ISSN: 0973-7510
E-ISSN: 2581-690X
The present study was conducted to identify the effectiveness of various antibiotics and antibiotic combinations in eliminating the black dots contamination in cell culture and to identify its effect on cell growth kinetics. Isolation of contaminant organism (Black dots) observed in our laboratory, was attempted by inoculation of harvested cell supernatant and pellet into Nutrient broth, Luria broth, and Pleuropneumonia-like organisms’ broth but no visible growth was observed. To analyze the effect of antibiotics in elimination of contamination, the cell monolayer was incubated with different antibiotics and combination of antibiotics till 120 hrs. Withdrawal effect of antibiotics was observed by further incubation cells in maintenance media for another 48 hrs. To investigate the effect of different antibiotics on cell growth kinetics, cell seeding was done in media containing antibiotics. A subset of cells was harvested every 24 hrs and counted till 120 hrs. Among all antibiotics, Ampicillin reduced the contamination, the Piperacillin and Tazobactam combination diminished the contamination to a great extent. At the studied concentrations of the antibiotic trio of piperacillin, tazobactam, and ampicillin, black dot contamination was eliminated and found 100% effective in inhibiting contamination within 120 hrs of the study period; but, after 48 hrs of antibiotic withdrawal, contamination reappears in cell culture. The growth kinetics showed no significant impact of antibiotics treatment on the cell growth curve. The growth kinetics showed that cell growth was not interfering significantly by individual or combination of antibiotics. Therefore, in the presence of black dot, a combination of three antibiotics (piperacillin, tazobactam, and ampicillin) should be effective in cell culture.
Cultured Cells, Black Dots, Contamination, Antibiotic Treatment
Cell culture contamination is a universal problem that most researchers face at some point in their careers. Failure of experiments, wastage of time, and frustration are clear consequences of contamination. Contamination is difficult to check, especially when working with a transient workforce, which is common in research, mainly when too many people use the same culture lab. However, if cell culture contamination is not recognized, it might lead to incorrect results caused by the cellular response to the contamination rather than the treatments 1. Researchers employ several aseptic culture handling techniques to keep the cultures free of undesirable organisms 2. Sometimes, researchers work with cell lines that are not commercially available and hard to obtain or must be isolated from primary cells. The most common contaminants in cell culture are well addressed by researchers 3, but floating or swimming black dots grow in cell culture without creating many problems, causing great concern. Viral and mycoplasma contamination do not cause noticeable turbidity in the media, have a lower pH 4,5, and are better tolerated by cells. They are only excluded from cell lines if they are periodically checked and subjected to suitable processing and modification requirements 6. The black spots in the cultured cells are remarkably little dots. Their origin and identification are still a point of argument. They are difficult to remove and have a negative influence on cell research. However, there is very little information about the use of antibiotics to treat these issues. This study was conducted to evaluate the effectiveness of different antibiotics in eliminating black dot contamination and the effect of antibiotic treatment on cell growth kinetics.
Cell culture
The Vero cell line (clone CCL81) was propagated in Eagle Minimum Essential Medium (EMEM) supplemented with 10% fetal bovine serum (FBS, Invitrogen). Once the 100% confluency was achieved, the cell culture was further maintained in maintenance media containing EMEM with 2% FBS in a humidified CO2 incubator at 37°C with 5% CO2.
Contamination detection in the culture broth
While sub culturing the black dot contaminated Vero cells all possible precautions were taken to avoid the spread of contamination. The culture media was removed separately, and cells were washed twice with PBS and then treated with trypsin for detachment from the flask. The detached cells were collected separately in a new Eppendorf tube and centrifuged at 300g for 10 minutes. The pellet was collected for further analysis. The contaminant pellet and culture media were added separately to the Nutrient broth (NB), Luria broth (LB), and Pleuropneumonia-like organisms broth (PPLOB) for detection of bacteria and mycoplasma, respectively. Inoculated NB and LB were incubated for 48 hrs and PPLOB for 120 hrs at 37°C, respectively. Broths were examined via visual observation for the appearance of any growth every 24 hrs.
Antibiotic treatment
Antibiotics were used for the decontamination of black dots in cell culture. Different antibiotics with respective concentrations used in this study are given in table. The concentration of antibiotics used in the present study was based on published reports 7-9. Every 48 hrs, the maintenance media was replaced by new maintenance media containing antibiotics. The decontamination effect was evaluated every 24 hrs by microscopic evaluation of the cell culture for a period of 120 hrs. After maintaining the cells under the influence of antibiotic for 2-3 passages, the subcultured Vero cells were further maintained for another 48 hrs in maintenance media without antibiotics to study the withdrawal effect of antibiotics on black dot contamination.
Growth kinetics analysis
To investigate the impact of antibiotics on the cell growth kinetics, 1ml of Vero cell suspension containing approximately 5×103 cells/ml was seeded in the 24 well culture plate and propagated in EMEM containing different antibiotics/antibiotics combinations (Table ) supplemented with 10% FBS. Plates were kept in a humidified CO2 incubator at 37°C with 5% CO2. In addition, the effects of antibiotics/ antibiotic combinations in Vero cells growth kinetics without black dot contamination were also investigated. The cells were harvested and counted at 24 hrs intervals starting from 24 hrs to 120 hrs of incubation, and the cells of three wells were taken for average cell count.
Table :
Antibiotics used in this study and impact on the decontamination of the black dots in cell culture
| Antibiotics | Antibiotics concentration | Visual observation and interpretation of decontamination effect at different time intervals | Antibiotics withdrawal effect on black dot contamination (After 48hrs) | |||||
|---|---|---|---|---|---|---|---|---|
| Time (hrs) | Remark (Black dot contamination) | |||||||
| 24 | 48 | 72 | 96 | 120 | ||||
| Piperacillin, Tazobactam and Ampicillin | 112.5 + 100 µg/ml | – | + | ++ | +++ | +++ | Completely cleared | Reappeared |
| Piperacillin and Tazobactam | 112.5 µg/ml | – | + | + | ++ | ++ | Reduced to a greater extent | Reappeared |
| Ampicillin | 100 µg/ml | – | – | – | + | + | Reduced | Reappeared |
| Kanamycin | 100 µg/ml | – | – | – | – | – | No effect | No effect |
| Gentamicin | 100 µg/ml | – | – | – | – | – | No effect | No effect |
| Penicillin | 1000U/ml | – | – | – | – | – | No effect | No effect |
| Streptomycin | 10 µg/ml | – | – | – | – | – | No effect | No effect |
| Penicillin and Streptomycin | 1000U/ml +10 µg/ml | – | – | – | – | – | No effect | No effect |
‘+++’: Completely cleared; ‘++’: Reduced to greater extent; ‘+’: Reduced; ‘-’: No effect
The study was conducted to investigate the effects of the antibiotics and combinations of antibiotics on a mysterious type of contamination appearing like swimming blackish dots observed in cell culture during routine maintenance of cell lines in our laboratory. The contaminated cell pellet and media were inoculated in NB, LB, and PPLOB were observed for the appearance of any growth. Till the end of the incubation period, no visible growth was observed.
Elimination of Black dots in cell culture via antibiotic treatment
Effect of antibiotics treatment was observed at 24 hrs, 48 hrs, 72 hrs, 96 hrs, and 120 hrs of incubation and also it was observed in the second and third passage of the cells under the effect of the antibiotic. On microscopic examination of the Vero cell line an appreciable effect of antibiotic in considerable reduction in black dots, started appearing after 48 hrs of treatment with Piperacillin and Tazobactam, the result was very apparent when Ampicillin was also added to media, hence Piperacillin, Tazobactam, and Ampicillin combinations was more effective. The latter combination completely cleared the contamination within 92 hrs of incubation (Figure 1). However, the combination of the Piperacillin and Tazobactam reduced the contamination to a greater extent within the 92 hrs of incubation. Still, it failed to clear the contamination entirely by the end of the incubation period. The individual Ampicillin treatment was also able to reduce the black dots contamination by the 92 hrs of incubation, and the effect remained the same throughout the incubation period. Other antibiotics and antibiotic combinations tested showed no inhibitory effect on black dots. Maintaining the cells under this antibiotic treatment for 3 passages apparently cleared the cell line with all the visible black dot contaminants. After 48 hrs of withdrawal of antibiotics treatment, the black dots contamination reappeared fully in the cell culture (Figure 1).
Figure 1. Black dot decontamination effects of treatment with Piperacillin, Tazobactam, and Ampicillin combination in Vero cells observed under the light microscope. [A], [B] Vero cells with Black dots (No effect of antibiotics treatment at 24 hrs); [C], [D] Vero cells with reduced Black dots at 48 hrs; [E], [F] Vero cells with completely cleared black dots at 96 hrs; [G], [H] Reappearance of Black dots in Vero cells after 48 hrs of withdrawal of antibiotics
Antibiotics treatment and its effect on cell growth kinetics
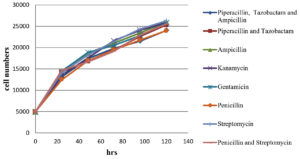
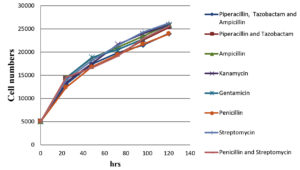
The impact of antibiotics and a combination of antibiotics on normal Vero cells (without black dots contaminations); on black dot contaminated Vero cells growth kinetics were evaluated. The cell number counted after trypsinization at an interval of 24 hrs, 48 hrs, 72 hrs, 96 hrs, and 120 hrs post-seeding are shown in Figure 2, 3. The growth curve for contaminated cells maintained in different antibiotics and antibiotic combinations did not show any significant variation from normal cells without contamination maintained in media without any antibiotics and with antibiotics/ antibiotic combinations. Microscopic evaluation of cells did not show any substantial changes in cell morphology and characteristics with any antibiotics or combinations of antibiotics tested.
Figure 2. Graphical representation of Vero cell growth kinetics with the treatment of different antibiotics/ antibiotics combination over a period of 120 hrs
The Vero cells were propagated and maintained in the lab for use, but while observing under an inverted microscope some floating black dots were observed in the flask. The appearance of these black dots was also not uniform but appeared as black dots under 10x. The number of these contaminants also kept on increasing when incubating the cells at 370C. After sub-culture, these contaminants appear and increase in number within 8-10 hrs. Surprisingly even after increase in number of black dots the media did not show any change in pH nor their considerable turbidity as it happens when there is bacterial contamination 10,11. A similar type of fidgets or swim-like contaminants, which are termed black dots, has been observed by many researchers during the maintenance of various cell lines. Gary et al 9 observed black dot-like contamination in the young adult murine colon epithelial and immorto-min mouse colon epithelial cells. Yang et al 12. and Xu et al 8 also observed a similar type of black dot contamination in the perivitelline space of zygotes and the A549 cell lines, respectively. Gary et al 9 reported black dot contamination as an intracellular Achromobacter by using 16S rDNA analysis. But they fail to specify whether the contaminants were present in the cells at the time of receipt or were the result of a handling fault. In addition to contaminant importation, intracellular variation in some indigenous particles can be mistaken for bacteria at times 13,14. Other intracellular organism of unclear nature which may have a symbiotic association with cell line has also been linked to cell line contamination 6. Despite black dot contamination, cell growth and morphology were healthy. Similar findings have been reported in other studies 9,15.
In this study, attempt was made to isolate the black dot contamination using different broths, but it was unsuccessful. Other better isolation methodologies can be explored for successful isolation. Barkwan et al 5 tried to identify similar contamination by inoculating supernatant culture and cell lyses in various agar plates like NB, Muller Hinton agar, Blood-brain and heart infusion, and MacConky agar plates. But all these agar plates show no visible colonies.
One of the most common precautionary measures used during in-vitro culture is antibiotics to prevent bacterial contamination 8. Antibiotics have unique impacts on microorganisms that are developing. For instance, an antibiotic may interfere with the development of cell walls, alter chromosomal topology by affecting DNA gyrase, and prevent the production of DNA, RNA, and proteins 16. It is widely accepted that using antibiotics in cultured cells has little/no effect on gene expression 17,18. Some research groups reported the elimination of black dots from cell culture by using Ampicillin and Gentamicin at the concentration of 50 μg/ml alone and combining these antibiotics at 100 μg/ml for five days 9,19. In our experiment, we used Ampicillin at 100 μg/ml concentration for the 120 hrs. It reduced the contamination but failed to eliminate it.
Contrary to the previous report, gentamicin used at 100 μg/ml concentrations for 120 hrs failed to show any significant inhibitory effects on black dot contamination. In the experiments of Gary et al 9, the combination of Piperacillin and Ciprofloxacin, both at a concentration of 10 µg/ml, worked more efficiently to eliminate the black dots from the cell culture. In our study, among all other antibiotics and combinations of antibiotics tested, the combination of Piperacillin and Tazobactam (stock solution (1.125g): Piperacillin- 1000mg; tazobactam- 125 mg) at 112.5µg/ml concentration worked more efficiently. When another antibiotic that is Ampicillin was also added to this combination there was remarkable reduction in the contaminant. The Piperacillin with Tazobactam along with the Ampicillin antibiotic combination inhibits the black dot contamination completely during the maintenance period and subsequent 2-3 passages. The cell line appeared completely free from this extraneous contaminant but still, once these antibiotics were removed from the media, the black dot contamination reappears. Xu et al 8 also reported that contamination in the cell culture reappeared after the withdrawal of antibiotics. It indicates that these antibiotics has bacteriostatic effect and is not bactericidal, it acts as a temporary solution and do not permanently eliminate contamination from the cell culture. Other antibiotics used in our study showed no inhibitory effects on black dot contamination.
A growth kinetic study was conducted to evaluate the impact of antibiotics and antibiotic combinations on the cells to treat black dot contamination and on normal Vero cells also and we conclude that there was no significant difference in the cell growth rate difference in the presence of antibiotics in the cell culture media with/ without contamination. Besides, there were no significant changes also observed in between each individual antibiotic and antibiotic combination. Along with the cell count, cell morphology was examined microscopically at each time interval. This microscopic evaluation shows no substantial changes in the cell morphology in the presence and/or absence of antibiotics and antibiotic combinations throughout the study.
In accordance with this experiment, this black dot contamination occurs in cell culture, which doesn’t interfere significantly with the cell morphology and growth pattern. It exists along with cells in a symbiotic association. In this study, the contamination was primarily observed by visual and microscopic observation. This experiment showed that Ampicillin reduces the contamination but not completely, whereas the combination of Piperacillin and Tazobactam reduced the contamination to a greater extent in the cell culture. The combination of Piperacillin, Tazobactam, and Ampicillin antibiotics at tested concentration clears black dot contamination completely in the cell culture system. The tested individual antibiotics and a combination of antibiotics did not significantly impede cell growth, according to the growth kinetics. The reemergence of contamination in cell culture after antibiotic removal demonstrated that the tested antibiotics do not entirely eradicate contamination.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
BM and CLP conceptualized and designed the study. NB performed wet-lab and dry lab work. NB, JS, GM and BM wrote the manuscript. NB, KD, JS, GM, BM, CLP, KB, AD, DV and BPM performed proofreading and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
This study was funded by CAAST-ACLH (Grand number: 8776-IN).
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
Not applicable.
- D Lehmann, S Jouette, F Olivieri, et al. Novel sample preparation method for molecular detection of Mollicutes in cell culture samples. Journal of microbiological methods. 2010;80(2):183-9.
Crossref - RJ Hay. Operator-induced contamination in cell culture systems. Developments in biological standardization, 1991;75:193-204.
- JA Ryan. Understanding and managing cell culture contamination, Corning Incorporated. 1994.
- OWJC Merten. Virus contaminations of cell cultures–a biotechnological view, 2002;39(2):91-116.
Crossref - S Barkwan, H Rasheed, O Spoloso. Preliminary evidence of a new microbial species capable of sustainable intracellular survival and transfer in mammalian cell lines, PeerJ PrePrints, 2014.
Crossref - SN Hassan, FJGlTD Ahmad. The relevance of antibiotic supplements in mammalian cell cultures: Towards a paradigm shift, 2020:62(4);224.
Crossref - IJC Kuhlmann. The prophylactic use of antibiotics in cell culture, 1995;19(2):95-105.
Crossref - X Xu, Y Lai, W Zhou, L Wu, ZJB Hua. Biochemistry, Quantification of a cell culture contaminant using 16S rDNA, 2019;66(5):815-822.
Crossref - JS Gray, JM Birmingham, JIJB Fenton. Got black swimming dots in your cell culture? Identification of Achromobacter as a novel cell culture contaminant, 2010;38(2):273-277.
Crossref - C Farrell, F Hassard, B Jefferson, T Leziart, A Nocker, PJ Sotte. Turbidity composition and the relationship with microbial attachment and UV inactivation efficacy, 2018;624;638-647.
Crossref - D Nurjadi, S Boutin, K Schmidt, M Ahmels, DJM Hasche. Identification and Elimination of the Clinically Relevant Multi-Resistant Environmental Bacteria Ralstonia insidiosa in Primary Cell Culture, 2020;8(10);1599.
Crossref - X Yang, X Jiang, W An, et al. Black swimming dots in cell culture: the identity, detection method and judging criteria, 2018;366906.
Crossref - V Zimorski, C Ku, WF Martin, SBJCoim Gould. Endosymbiotic theory for organelle origins, 22 (2014) 38-48.
Crossref - M Lynch, GKJe Marinov. Membranes, energetics, and evolution across the prokaryote-eukaryote divide, 2017;6:e20437.
Crossref - S Tang, X Yang, X Jiang, et al. Virus infection might cause cells producing black swimming dots, (2018) 431965.
Crossref - MA Kohanski, DJ Dwyer, JJJNRM Collins. How antibiotics kill bacteria: from targets to networks, 2010; 8(6);423-435.
Crossref - AH Ryu, WL Eckalbar, A Kreimer, N Yosef, NJSr. Ahituv. Use antibiotics in cell culture with caution: genome-wide identification of antibiotic-induced changes in gene expression and regulation, 2017;7(1): 1-9.
Crossref - C Lezin, P Mauduit, G Uzan, MEJAtLA Abdelgawad, An evaluation of different types of peptone as partial substitutes for animal-derived serum in Vero cell culture, 2022;50(5):339-348.
Crossref - S Garima, T Kumud, SJJAMR Singh. Curing of Mammalian Cell Lines from Severe Bacterial Contamination, 2020; 3;01-06.
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.