R.D. Anti-microbial susceptibility monitoring of Myco-plasma hyopneumoniae and Mycoplasma bovis isolated in Europe. Veterinary microbiology, 2017; 204: 188-193.
- Nicholas, R.A.J., Ayling, R.D. Mycoplasma bovis: disease, diagnosis, and control. Research in veterinary science, 2003; 74(2): 105-112.
- Ali, T., Zhang, L., Shahid, M., Zhang, S., Liu, G., Gao, J., Han, B. ESBL-producing Escherichia coli from cows suffering mastitis in China contain clinical class 1 integrons with CTX-M linked to ISCR1. Frontiers in microbiology, 2016; 7: 1931.
- Podolsky, S.H. The evolving response to antibiotic resistance (1945–2018). Palgrave Communi-cations, 2018; 4(1): 124.
- Gautier-Bouchardon, A.V., Reinhardt, A.K., Kobisch, M., Kempf, I. In vitro development of resistance to enrofloxacin, erythromycin, tylosin, tiamulin and oxytetracycline in Mycoplasma gallisepticum, Mycoplasma iowae and Myco-plasma synoviae. Veterinary microbiology, 2002; 88(1): 47-58.
- Hannan, P.C. Guidelines and recommendations for antimicrobial minimum inhibitory concen-tration (MIC) testing against veterinary myco-plasma species. Veterinary research, 2000; 31(4): 373-395.
- Loria, G.R., Sammartino, C., Nicholas, R.A.J., Ayling, R.D. In vitro susceptibilities of field isolates of Mycoplasma agalactiae to oxytetra-cycline, tylosin, enrofloxacin, spiramycin and lincomycin–spectinomycin. Research in Veterinary Science, 2003; 75(1): 3-7.
- Ayling, R.D., Bisgaard-Frantzen, S., March, J.B., Godinho, K., Nicholas, R.A.J. Assessing the in vitro effectiveness of antimicrobials against Myco-plasma mycoides subsp. mycoides small-colony type to reduce contagious bovine pleuro-pneumonia infection. Antimicrobial agents and chemotherapy, 2005; 49(12): 5162-5165.
- Duffy, L.B., Crabb, D., Searcey, K., Kempf, M.C. Comparative potency of gemifloxacin, new quinolones, macrolides, tetracycline and clinda-mycin against Mycoplasma spp. Journal of Antimicrobial Chemotherapy, 2000; 45(suppl_3): 29-29.
- Bartlett, J.G. Is activity against “atypical” pathogens necessary in the treatment protocols for community-acquired pneumonia? Issues with combination therapy. Clinical infectious diseases, 2008; 47(Supp._3): S232-S236.
- Chopra, I., Roberts, M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiology and Molecular Biology Reviews, 2001; 65(2): 232–260.
- Mehta, A. 2011. “Mechanism of Action of Tetracyclines”. Pharmaxchange.info. Archived from the original on 2012-06-05. Retrieved 2012-06-07.
- Thaker, M., Spanogiannopoulos, P., Wright, G.D. The tetracycline resistome. Cellular and Molecular Life Sciences, 2010; 67(3): 419-431.
- Taraskina, A.E., Savicheva, A.M., Akopian, T.A., Soroka, A.E., Momynaliev, K.T., Govorun, V.M. Drift of tetM determinant in urogenital micro-biocenosis containing mycoplasmas during treatment with a tetracycline antibiotic. Bulletin of experimental biology and medicine, 2002; 134(1): 60-63.
- Bebear, C.M., Kempf, I. Mycoplasmas: molecular biology pathogenicity and strategies for control. UK: Horizon Bioscience, 2005; pp 535-569.
- Dיgrange, S., Renaudin, H., Charron, A., Pereyre, S., Bיbיar, C., Bיbיar, C.M. Reduced susceptibility to tetracyclines is associated in vitro with the presence of 16S rRNA mutations in Mycoplasma hominis and Mycoplasma pneumoniae. Journal of antimicrobial chemotherapy, 2008; 61(6): 1390-1392.
- Gary, K. 2009. The Community College of Baltimore County Protein synthesis inhibitors: macrolides mechanism of action animation. Classification of agents Pharmamotion.
- Moore, S.D., Sauer, R.T. Revisiting the mechanism of macrolide-antibiotic resistance mediated by ribosomal protein L22. Proceedings of the National Academy of Sciences, 2008; 105(47): 18261-18266.
- Cao, B., Zhao, C.J., Yin, Y.D., Zhao, F., Song, S.F., Bai, L., Zhang, J.Z., Liu, Y.M., Zhang, Y.Y., Wang, H., Wang, C. High prevalence of macrolide resistance in Mycoplasma pneumoniae isolates from adult and adolescent patients with respiratory tract infection in China. Clinical infectious diseases, 2010; 51(2): 189-194.
- Morozumi, M., Ubukata, K., Takahashi, T. Macrolide-resistant Mycoplasma pneumoniae: characteristics of isolates and clinical aspects of community-acquired pneumonia. Journal of Infection and Chemotherapy, 2010; 16(2): 78-86.
- Leclercq, R. Mechanisms of resistance to macrolides and lincosamides: nature of the resistance elements and their clinical implications. Clinical Infectious Diseases, 2002; 34(4): 482-492.
- Alekshun, M.N., Levy, S.B. Molecular mechanisms of antibacterial multidrug resistance. Cell, 2007; 128(6): 1037-1050.
- Peuchant, O., Menard, A., Renaudin, H., Morozumi, M., Ubukata, K., Bebear, C.M., Pereyre, S. Increased macrolide resistance of Mycoplasma pneumoniae in France directly detected in clinical specimens by real-time PCR and melting curve analysis. Journal of antimicrobial chemotherapy, 2009; 64(1): 52-58.
- Xin, D., Mi, Z., Han, X., Qin, L., Li, J., Wei, T., Chen, X., Ma, S., Hou, A., Li, G., Shi, D. Molecular mechanisms of macrolide resistance in clinical isolates of Mycoplasma pneumoniae from China. Antimicrobial agents and chemotherapy, 2009; 53(5): 2158-2159.
- Dumke, R., von Baum, H., Luck, P.C., Jacobs, E. Occurrence of macrolide-resistant Mycoplasma pneumoniae strains in Germany. Clinical Microbiology and Infection, 2010; 16: 613–616.
- Principi, N., Esposito, S. Macrolide-resistant Mycoplasma pneumoniae: its role in respiratory infection. Journal of antimicrobial chemotherapy, 2012; 68(3): 506-511.
- Akaike, H., Miyashita, N., Kubo, M., Kawai, Y., Tanaka, T., Ogita, S., Kawasaki, K., Nakano, T., Terada, K., Ouchi, K., Atypical Pathogen Study Group. In vitro activities of 11 antimicrobial agents against macrolide-resistant Mycoplasma pneumoniae isolates from pediatric patients: results from a multicenter surveillance study. Japanese journal of infectious diseases, 2012; 65(6): 535-538.
- Matsuoka, M., Narita, M., Okazaki, N., Ohya, H., Yamazaki, T., Ouchi, K., Suzuki, I., Andoh, T., Kenri, T., Sasaki, Y., Horino, A. Characterization and molecular analysis of macrolide-resistant Mycoplasma pneumoniae clinical isolates obtained in Japan. Antimicrobial agents and chemotherapy, 2004; 48(12): 4624-4630.
- Steeve, G., Prescott, J.F., Dowling, P.M. Antimicrobial Therapy in Veterinary Antimicrobial activity. Edition 5th, Wiley-Blackwell, 2013; pp 704.
- Shah, M.K., Saddique, U., Ahmad, S., Hayat, Y., ur Rahman, S., Hassan, M.F., Ali, T. Isolation rate and antimicrobial susceptibility profiles of Mycoplasma mycoides subspecies capri field isolates from sheep and goats in Pakistan. Small ruminant research, 2017; 153: 118-122.
- Uphoff, C.C., Drexler, H.G. Eradication of mycoplasma contaminations from cell cultures. Current protocols in molecular biology, 2014; 106(1): 28-5.
- Antunes, N.T., Assunחדo, P., Poveda, J.B., Tavםo, M.M. Mechanisms involved in quinolone resistance in Mycoplasma mycoides subsp. capri. The Veterinary Journal, 2015; 204(3): 327-332.
- Kreizinger, Z., Grףzner, D., Sulyok, K.M., Nilsson, K., Hrivnבk, V., Benטina, D., Gyuranecz, M. Antibiotic susceptibility profiles of Mycoplasma synoviae strains originating from Central and Eastern Europe. BMC veterinary research, 2017; 13(1): 342.
- Lysnyansky, I., Ayling, R.D. Mycoplasma bovis: mechanisms of resistance and trends in anti-microbial susceptibility. Frontiers in microbiology, 2016; 7: 595.
- Hooper, D.C. Emerging mechanisms of fluoroquinolone resistance. Emerging infectious diseases, 2001; 7(2): 337.
- Khalil, D., Becker, C.A., Tardy, F. Alterations in the quinolone resistance-determining regions and fluoroquinolone resistance in clinical isolates and laboratory-derived mutants of Mycoplasma bovis: not all genotypes may be equal. Applied and Environmental Microbiology, 2016; 82(4): 1060-1068.
- Wetzstein, H.G., Schmeer, N., Karl, W. Degradation of the fluoroquinolone enrofloxacin by the brown rot fungus Gloeophyllum striatum: identification of metabolites. Applied and Environmental Microbiology, 1997; 63(11): 4272-4281.
- Dong-Ya, M., Chang-Jian, S., Jing-Bo, Y., Jun, M., Wen-Cheng, X. Molecular mechanism of fluoroquinolones resistance in Mycoplasma hominis clinical isolates. Brazilian Journal of Microbiology, 2014; 45(1): 239-242.
- Redgrave, L.S., Sutton, S.B., Webber, M.A., Piddock, L.J. Fluoroquinolone resistance: mechanisms, impact on bacteria, and role in evolutionary success. Trends in microbiology, 2014; 22(8): 438-445.
- Bיbיar, C.M., De Barbeyrac, B., Pereyre, S., Renaudin, H., Clerc, M., Bיbיar, C. Activity of moxifloxacin against the urogenital myco-plasmas Ureaplasma spp., Mycoplasma hominis and Mycoplasma genitalium and Chlamydia trachomatis. Clinical microbiology and infection, 2008; 14(8), pp.801-805.
- Mingeot-Leclercq, M.P., Glupczynski, Y., Tulkens, P.M. Aminoglycosides: activity and resistance. Antimicrobial agents and chemotherapy, 1999; 43(4): 727-737.
- Murray, B.E. New aspects of antimicrobial resistance and the resulting therapeutic dilemmas. Journal of Infectious Diseases, 1991; 163(6): 1185-1194.
- Davies, J., Wright, G.D. Bacterial resistance to aminoglycoside antibiotics. Trends in Microbiology, 1997; 5: 234–240.
- Shaw, K.J., Rather, P.N., Hare, R.S., Miller, G.H. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Micro-biology and Molecular Biology Reviews, 1993; 57(1): 138-163.
- Mingeot-Leclercq, M.P., Tulkens, P.M. Aminoglycosides: nephrotoxicity. Antimicrobial agents and chemotherapy, 1999: 43(5): 1003-1012.
- Lee, D.H., Miles, R.J., Inal, J.R.M. Antibiotic sensitivity and mutation rates to antibiotic resistance in Mycoplasma mycoides ssp. mycoides. Epidemiology & Infection, 1987; 98(3): 361-368.
- Ramirez, M.S., Tolmasky, M.E. Aminoglycoside modifying enzymes. Drug Resistance Updates, 2010; 13(6): 151-171.
- Mahairas, G.G., Minion, F.C. Transformation of Mycoplasma pulmonis: demonstration of homologous recombination, introduction of cloned genes, and preliminary description of an integrating shuttle system. Journal of bacteriology, 1989; 171(4): 1775-1780.
- Minion, C.F., Kapke, P.A. Transformation of Mycoplasmas, In: Mycoplasma Protocols. 1998; pp 227-234.
- Soehnlen, M.K., Kunze, M.E., Karunathilake, K.E., Henwood, B.M., Kariyawasam, S., Wolfgang, D.R., Jayarao, B.M. In vitro antimicrobial inhibition of Mycoplasma bovis isolates submitted to the Pennsylvania Animal Diagnostic Laboratory using flow cytometry and a broth microdilution method. Journal of Veterinary Diagnostic Investigation, 2011; 23(3): 547-551.
- Cheng, G., Hao, H., Xie, S., Wang, X., Dai, M., Huang, L., Yuan, Z. Antibiotic alternatives: the substitution of antibiotics in animal husbandry?. Frontiers in microbiology, 2014; 5: 217.
- Blum, C.A., Nigro, N., Briel, M., Schuetz, P., Ullmer, E., Suter-Widmer, I., Winzeler, B., Bingisser, R., Elsaesser, H., Drozdov, D., Arici, B. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. The Lancet, 2015; 385(9977): 1511-1518.
- Torres, A., Sibila, O., Ferrer, M., Polverino, E., Menendez, R., Mensa, J., Gabarrתs, A., Sellarיs, J., Restrepo, M.I., Anzueto, A., Niederman, M.S. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. Jama, 2015; 313(7): 677-686.
- Yang, E.A., Lee, K.Y. Additional corticosteroids or alternative antibiotics for the treatment of macrolide-resistant Mycoplasma pneumoniae pneumonia. Korean journal of pediatrics, 2017; 60(8): 245.
- March, J.B., Gammack, C., Nicholas, R. Rapid Detection of Contagious Caprine Pleuro-pneumonia Using a Mycoplasma capricolum subsp. Capripneumoniae Capsular Polysac-charide-Specific Antigen Detection Latex Aggluti-nation Test. Journal of clinical microbiology, 2000; 38(11): 4152-4159.
- Gupta, S.K., Singh, S., Gupta, M.K., Pant, K.K., Seth, P.K. Definition of Potential Targets in Mycoplasma Pneumoniae Through Subtractive Genome Analysis. Journal of Antivirals and Antiretrovirals, 1948; 2: 038-041.
- Medvedeva, E.S., Baranova, N.B., Mouzykantov, A.A., Grigorieva, T.Y., Davydova, M.N., Trushin, M.V., Chernova, O.A., Chernov, V.M. Adaptation of mycoplasmas to antimicrobial agents: Achole-plasma laidlawii extracellular vesicles mediate the export of ciprofloxacin and a mutant gene related to the antibiotic target. The Scientific World Journal, 2014; 2014: 150615.
- Krishnakumar, R., Assad-Garcia, N., Benders, G.A., Phan, Q., Montague, M.G., Glass, J.I. Targeted chromosomal knockouts in Mycoplasma pneumoniae. Applied and Environmental Micro-biology, 2010; 76(15): 5297-9.
- Lambert, T. Antibiotics that affect the ribosome. Revue Scientifique et Technique-OIE, 2012; 31(1): 57.
- Liu, X., Jiang, Y., Chen, X., Li, J., Shi, D., Xin, D. Drug resistance mechanisms of Mycoplasma pneu-moniae to macrolide antibiotics. BioMed research international, 2014; 2014: 320801.
- Gr ndel, A., Pfeiffer, M., Jacobs, E., Dumke, R. Network of surface-displayed glycolytic enzymes in Mycoplasma pneumoniae and their intera-ctions with human plasminogen. Infection and immunity, 2016; 84(3): 666-676.
- Voorde, J.V., Sabuncuoנlu, S., Noppen, S., Hofer, A., Ranjbarian, F., Fieuws, S., Balzarini, J., Liekens, S. Nucleoside-catabolizing enzymes in mycoplasma-infected tumor cell cultures compromise the cytostatic activity of the anticancer drug gemcitabine. Journal of Biological Chemistry, 2014; 289(19): 13054-13065.
- Voorde, J.V., Gago, F., Vrancken, K., Liekens, S., Balzarini, J. Characterization of pyrimidine nucleoside phosphorylase of Mycoplasma hyorhinis: implications for the clinical efficacy of nucleoside analogues. Biochemical Journal, 2012; 445(1): 113-123.
- Schmidl, S.R., Otto, A., Lluch-Senar, M., Piסol, J., Busse, J., Becher, D., St lke, J. A trigger enzyme in Mycoplasma pneumoniae: impact of the glycerophosphodiesterase GlpQ on virulence and gene expression. PLoS pathogens, 2011; 7(9): 1002263.
- Blצtz, C., St lke, J. Glycerol metabolism and its implication in virulence in Mycoplasma. FEMS Microbiology Reviews, 2017; 41(5): 640-652.
- Hames, C., Halbedel, S., Hoppert, M., Frey, J., St lke, J. Glycerol metabolism is important for cytotoxicity of Mycoplasma pneumoniae. Journal of bacteriology, 2009; 191(3): 747-753.
- Gro hennig, S., Schmidl, S.R., Schmeisky, G., Busse, J., St lke, J. Implication of glycerol and phospholipid transporters in Mycoplasma pneu-moniae growth and virulence. Infection and immunity, 2013; 81(3): 896-904.
- You, X.X., Zeng, Y.H., Wu, Y.M. Interactions between mycoplasma lipid-associated mem-brane proteins and the host cells. Journal of Zhejiang University Science B, 2006; 7(5): 342-350.
- He, J., Wang, S., Zeng, Y., You, X., Ma, X., Wu, N., Wu, Y. Binding of CD14 to Mycoplasma genitalium-derived lipid-associated membrane proteins upregulates TNF-ב. Inflammation, 2014; 37(2): 322-30.
- Szczepanek, S.M., Frasca, S., Schumacher, V.L., Liao, X., Padula, M., Djordjevic, S.P., Geary, S.J. Identification of lipoprotein MslA as a neoteric virulence factor of Mycoplasma gallisepticum. Infection and immunity, 2010; 78(8): 3475-3483.
- Shimizu, T., Kida, Y., Kuwano, K. Triacylated lipoproteins derived from Mycoplasma pneu-moniae activate nuclear factor ךB through toll like receptors 1 and 2. Immunology, 2007; 121(4): 473-483.
- Liu, Y.C., Lin, I.H., Chung, W.J., Hu, W.S., Ng, W.V., Lu, C.Y., Huang, T.Y., Shu, H.W., Hsiao, K.J., Tsai, S.F., Chang, C.H. Proteomics characterization of cytoplasmic and lipid-associated membrane proteins of human pathogen Mycoplasma fermentans M64. PloS one, 2012; 7(4): e35304.
- Fonseca-Aten, M., Salvatore, C.M., Mejםas, A., Rםos, A.M., Chבvez-Bueno, S., Katz, K., Gףmez, A.M., McCracken, G.H., Hardy, R.D. Evaluation of LBM415 (NVP PDF-713), a novel peptide deformylase inhibitor, for treatment of experimental Mycoplasma pneumoniae pneumonia. Antimicrobial agents and chemotherapy, 2005; 49(10): 4128-4136.
- Andrיs, E., Martםnez, N., Planas, A. Expression and Characterization of a Mycoplasma genitalium Glycosyltransferase in Membrane Glycolipid Biosynthesis potential target against myco-plasma infections. Journal of Biological Chemistry, 2011; 286(41): 35367-35379.
- Bose, S., Segovia, J.A., Somarajan, S.R., Chang, T.H., Kannan, T.R., Baseman, J.B. ADP-ribosylation of NLRP3 by Mycoplasma pneumoniae CARDS toxin regulates inflammasome activity. MBio, 2014; 5(6): e02186-14.
- Kannan, T.R., Baseman, J.B. ADP-ribosylating and vacuolating cytotoxin of Mycoplasma pneumoniae represents unique virulence determinant among bacterial pathogens. Proceedings of the National Academy of Sciences, 2006; 103(17): 6724-6729.
- Kannan, T.R., Musatovova, O., Balasubramanian, S., Cagle, M., Jordan, J.L., Krunkosky, T.M., Davis, A., Hardy, R.D., Baseman, J.B. Mycoplasma pneumoniae Community Acquired Respiratory Distress Syndrome toxin expression reveals growth phase and infection dependent regulation. Molecular microbiology, 2010; 76(5): 1127-1141.
- Johnson, C., Kannan, T.R., Baseman, J.B. Cellular vacuoles induced by Mycoplasma pneumoniae CARDS toxin originate from Rab9-associated compartments. PLoS One, 2011; 6(7): e22877.
- Sethi, S., Zaman, K., Jain, N. Mycoplasma genitaliuminfections: current treatment options and resistance issues. Infection and Drug Resistance, 2017; 10: 283-292.
- Read, T.R.H., Fairley, C.K., Murray, G.L., Jensen, J.S., Danielewski, J., Worthington, K., Doyle, M., Mokany, E., Tan, L., Chow, E.P.F., Garland, S.M., Bradshaw, C.S. Outcomes of resistance-guided sequential treatment of Mycoplasma genitalium infections: A prospective evaluation. Clinical Infectious Disseases, 2019; 68(4): 554-560.
- Zhang, Y.M. San Ao Tang auxiliary treatment for children mycoplasma pneumonia infection observation of curative effect,” Chinese Traditional and Herbal Drugs, 2012; 43(2): 341–342.
- Yang, Q., Wu, D., Mao, W., Liu, X., Bao, K., Lin, Q., Lu, F., Zou, C., Li, C. Chinese medicinal herbs for childhood pneumonia: a systematic review of effectiveness and safety. Evidence-Based Comple-mentary and Alternative Medicine, 2013; 203845.
- Wang, H.M., Ge, J.L., Mo, D. Azithromycin combined with traditional Chinese Ma Xing Shi Gan Tang for children pneumonia and mycoplasma in 106 cases. Medical Journal of Chinese People’s Health, 2009; 21(19): 2387–2389.
- Shi, Y.F. Combination of traditional Chinese and Western medicine for childhood mycoplasma pneumonia in 40 cases. Chinese Pediatrics of Integrated Traditional and Western Medicine, 2009; 1(4): 364–365.
- Dhama, K., Chakraborty, S., Tiwari, R., Verma, A.K., Saminathan, M., Amarpal, Malik, Y.S., Nikousefat, Z., Javdani, M., Khan, R.U. A concept paper on novel technologies boosting production and safeguarding health of humans and animals. Research Opinion in Animal Veterinary Science, 2014; 4(7): 353-370.
- Dybvig, K., Tu, A., Clapper, B. Mycoplasma Phages, p 223-237. In Waldor M, Friedman D, Adhya S (ed),Phages. ASM Press, Washington, DC. doi: 10.1128/9781555816506.ch11. 2005.
- Tiwari, R., Chakraborty, S., Dhama, K., Wani, M.Y., Kumar, A., Kapoor, S. Wonder world of phages: potential biocontrol agents safeguarding biosphere and health of animals and humans- current scenario and perspectives. Pakistan Journal of Biological Sciences, 2014; 17: 316-328.
- Arkilo, D., Pierce, B., Ritter, F., Doescher, J.S., Frost, M. Diverse seizure presentation of acute Mycoplasma pneumoniae encephalitis resolving with immunotherapy. Journal of Child Neurology. 2014; 29(4): 564-566.
- Salvatore, C.M., Fonseca-Aten, M., Katz-Gaynor, K., Gomez, A.M., Hardy, R.D. Intranasal interleukin-12 therapy inhibits Mycoplasma pneumoniae clearance and sustains airway obstruction in murine pneumonia. Infection Immunity, 2008; 76(2): 732-738.
- Dhama, K., Chakraborty, S., Wani, M.Y., Tiwari, R. and Barathidasan, R. Cytokine therapy for combating animal and human diseases – A review. Research Opinion in Animal Veterinary Science, 2013; 3(7): 195-208.
- Dhama K, Saminathan, M., Jacob, S.S., Singh, M., Karthik, K., Amarpal, Tiwari, R., Sunkara, L.T., Malik, Y.S., Singh, R.K. Effect of immuno-modulation and immunomodulatory agents on health with some bioactive principles, modes of action and potent biomedical applications. International Journal of Pharmacology, 2015; 11(4): 253-290.
- Tiwari, R., Latheef, S.K., Ahmed, I., Iqbal, H.M.N., Bule, M.H., Dhama, K., Samad, H.A., Karthik, K., Alagawany, M., El-Hack, M.E.A., Yatoo, M.I., Farag, M.R. Herbal immunomodulators – A remedial panacea for designing and developing effective drugs and medicines: current scenario and future prospects. Current Drug Metabolism, 2018; 19(3): 264-301. doi: 10.2174/1389200219666180-129125436.
- Prasad, M., Lambe, U.P., Brar, B., Shah, I., Manimegalai, J., Ranjan, K., Rao, R., Kumar, S., Mahant, S., Khurana, S.K., Iqbal, H.M.N., Dhama, K, Misri, J., Prasad, G. Nanotherapeutics: An insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomedicine & Pharmacotherapy, 2018; 97:1521-1537.
- Zhu, X., Radovic-Moreno, A.F., Wu, J., Langer, R., Shi, J. Nanomedicine in the management of microbial infection-overview and perspectives. Nano today, 2014; 9(4):478-498.
- Yatoo, M.I., Saxena, A., Malik, M.H., Kumar, M.K. Dimri, U. Nanotechnology based drug delivery at cellular level: a review. Journal of Animal Science Advances, 2014; 4(2):705-709.
© The Author(s) 2019. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.