ISSN: 0973-7510
E-ISSN: 2581-690X
Recent emergence of carbapenem resistant non-fermenting Gram negative bacteria (CRNFGNB) predominantly Pseudomonas and Acinetobacter species are responsible for significant proportion of nosocomial infections with increased mortality. Of the various mechanisms known, carbapenemases especially metallo beta lactamase (MBL) mediated resistance is the most concerning because of its easy transmissibility via mobile genetic elements and lack of MBL inhibitors for clinical use. In the present study we determined to estimate the prevalence of carbapenem resistant Pseudomonas and Acinetobacter species, their resistance mechanisms by phenotypic tests and synergistic studies with Colistin and carbapenems combination by checkerboard assay. Carbapenem resistance among these two bacteria is 53.2% being isolated predominantly from pus and endotracheal secretions and from patients within the age group of less than 9 years (44%) and more than 60 years (23%). The incidence of carbapenemase and MBL production in NFGNB is 89.8% and 87.9%, respectively. Only Colistin and Tigecycline show significant antibacterial activity while most of the tested antibiotics were found to be least effective against carbapenem resistant NFGNB. Colistin and Imipenem combination demonstrated synergistic activity in majority of the NFGNB species; however, translation of such in vitro efficacy models into highly variable in vivo conditions could be possible only with strong clinical support.
Non-fermenting GNB, Carbapenemase, Metallo Beta Lactamases, Checker Board Assay
The emergence of carbapenem resistant Gram negative bacteria (CRGNB) including non-lactose fermenting GNB (NFGNB) such as, Pseudomonas and Acinetobacter species is a serious global health concern with increased morbidity and mortality. They are responsible for a significant proportion of hospital acquired infections associated with prolonged hospitalization imposing additional financial burden. As the intrinsic resistance to routinely used antibiotics is common in NFGNB, carbapenems were used at high frequency, which further enhance carbapenem resistance (CR). Often the NFGNB colonize various body sites such as respiratory and gastrointestinal tract along with CR Enterobacteriaceae, hence facilitating the horizontal spread of resistance between them. Therefore, stringent surveillance strategies and effective infection control practices encompasses early detection of CRGNB carriers, strict contact isolation, judicious use of carbapenems is critically important to limit their spread. Also screening for CRGNB in patients with existing co morbidities prior to any major health implications could be potential lifesaving clinical practice by reducing hospital infection related mortality.
The propensity of Pseudomonas and Acinetobacter species to survive virtually on any surfaces and inanimate objects, diverse ecological conditions and natural resistance to routine decontamination practices are the most difficult criteria in the strategy for hospital infection control.
Despite the fact that there are numerous pathways contributing to carbapenem resistance in NFGNB production of carbapenemases is the most concerning method due to its high prevalence, easy transmissibility being carried on mobile genetic elements and their association with genes conferring resistance to other antimicrobials therefore spread of multiple drug resistance.1,2 Of the various carbapenemases that are well characterized, class B enzymes are known as metallo beta lactamase (MBLs)as they need Zinc ions for their catalytic activity and predominantly encountered in Pseudomonas and Acinetobacter species.3,4 Though, the mechanisms for CR in NFGNB vary geographically, MBL mediated resistance poses a significant risk to human health because of lack of MBL inhibitors for clinical use as well as their spread noticed across the continents.2,5
Colistin and Tigecycline are commonly used to treat life threatening infections caused by CRGNB, however, monotherapy often results in clinical failure for a variety of reasons. Therefore, the present study aims to determine the prevalence of carbapenem resistant Pseudomonas and Acinetobacter species, resistance mechanisms using phenotypic approaches and to determine the synergistic activity of Colistin with Imipenem and Meropenem combinations against these isolates.
The study was conducted over a period of one year from January 2022 to December 2022 at SVS Medical College and Hospital Mahabubnagar, Telangana, India. The Institutional Ethical Committee clearance was duly obtained before start of the study (06/2020-624). Various clinical samples in suitable containers received in clinical bacteriology lab were processed as per the standard protocols (CLSI). The identification and antibiotic sensitivity testing of isolated bacteria was performed using automated Vitek-2 compact (Bio-merieux) system. Only carbapenem resistant Pseudomonas and Acinetobacter species were included in the study. Culture media, antibiotic discs and drugs in pure powder form and other chemicals were obtained from Hi Media laboratories.
Detection of carbapenemases by Rapidec carba NP test
It is a simple, sensitive and ready to use calorimetric test for the detection of carbapenemases. When bacterial lysate hydrolysis the carbapenem, the carboxylic acid derivative formed acidifies the medium which result in the color change of the pH indicator red to yellow indicating the presence of carbapenemase. Organisms positive in this test were further screened for metallo beta lactamases (MBL) by double disc synergy test.6
Detection of MBLs by double disk synergy test (DDST)
Filter paper disc containing 10μl EDTA (0.5M) was placed in the center of the Mueller Hinton agar plate containing test inoculum and Imipenem disc (10μg) was placed at a distance of 20mm and incubated at 24hrs at 37°C. Enhancement of the zone of inhibition in the area between Imipenem and the EDTA disc in comparison with the zone of inhibition on the far side of the drug was interpreted as a positive result for MBL production.7 Organisms positive for MBL production were used for synergy testing by checkerboard assay.
Checkerboard assay to determine synergistic drug combinations
Colistin is used as the drug of choice to treat infections caused by CRGNB, hence it is selected to test (drug A) in combination with Imipenem and Meropenem (drug B). Briefly, two-fold dilutions of each drug was added in 100µl of Muller Hinton broth in 96 well microtiter plate and 10µl bacterial inoculum was added in all the wells followed by incubation for 18-24hrs at 370C. A fractional inhibitory concentration index (FICI) was used to interpret the results. Synergy is more likely to be expressed when the ratio of the concentration of each antibiotic to the MIC of that antibiotic was same for all components of the mixture. The combination is considered synergy when the FICI is ≤ 0.5, additive ≥0.5-1, indifference ≥ 1-4, and antagonism ≥ 4.8
Statistical analysis
Graph Pad Prism and SPSS (version 23.0) were used to analyze the data in Microsoft Excel. Chi-square test was run (IBM Crop., Armonk, NY, USA) with P-value cutoff for statistical significance was ≤0.05.
A total of 295 non-lactose fermenting gram negative bacteria (NFGNB)were isolated from clinical samples (n=2268) during the study period.
A vast majority of these (254, 86%) were from inpatients, while remaining 41 samples were from outpatients (14%). Maximum number of organisms were isolated from 0-9 years of age (44%), followed by the age group of more than 60 years (23.0%) and the least number of organisms were from the age group of 20-29 (3.3%).
Pseudomonas spp., are the most predominant bacteria isolated with a frequency of 41.3% (122 out of 295), followed by Acinetobacter spp., at 31.9% (94) while the remaining 26.8% (79) comprises of Moraxella, Bordetella, Legionella, Burkholderia, Stenotrophomonas accounting for a total of 295 isolates. The overall prevalence of carbapenem resistant NFGNB amongst total clinical samples processed is 6.9% (157 out of 2268 samples) whereas, carbapenem resistant strains among NFGNB is 53.2% (157 out of 295) being largely contributed by Pseudomonas spp., with 54.8% (86 out of 157) followed by Acinetobacter spp., 45.2% (71 out of 157) indicating the high incidence of carbapenem resistance in these bacteria.
As shown in Table 1, a significant proportion of these carbapenem resistant isolates were mostly obtained from clinical samples relevant to nosocomial nature of infections. Pseudomonas spp., were isolated from pus samples (53.5%) followed by ET secretions (19.8%), whereas Acinetobacter spp., were isolated from ET secretions (50.7%) followed by blood (33.8%).
Table (1):
Incidence of carbapenem resistant NFGNB in different clinical samples
Samples |
Pseudomonas spp., n=86 (%) |
Acinetobacter spp., n=71 (%) |
|---|---|---|
Pus |
46 (53.5) |
5 (7) |
Blood |
15 (17.4) |
24 (33.8) |
Urine |
4 (4.6) |
3 (4.2) |
ET* |
17 (19.8) |
36 (50.7) |
Tissue biopsy |
1 (1.2) |
0 |
BAL* |
3 (3.5) |
2 (2.9) |
CSF* |
0 |
1 (1.4) |
(*ET-Endotracheal secretion, BAL-Broncho alveolar lavage, CSF-Cerebrospinal fluid)
As shown in Table 2, the incidence of carbapenemase production in Pseudomonas spp., is 86% (74 out of 86) and MBL detection is 85.1% (63 out of 74), whereas in Acinetobacter spp., the carbapenemase was 94.4%(67 out of 71) and MBL production is 91% (61 out of 67). The overall carbapenemase production is seen in 89.8% (141 out of 157) and MBL detection is 87.9%(124 out of 141).
Table (2):
Incidence of carbapenemase (Rapidec carba NP)and MBL (DDST) production in Pseudomonas and Acinetobacter species
| Organism | Carbapenem resistant | Rapidic carba NP test | DDST method | ||
|---|---|---|---|---|---|
| Positive (%) | Negative (%) | Positive (%) | Negative (%) | ||
| Pseudomonas spp., | 86 | 74 (86) | 12 (14) | 63 (85.1) | 11 (14.9) |
| Acinetobacter spp., | 71 | 67 (94.4) | 4 (5.6) | 61 (91) | 6 (9) |
| Total | 157 | 141 (89.8) | 16 (10.2) | 124 (87.9) | 17 (12.1) |
As shown in Table 3, both the species were 100% resistant to Imipenem and Meropenem, in addition Pseudomonas spp., also shows 100% resistance to Ceftazidime, Ciprofloxacin and Acinetobacter spp., shows 100% resistance to Amikacin and Levofloxacin. However, Colistin and Tigecycline shows significant efficacy while most of the tested antibiotics were found to be least effective against these CR isolates.
Table (3):
Antibiotic susceptibility pattern of MBL producing Pseudomonas and Acinetobacter species (by Vitek-2 compact)
| Antibiotics | Pseudomonas spp., n=63 (%) | Acinetobacter spp., n=61 (%) | |
|---|---|---|---|
| Aztreonam | S | 14 (22.2) | 20 (32.3) |
| R | 49 (77.8) | 41 (67.7) | |
| Cefoperazone/Sulbactam | S | 11 (17.5) | 7 (11.5) |
| R | 52 (82.5) | 54 (88.5) | |
| Piperacillin/Tazobactam | S | 3 (4.8) | 2 (3.3) |
| R | 60 (95.2) | 59 (96.7) | |
| Ceftazidime | S | 0 (0) | 15 (24.6) |
| R | 63 (100) | 46 (75.4) | |
| Ceftriaxone | S | 7 (11.1) | 1 (1.6) |
| R | 56 (88.9) | 60 (98.4) | |
| Amikacin | S | 2 (3.2) | 0 (0) |
| R | 61 (96.8) | 61 (100) | |
| Gentamicin | S | 4 (6.3) | 2 (3.3) |
| R | 59 (93.7) | 59 (96.7) | |
| Ciprofloxacin | S | 0 (0) | 8 (13.1) |
| R | 63 (100) | 53 (86.9) | |
| Levofloxacin | S | 1 (1.7) | 0 (0) |
| R | 62 (98.4) | 61 (100) | |
| Minocycline | S | 31 (49.2) | 25 (41) |
| R | 32 (50.8) | 36 (59) | |
| Tigecycline | S | 49 (77.8) | 57 (93.5) |
| R | 14 (22.2) | 4 (6.5) | |
| Trimethoprim Sulphamethoxazole | S | 2 (3.2) | 0 (0) |
| R | 61 (96.8) | 61 (100) | |
| Imipenem | S | 0 (0) | 0 (0) |
| R | 63 (100) | 61 (100) | |
| Meropenem | S | 0 (0) | 0 (0) |
| R | 63 (100) | 61 (100) | |
| Colistin | S | 53 (84.1) | 46 (75.4) |
| R | 10 (15.9) | 15 (24.6) |
Varying range of drug concentrations based on their MIC has been tested. Colistin was used in the range of 0.25 to 256µg/ml whereas, Imipenem and Meropenem was from 0.5 to 32 µg/ml. As shown in Figure 1A, combination of Colistin and Imipenem (Cl+Imp)shows synergistic activity in 65.1% (41 out of 63)while the combination of Colistin and Meropenem (Cl+Mrp) shows synergy only in 8.6%(18 out of 63) of MBL positive Pseudomonas spp., indicating that Cl+Imp combination found to be significant (p≤0.05). Of the various combinations tested, synergy was significantly observed at 1+4µg/ml for Cl+Imp and 1+8µg/ml for Cl+Mrp combination, respectively.
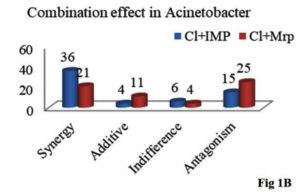
Figure 1. Colistin and carbapenems combination by checkerboard assay in MBL producing Pseudomonas and Acinetobacter species
As shown in Figure 1B, combination of Cl+Imp shows synergistic activity in 59% (36 out of 61) while the combination of Cl+Mrp shows synergy in 34.4% (21out of 61) MBL positive Acinetobacter spp., The most efficacious combination for majority of the tested isolates is0.5+2µg/ml for Cl+Imp and 8+16µg/ml for Cl+Mrp combination, respectively (p≤0.05).
Pseudomonas and Acinetobacter species are the most predominant members of the NFGNB group,9,10 which also encompasses numerous clinically significant bacteria with diverse etiology. The exponential spike in their clinical significance since a decade is partly due to the advent of automation in clinical microbiology lab. Due to their multiple resistance mechanisms to many antibiotics including carbapenems they are often associated with life threatening infections of nosocomial origin with increased in hospital mortality.11 A significant proportion of these organisms (86%) were isolated from clinical samples received from admitted patients indicating the nosocomial nature and also severity of the infection. The clinical site of infection is another important epidemiological indicator. Surgical site infections were the most common followed by lower respiratory tract infections as also noted in this study by isolation of more than 50% organisms from pus and ET secretions alone. Prolonged hospital stay, excess instrumentation and surgical interventions are known predetermined factors for NFGNB infections.
The prevalence of NFGNB infections among children less than 9 years (44%) and elderly (23%) age group of more than 60 years strongly relate their clinical significance with compromised innate defense mechanisms and related co-morbidities, however, the underlying associated mechanisms needs to be elucidated. Both of these findings corroborate with existing data.12,13
The incidence of CR Pseudomonas and Acinetobacter spp., vary geographically. A study from India shows their prevalence ranging from as low as 18.2% to as high as 72.5%. Hospitals across the world including Asia Pacific and Latin America also report almost in the similar trend.14-17
Of the various mechanisms described for CR, production of carbapenemases and MBLs were found to be the most predominant mechanisms reported in the present study the results of which are in concordance with others.18,19 The identification of carbapenemase producing bacteria is difficult challenge for clinical laboratories coping with the current global spike in these CRGNB. Since many clinical laboratories are unable to use genomic assays on routine due to its cost and technical complexity, therefore phenotypic tests such as Rapidec carba NP found most suitable for rapid and reliable detection with wide spread usage.8
Despite the fact that Colistin is the last resort drug especially in CRNFGNB infections, soon NFGNB strains resistant to Colistin have been developed and circulating in the community.20 Present study also shows Colistin resistance at a frequency of 15.9% and 24.6% among MBL positive Pseudomonas and Acinetobacter spp., respectively. In such a critical scenario, combination therapies have been proved to be efficacious than monotherapy. In the present study also Colistin and carbapenems combination by checkerboard assay proved to be synergistic and additive effect in majority of the MBL positive strains. Of the various combinations tested, Cl+Imp combination demonstrated the maximum synergistic effect in our study.
Synergistic drug combinations could be the present therapeutic scope; however, since in-vitro models cannot accurately represent in vivo variables such as antibiotic concentrations at the site of action, host immune response in the body, bacterial load and resistance, we cannot assume that they will be effective in clinical settings. Therefore, more clinical studies are required to ascertain the therapeutic potential of various drug combinations and their synergistic mechanisms.
Limitations of the study
Colistin resistant patients could have been followed up to monitor clinical prognosis and the efficacy of drug treatment to substantially validate the resistant patterns of the organisms isolated.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, SVS Medical College, Yenugonda, India, with reference number SVSMC-IEC-2022(1)/005/2022.
- King D, Strynadka N. Crystal structure of New Delhi metallo- β-lactamase reveals molecular basis for antibiotic resistance. Protein Science. 2011;20(9):1484-1491.
Crossref - Bello-Lopez JM, Cabrero-Martinez OA, Ibanez-Cervantes G, et al. Horizontal Gene Transfer and its Association with Antibiotic Resistance in the Genus Aeromoonas spp. Microorganisms.2019;7(9):363.
Crossref - Perry JD, Naqvi SH, Mirza LA. Prevalence of fecal carriage of Enterobacteriaceae with NDM-1 carbapenemase at military hospital in Pakistan,and evaluation of two chromogenic media. J Antimicrob Chemother. 2011;66(10):2288-2294.
Crossref - DAndrea MM, Venturelli C, Giani T. Persistent carriage and infection by multidrug-resistant Escherichia coli ST405 RODUCING NDM-1 carbapenemase: report on the first Italian cases. J Clin Microbiol. 2011;49(7):2755-2758.
Crossref - Hrabak J, Cervena D, Izdebski R, Duljasz W, Gniadkowski M, Fridrichova M. Regional spread of Pseudomonas aeruginosa ST 357 producing IMP-7 metallo-beta-lactamase in Central Europe. J Clin Microbiol. 2011;49(1):474-475.
Crossref - Vamsi KS, Ramamoorthy S, Murali TS, Singh M, Kabra V. Utility of Carba NP test (Inhouse/RAPIDEC Commercial Kit) in the identification of Carbapenemase Producing Clinical Isolates in a Tertiary Care Hospital. J Clin Diagn Res.2019;13(12):19-22.
Crossref - Kanchanadevi P, Sekaran SC. Importance of EDTA in the detection of Metallo Beta-Lactamase from imipenem resistant gram-negative bacilli. Int J Curr Microbiol App Sci. 2016;5(11):702-706.
Crossref - Laishram S, Pragasam AK, Bakthavathchalam YD. An update on technical, interpretative and clinical relevance of antimicrobial synergy testing methodologies. Indian J Med Microbiol. 2017;35(4):445-468.
Crossref - Rahbar M, Mehragan H, Akbari AN. Prevalence of drug resistance in nonfermenter gram negative bacilli. Iran J Pathol. 2010;5:90-96.
- Sarkar M, Jenaj, Pattnaik D. Mallick.Prevalence of nonfermentative gram -negative bacilli and their antimicrobial susceptibility profiles in a tertiary care hospital of Eastern India. Int J Adv Med. 2018;5(2):366-370.
Crossref - Rit K, Nag F, Raj HK, Maity PK .Prevalence and susceptibility profiles of non fermentative gram-negative bacilli infection in a tertiary care hospital of Eastern India. Indian Journal of Clinical Practice. 2013;24(5):451-455.
- Al Samawi MS, Khan FY, EldeebY, et al. Acinetobacter infections among Adult patients in Qatar:A2-Year Hospital-Based study. Can J Infect Dis Med Microbiol. 2016;687-689.
Crossref - Veena M, Vadnal RS, Asha B, Vedalaveni J. Prevalence of multidrug resistant non-fermenters in a tertiary care center. Asian J Med Sci. 2022;13(10),176-182.
Crossref - Gandra S, Joshi J, Trett A, Lamkang AS, Laxminarayan R. Scoping report on antimicrobial resistance in India. Washington, DC: Center for Disease Dynamics, Economics and Policy. 2017.
- Bajpai V, Govindaswamy A, Khurana S, et al. Phenotypic genotypic profile of antimicrobial resistance in Pseudomonas species in hospitalized patients. Indian J Med Res. 2019;149(2):216-221.
Crossref - Verma N,Prahraj AK, Mishra Behera B,Gupta K. Detection of carbapenemase- producing Pseudomonas aeruginosa by phenotypic and genotypic methods in a tertiary care hospital of East India. J Lab Physicians. 2019;11(4):287-91.
Crossref - Gales AC, Seifert H, Gur D, Castanheira M, Jones RN, Sader HS. Antimicrobial susceptibility of Acinetobacter calcoaceticus-Acinetobacter baumannii complex and Stenotrophomonas maltophila clinical isolates:results from the SENTRY antimicrobial surveillance program (1997-2016). Open Forum Infect Dis. 2019;6(1):34-46.
Crossref - Kabir MH, Meunier D, Hopkins KL, Giske CG, Woodford N. A Two-centered evaluation of PAPIDIC CARBA NP for carbapenemase detection in Enterobacteriaceae,Pseudomonas aerugionosa and Acinetobacter spp. J Antimicrob Chemother. 2016;71(5):1212-1216.
Crossref - Sajjad A, Malik M, JavedI, Mushtaq S, Imran F, Jamil R. Detection of metallo beta lactamase production in imipenem resistant gram negative bacilli non fermenters isolated in a Tertiary Care Hospital. Professional Med J. 2019;26(12):2080-2084.
Crossref - Aarthi M, Subramanian S, Krishnan P. Colistin resistance among multidrug resistant gram-negative bacteria isolated from cancer patients from Chennai, South India. Int J Infect Dis. 2021;101(S1):39.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.