ISSN: 0973-7510
E-ISSN: 2581-690X
The effects of Candida sp. and Blastobotrys sp. starters on fermentation of cocoa beans were studied to determine the potential antibacterial activity of its extracts against foodborne pathogens. The fermentations of cacao beans using Candida sp. and Blastobotrys sp. were conducted for seven days. The observed parameters including pH and temperature monitoring during fermentation, detected active compounds, and in-vitro antibacterial activity against several foodborne pathogens at on sampling day 0, 3 and 7. Spontaneous fermentation (without starter culture added) was used as control. The pH during fermentation increased from pH 3.00 to 7.97, pH 3.00 to 7.68, and pH 3.00 to 7.54 for spontaneous, Candida sp. and Blastobotrys sp. fermentation respectively. The temperatures of fermentation ranged from 28oC to 33oC, 28oC to 32oC, and 28oC to 32oC for fermentation by spontaneous, Candida sp. and Blastobotrys sp., respectively. Gas Chromatography Mass Spectrometry (GC-MS) analyses showed several active compounds including caffeine, theobromine, gamma-tocopherol, stigmasterol and beta-sitosterols in all three fermentations. Caffeine content was the highest (74.59%) in control fermentation in earlier process. Theobromine content was higher for control fermentation compared to other Candida sp. and Blastobotrys sp. fermentation. Generally, gamma-tocopherol, stigmasterol and beta-sitosterols contents declined in the middle of the fermentation period but increased again towards the end. Fermented cocoa beans extract exhibited antibacterial activity against Bacillus cereus, Bacillus subtilis, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Salmonella enteritica, and Staphylococcus aureus. However, the extracts did not show any antibacterial activity against Listeria monocytogenes and Pseudomonas aeruginosa. In summary, the addition of starter cultures namely Candida sp. and Blastobotrys sp. in fermentation of cocoa beans able to trigger off the active compounds and show potent antibacterial activity against several foodborne pathogens.
Blastobotrys sp.; Candida sp.; Theobroma cacao L; cocoa beans; fermentation; foodborne pathogens.
In food industry, safety, quality, and taste are among the substantial criteria that were concerned most to produce excellent characteristic of food products apart from promising health-promoting foods. Unfortunately, foodborne pathogens are one of the potent roots of food contaminations and spoilage that cause countless foodborne outbreaks. In 2005, 1.8 million people reported died from diarrheal diseases largely due to consumption of contaminated food and drinking water (Newell et al., 2010). Nowadays numerous researches have proved that natural products especially plant sources are plenteous with preservative properties that could reduce the pathogens growth at the same time prolong the shelf life of the products (Calatayud et al., 2013).
Cocoa (Theobroma cacao L.) in Greek means ‘Food for God’ (Knight, 1999) which was first bred in 250-900 AD by the Mayans and Aztec in Mesoamerican regions (Knight, 1999). It is regarded as one of the precious food for preserving healthy life and longevity as well as other uses ranging from medicines to currency (Dillinger et al., 2000). Approximately 10% of the dry weight of the gross bean enriched with polyphenols contents making them one of the major contributors of antioxidants to the American diet after vegetables and fruits (Rusconi and Conti, 2010). Fermentation of the cocoa beans is a crucial step to discard the mucilaginous pulp and develop the flavor precursors of the chocolate (Fowler, 2009; Thompson et al., 2013). The microbial ecology in the fermentation system works wondrous and intricate at the same time. Recent research has found that some strains present in the microbial ecology of the fermentation could inhibit the growth of pathogens namely L. monocytogenes, E. coli and Salmonella sp. (Adimpong et al., 2010; Saltini et al., 2013). Fermentation of cocoa beans was reported to enhance the development of flavor, aroma and color precursors (Saltini et al., 2013). It was also reported to improve the rate and potential yield of cocoa sweating and shorten the fermentation duration (Dzogbefia et al., 1999). Yeast, lactic acid bacteria, and acetic acid bacteria are among the proclaimed microflora involved in the fermentation of the cocoa beans (Gálvez et al., 2007; Schwan and Wheals, 2010; Lima et al., 2011). Microflora such as Kloeckera apiculata and Saccharomyces cerevisiae var. chevalieri were reported to produce volatile compounds such as isopropyl acetate, ethyl acetate, 1-propanol, isoamyl alcohol, 2,3-butanediol, diethyl succinate and 2-phenylethanol during fermentation (Rodriquez-Campos et al., 2012). Hence, in this study the antibacterial activity of the fermented cocoa beans extracts using starter Candida sp. and Blastobotrys sp. starter cultures with regards to fermentation time was analyzed against several foodborne pathogens. Therefore, it is imperative to know the potential of the fermented cocoa beans extracts in impede growth of foodborne pathogens in order to exploit the resourcefulness of the natural products as food preservative.
Preparation of starter culture
Candida sp. and Blastobotrys sp. were acquired from Barry Callebout Services Asia Pacific Sdn. Bhd., Pahang, Malaysia. The cultures were cultivated in yeast peptone dextrose broth media for 24 hours at 30°C prior to fermentation process (Barry Callebout). The cultures were then transferred into molasses-yeast media and finally diluted in sterilized distilled water of ratio 1:9.
Sample preparation (cocoa beans)
Cocoa pods (Theobroma cacao L.) were purchased from cocoa plantation, Lembaga Koko Malaysia, Jengka, Pahang, Malaysia. Fresh cocoa pods were opened to obtain the beans for further fermentation process. Briefly, cocoa beans were piled into basket with drainage-holes to allow air and for fluids to flow out. Banana leaves were laid over the base of the baskets. Three separate heaps of cocoa beans were contrived for three different treatments namely spontaneous fermentation (control), fermentation with starter culture Candida sp. and lastly fermentation with starter culture Blastobotrys sp. The heaps were covered with banana leaves to content the heat in the system along with the observation of pH and temperature in the center of the heap. Samplings of cocoa beans were done at day 0, 3, and 7. Afterwards, the beans were oven dried at 50°C for 48 hours. Then the dried fermented cocoa beans were manually dehusked and grinded for further extraction process. Extractions of fermented cocoa beans were based on Rukayadi et al. (2008) with slight modification. 100 g of cocoa beans were soaked in 400 ml methanol (R&M Marketing, Essex, UK) for four days with occasionally shake. The solutions were filtered and evaporated to obtain crude extracts. The extracts were dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, Saint Louis, MO, USA) for further analysis.
Bacterial strains
Bacterial strains namely Bacillus cereus ATCC 21772, Bacillus subtilis ATCC 6633, Escherichia coli ATCC 25922, Klebsilla pneumoniae ATCC 13773, Listeria monocytogenes ATCC 15313, Proteus mirabilis ATCC 21100, Pseudomonas aeruginosa ATCC 27853, Salmonella enterica ATCC 13311, and Staphylococcus aureus ATCC 29737 were obtained from the American Type Culture Collection (Rockville, MD). Bacterial strains were growth in Mueller-Hilton broth (MHB) (Difco Becton Dickinson, Sparks, MD) at 37°C overnight prior to antibacterial-activity analysis.
In-vitro susceptibility test
Each extract was tested for antibacterial-activity using the disc-diffusion method based on Clinical and Laboratory Standard Institute (CLSI, 2012). The pathogenic microorganism is grown on Mueller-Hinton agar (MHA) in the presence of 10ìl of 10% extract impregnated onto disk of filter paper. Evidence of clear zone after 24 hours incubation surrounding the filter paper disk indicates as an indirect measure of the ability of that compound to inhibit the microorganism. Chlorhexidine was used as positive control. The plates were incubated at 37°C for 24 hours and observed for clear zone surrounding the disc.
GC-MS analysis
GC-MS analysis was carried out employing the following condition: column BP5MS with length 30.0 m × 0.25 mm in diameter and 0.25 µl film thickness. Helium was used as carrier gas with 37.1 kPa pressure, 19.8 ml/min total flow and 0.80 ml/min column flow. The linear velocity was 32.4 cm/sec with 3.0 ml/min purge flow. GC was performed in the splitless mode and the split ratio 20:1. The oven temperature was programmed from 50°C with and increased to 300°C at 3°C/min and held for 10 min. The injection temperature was 250°C and ion-source temperature was 200°C.
Temperature and pH
In this study, each crude extract from respective fermentations was observed for its antibacterial-activity. Cocoa beans were fermented for seven days. During the fermentation period the temperatures (Table 1) and pH (Table 2) were observed simultaneously for every 24 hours. Figure 1 illustrates the average fermentation temperature of cocoa beans while Figure 2 represent the average fermentation pH of cocoa beans. For spontaneous fermentation, the temperature increased gradually from 28oC to the highest at day four, which was 41.7oC. and started to decline at day 5 until the end of fermentation. Meanwhile, Blastobotrys sp. fermentation and Candida sp. fermentation have showed its highest temperature both at day three, 40.0oC and 43.0oC respectively. The temperature of all three fermentation systems exhibited a maximum value in the middle of the fermentation period and started to decline as the fermentation ends. The initial pH (3.0) of fresh cocoa pulps was similar to all three fermentations. Trend of increment in pH was observed as the fermentation days increase for Blastobotrys sp. fermentation. However, for spontaneous and Candida sp. fermentation a slight decreased in pH value (Table 2) was observed in day five. In all cases the pH increments were gradual again throughout the fermentation. At the end of the fermentation duration, all pH generally maintained at 7 for all three fermentations.
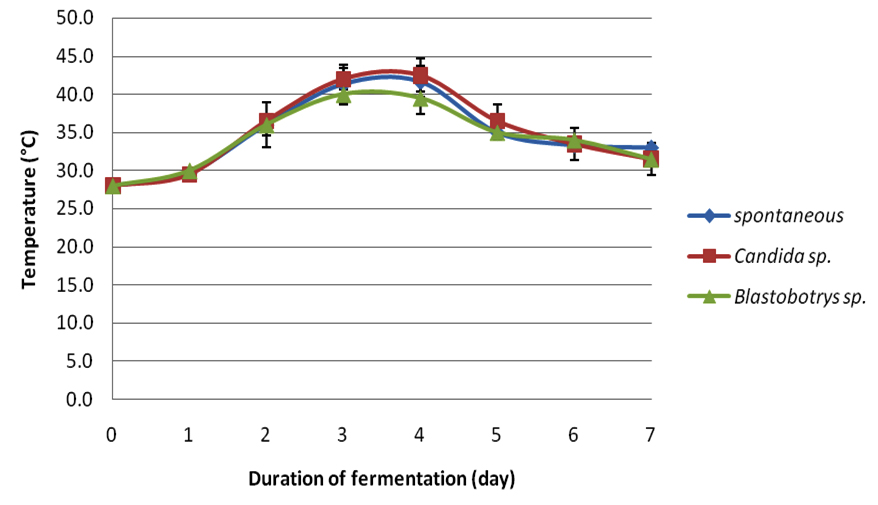 Fig. 1. Average fermentation temperature of cocoa beans
Fig. 1. Average fermentation temperature of cocoa beans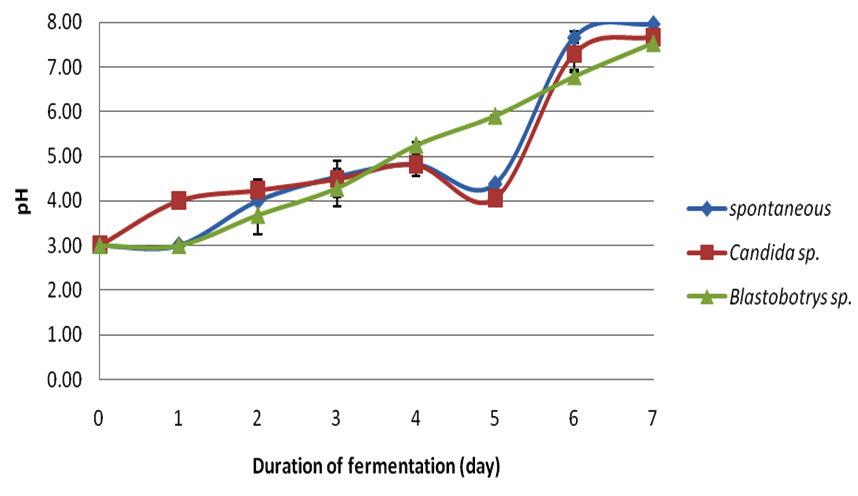 Fig. 2. Average fermentation pH of cocoa beans
Fig. 2. Average fermentation pH of cocoa beansTable (1):
Average fermentation temperature of cocoa beans .
Day |
Spontaneous (oC) |
Blastobotrys sp. (oC) |
Candida sp. (oC) |
|---|---|---|---|
0 |
28.8 |
28.0 |
28.0 |
1 |
29.7 |
30.0 |
29.5 |
2 |
36.0 |
36.0 |
35.5 |
3 |
41.3 |
40.0 |
43.0 |
4 |
41.7 |
39.5 |
42.5 |
5 |
35.0 |
35.0 |
42.5 |
6 |
33.3 |
34.0 |
36.5 |
7 |
33.0 |
31.5 |
33.5 |
Table (2):
Average fermentation pH of cocoa beans.
Day |
Spontaneous (oC) |
Blastobotrys sp. (oC) |
Candida sp. (oC) |
|---|---|---|---|
0 |
3.00 |
3.00 |
3.00 |
1 |
3.00 |
3.00 |
4.00 |
2 |
4.00 |
3.68 |
4.24 |
3 |
4.54 |
4.29 |
4.49 |
4 |
4.82 |
5.25 |
4.81 |
5 |
4.38 |
5.91 |
4.06 |
6 |
7.66 |
6.79 |
7.29 |
7 |
7.97 |
7.54 |
7.68 |
GC-MS analysis
GC-MS analysis from the crude extracts yielded five compounds that were detected (Table 3), namely caffeine, theobromine, gamma-tocopherol, stigmasterol and beta-sitosterol. Among these compounds detected, caffeine and theobromine were the two compounds that showed the highest percentage in early fermentation process. In general, caffeine and theobromine content (in percentage) decreased as the fermentation continues to day three but rose again when it reached day seven for all three fermentations. Spontaneous fermentation showed the highest percentage of caffeine and theobromine with 74.59% and 37.71% at early fermentation period respectively. However, in Candida sp. fermentation theobromine was not detected as the fermentation end. Stigmasterol and beta-sitosterol were another two major compounds markers in the GC-MS analysis in this study. The percentage of these two compounds implies that they were reduced at day three and regained the concentration as the fermentation reached day seven. These phytosterols showed the same guise for all three fermentations along the seven fermentation days. Gamma-tocopherol content in the spontaneous-fermentation was not detected in the early spontaneous fermentation but emerged at the end of fermentation. Meanwhile, for other two fermentations (Candida sp. and Blastobotrys sp. fermentation) the presences of gamma-tocopherol were at moderate concentration at early fermentation until day three and eventually regained the concentration as the fermentation day continues.
Table (3):
Percentage of targeted active compounds in fermented cocoa beans.
| Targeted active compounds (%) | Control (Spontaneous-fermentation) | Blastobotrys sp. fermentation | Candida sp. fermentation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day3 | Day 7 | Day 0 | Day 3 | Day 7 | Day 0 | Day 3 | Day 7 | ||||
| Caffeine | 74.59 | 18.26 | 38.45 | 46.64 | 20.26 | 30.59 | 44.15 | 18.23 | 54.04 | |||
| Theobromine | 37.71 | 17.59 | 26.26 | 35.47 | 19.22 | 25.66 | 34.08 | 16.53 | ND | |||
| γ-tocopherol | ND | ND | 3.63 | 0.95 | 0.43 | 1.29 | 1.11 | 0.88 | 5.38 | |||
| Stigmasterol | 0.51 | 0.16 | 1.46 | 0.69 | 0.28 | 0.70 | 0.58 | 0.49 | 1.64 | |||
| β-sitosterol | 1.07 | 0.22 | 1.74 | 1.31 | 0.41 | 1.17 | 0.95 | 0.64 | 3.04 | |||
In-vitro susceptibility test
This study was carried out to evaluate the antibacterial properties of the crude cocoa beans extracts by means of Kirby-Bauer disc diffusion susceptibility test. Diameter of the clear zones (in mm including disk diameter) of the crude extracts (Table 4) indicates the inhibition growth against several foodborne pathogens for crude extracts from three fermentations. The results showed that crude extracts for all three fermentations have higher inhibition zones in early fermentation against B. cereus, B. subtilis, E. coli, K. pneumoniae, and S. aureus but the inhibition became weaker as the fermentations continued. On the other hand, L. monocytogenes and P. aeruginosa appeared to have hight resistance to the extracts of which no antibacterial activity were observed on the early fermentation period for all three fermentations and generally implied modest inhibition at day three and seven of fermentation. Concurrently, the crude extracts from the spontaneous fermentation of sampling day three and seven appeared to be resistant against S. enterica. The inhibition activity against S. enterica by the crude extracts of Blastobotrys sp. and Candida sp. fermentations at early fermentation executed potent inhibitory however it diminished as fermentation ends. This inclination was collateral for all extracts against S. aureus.
Table (4):
Average diameter of inhibition zone (in mm) of fermented cocoa bean extracts.
| Extract Bacteria | Spontaneous Fermentation (mm) | Candida sp. fermentation (mm) | Blastobotrys sp. fermentation (mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 7 | Day 0 | Day 3 | Day 7 | Day 0 | Day 3 | Day 7 | ||
| Bacillus cereus | 13.0 | 10.0 | 9.5 | 12.5 | 9.0 | 8.0 | 12.5 | 8.0 | 8.0 | |
| Bacillus subtilis | 12.5 | 10.0 | 10.0 | 12.0 | 10.0 | 10.0 | 12.5 | 9.5 | 9.5 | |
| Escherichia coli | 12.0 | 8.0 | 7.5 | 11.5 | 8.5 | 8.0 | 11.0 | 10.0 | 8.0 | |
| Klebsiella pneumoniae | 12.5 | 8.5 | 8.5 | 13.0 | 9.5 | 9.0 | 13.0 | 8.5 | 8.5 | |
| Listeria monocytogenes | NA | 7.0 | 7.0 | NA | 7.0 | 7.0 | NA | 7.0 | 7.0 | |
| Pseudomonas aeruginosa | NA | 6.5 | 6.5 | NA | 6.5 | 6.5 | NA | 7.0 | 7.0 | |
| Salmonella enterica | 10.0 | NA | NA | 10.0 | 7.5 | 7.0 | 13.0 | 8.5 | 8.5 | |
| Staphylococcus aureus | 12.0 | 7.0 | 7.0 | 12.0 | 7.0 | 7.0 | 12.0 | 7.0 | 7.0 | |
NA – no antibacterial activity.
The compelling inhibitory activity of the extracts of fermented cocoa beans fermented with Blastobotrys sp. and Candida sp. as starter cultures have yet to unleash its competency. The fresh cocoa pulps were heaped in the present of yeast (Blastobotrys sp. and Candida sp.) for seven days. Samplings were taken at day zero, three and seven. Sampling populations were oven-dried as to achieve even drying throughout the process. Afterwards, the dry cocoa beans were dehusked and grinded for further analysis. During the fermentation, the measurement of temperature and pH were taken simultaneously every 24 hours. The temperature of the fermented cocoa beans for all three fermentations started approximately at 28.0°C. In the spontaneous fermentation, the temperature increased gradually until it reached its highest at day four (41.7°C) and moderately decreased as the fermentation reached the end. Meanwhile for the Blastobotry sp. and Candida sp. fermentation reached its maximum temperature both at day three which was 40.0°C and 43.0°C respectively. The increase in the temperature was also regularly reported in other cocoa fermentations (Schwan and Wheels, 2004; Jesperson et al., 2005; Papalexandratau et al., 2011; Ouattara et al., 2014). The rise in temperature was a result of exothermic reaction by alcoholic fermentation of yeast to produce ethanol and carbon dioxide (Gálvez et al., 2007) and appearance of lactic acid bacteria and acetic acid bacteria (Schwan and Wheals, 2004). At the same time aerobic spore-forming bacteria starting to grow once the acid bacteria decreases (Schwan and Wheals, 2004). The rising of temperature is also important in cocoa fermentation in order to kill the beans (Gálvez et al., 2007). During our fermentation monitoring, the highest temperatures ranged from 40.0°C to 43.0°C. This temperature range parallel with study by Ouattara et al., (2008). However, the temperature started to decline as the fermentation days increased plausibly due to degeneration of microbial activity is starting to take place (Thompson et al., 2001; Schwan and Wheals, 2004; Ouattara et al., 2014). This trend conformed for other two fermentations. The initial pH (3.0) of fresh cocoa pulps was similar to all three fermentations basically due to the high concentration of citric acid (Nielsen et al., 2006). This condition is favorable for yeast to grow due to high sugar content and its pH (Thompson et al., 2001; Nielsen et al., 2006). Study by Ouattara et al. (2008) reported the fluctuation of pH in their fermentation ranged from 3.5 to 5.5. This is also corresponding to study by Ganeswari et al. (2014) where the pH begins at 4. This low pH value was known to be contributed by the presence of citric acid. However, a slight pH value decreased was detected for spontaneous and Candida sp. fermentations at day five (Table 2). This changes might be consequently due to the difference in microflora activity of the microbial community in the system since the mass size of the cocoa beans may influence the fermentation process subsequently differ in their metabolic vitality (Schwan and Wheals, 2004; Saltini et al., 2013). Similar trend in pH changes were reported in studies by Sanchez et al. (1984), Dzogbefia et al. (1999) and Ouattara et al. (2014).
The GC-MS analysis was conducted to detect the percentage-content of available compounds during the fermentation systems of different starter cultures. Numerous studies have reported several significant bioactive groups in the cocoa and cocoa products including polyphenols (epicatechin, catechin and procyanidin) (Afoakwa et al., 2008; Arlorio et al., 2008; Ariza et al., 2014; Nsor-Atindana et al., 2012; Hii et al., 2009; Kim et al., 2014), methylxanthines (theobromine, caffein and theophylline) (Caudle et al., 2001; Mhd-Jalil and Ismail, 2008), peptides (albumins, globulins, prolamin, and glutenin) (Voight et al., 1993; Mhd-Jalil and Ismail, 2008) lipids and sterols (Schwan and Wheals, 2004; Lim, 2012), fiber (Paoletti et al., 2012) as well as minerals (magnesium, copper and selenium). In this study, five targeted active compounds (Table 3) were detected in all three fermentation systems; caffeine, theobromine, gamma-tocopherol, stigmasterol and beta-sitosterol. In this findings, caffeine and theobromine were the major compounds in cocoa as supported by Maleyki and Ismail (2008). The percentage detected-content of these compounds decreased in the middle of the fermentation however it increased back as the fermentation ends. Nevertheless, in Candida sp. fermentation the undetectable content of theobromine was still vague and unclear. Study by Adamafio et al. (2011) and Bentil et al. (2015) reported that the usage of fungi (Aspergillus niger) able to bio-detheobrominate the concentration of theobromine in cocoa waste to be used as animal feed. In the study by Adamafio et al. (2013) other yeast used was identified as Candida krusei as the bio-detheobrominator. This perhaps elucidates the non-traceable of theobromine in Candida sp. fermentation. However, based on Adamafio et al. (2011), this method is not feasible in conventional agricultural practices and therefore not applied by local farmers. Cocoa beans are prosperous with plant sterols including beta-sitosterols and stigmasterols specifically in cocoa butter (Steinberg et al., 2003). Fedeli et al. (1966) reported that beta-sitosterols and stigmasterols have been identified in the cocoa butter (Lim, 2012). These two are phytosterols that occur naturally in plant which able to diminish the intestinal absorption of diet and biliary cholesterols (Fernandes and Cabral, 2007; Lengyel et al., 2012; Botelho et al., 2014). These sterols also effective in lowering circulating cholesterol levels (Thompson et al., 2005). Stigmasterol and beta-sitrosterol were numerously reported in previous cocoa beans studies; nonetheless no study has reported the exact amount or values for these plant sterols. Study by Singh et al. (2012), reported that several plant sterols from Withania somnifera, Euphorbia hirta and Terminalia chebula were tested against pathogens and found that it is susceptible against E. coli and Enterobacter aerogens (Singh et al., 2012). The fluctuations of both sterols in all three fermentations are still unclear thus study needed to be conducted further on this matter. On the other hand, gamma-tocopherol was not detected in the spontaneous fermentation. Plausible explanation on this matter could be because of the absence of yeast starter culture in the control fermentation. This is conformed to the study of antioxidant properties in fermented soybean by Hubert et al. (2008), where the tocopherol content in the fermented soybean experienced some losses compared to the unfermented soybeans. Study by Erickson et al. (1973), cocoa butter was rich with vitamin E such as alpha-tocopherol, beta-tocopherol, and gamma-tocopherol (Jahurul et al., 2013). Cocoa butter is one of the important ingredients in producing chocolate (Zyzelewicz et al., 2014). Based on the result, generally the additional of starter cultures have improved the content of bioactive compounds in the cocoa beans fermentation.
The antibacterial potentialities of the crude extracts from all three fermentations were screened using the method of Kirby-Bauer disc diffusion susceptibility test (Das et al., 2010). Based on the result (Table 4), B. cereus and B. subtilis were susceptible to all crude extracts of different sampling days from all three fermentations. The diameter of clear zones was generally higher for some extracts in the early fermentation process and eventually reduced to the end of fermentation. The inhibition potential reduced perhaps due to the excessive appearance of Bacilli at later stage of the fermentation suppressing the antibacterial activity of the extracts (Ardhana and Fleet, 2003; Papalexandratou et al., 2011). However, Lima et al. (2011) have mentioned that the role of Bacillus sp. in fermentation system is still unclear although some report suggested that they contribute to the yield of pectinolytic enzymes (Ouattara et al., 2008). The same trend goes to inhibition against E. coli, K. pneumoniae, and S. aureus where inhibition was greater at early fermentation stage of which microbial metabolic activity is about to begin. Research by Smullen et al. (2007) mentioned that the unfermented cocoa extracts have displayed a greater antibacterial activity than fermented cocoa extract against Streptococcus mutans Ingbritt. This could be the plausible reason of the antibacterial potential where the fermented beans consist lesser low molecular weight phenolics compared to the unfermented cocoa beans (Smullen et al., 2007). Howbeit, the resistance of L. monocytogenes and P. aeruginosa against all crude extracts were observed at early fermentations though some inhibition were marked at the end of fermentations. Calatayud et al. (2013) have reported that cocoa extract in the biofilm displayed bactericidal effects against L. monocytogenes whereby higher concentration of cocoa extract is required in food-application compared to broth media due to the food matrix. Similar study conducted also showed that cacao pulp crude extract was inactive against P. aeruginosa ATCC 27853 (Panganiban et al., 2012). Several reports have also emphasized the potential antibacterial activity of cocoa extracts that perhaps due to high concentration of flavonoids (Cushnie et al., 2005). Nevertheless, further study needs to be implemented as to discover the mechanism of bactericidal of the crude fermented cocoa extracts and compound/s that are responsible in the inhibition against foodborne pathogens.
The pHs of cocoa beans during fermentation ranged from pH 3.00 to 7.97 and its temperature started at 28oC and ended at 33oC. pH and temperature of these fermentations showed no outstanding distinction between each other. Caffeine and theobromine content decreased in spontaneous fermentation, Blastobotrys sp. and Candida sp. fermentation, but caffeine increased in Candida sp. fermentation. Gamma-tocopherol, stigmasterol and beta-sitosterol increased along fermentation process. Fermentation process is suggested to be less than seven days as caffeine content could be increased. Fermentation of cocoa beans in additional of starter cultures Candida sp. and Blastobotrys sp. have showed potential bactericidal effect compared to the control fermentation.
ACKNOWLEDGMENTS
This work was supported by UPM Grand Putra – IPS to Yaya Rukayadi with project number GP-IPS/2014/9446800
- Adamafio, N.A., Ayombil, F., Tano-Debrah, K. Microbial detheobromination of cocoa (Theobroma cacao) pod husk. Asian J Biochem, 2011; 6: 200-207.
- Adamafio, N.A., Kolawole, O.M., Oduro-Mensah, D. Theobromine-degrading potential of yeast strain isolated from tomato (Lycopersicon esculentum) fruit. Int J Biol Chem, 2012; 6: 103-112.
- Adamafio, N.A, Kyeremeh, K., Matey, A.T., Mingle, C., Kolawole, O.M. In-situ degradation of cocoa (Theobroma cacao) pod husk theobromine by Candida Krusei. Int J Biotech Biochem, 2013; 9: 135-144.
- Adimpong, B.D., Nielsen, D.S., Sørensen, K.I., Derkx, P.M.F., Jeperson. Genotypic and technological characterization of lactic acid bacteria from indigenous fermented African foods. In FOODS Denmark PHd congress 2010: Functional foods and sustainable food production. FOOD Denmark Research School, Copenhagen, pp 28.
- Afoakwa, E.O., Paterson, A., Fowler, M., Ryan, A. Flavor formation and character in cocoa and chocolate: A critical review. Food Sci Nutr, 2008; 48: 840-857.
- Ardhana, M., Fleet, G. The microbial ecology of cocoa bean fermentations in Indonesia. Int J Food Microbiol, 2003; 86: 87-99.
- Ariza, B.T.S., Mufida, D.S., Fatima, N.N., Hendrayati, T.I., Wahyudi, T., Misnawi. In-vitro antibacterial activity of cocoa ethanolic extract against Escherichia coli. Int Food Res J, 2008; 21: 935-940.
- Arlorio, M., Locatelli, M., Travaglia, F., Coisson, J.D., Grosso, E.D., Minassi, A., Appendino, G., Martelli, A. Roasting impact on the content of clovamide (N-caffeoyl-L-DOPA) and the antioxidant activity of cocoa beans (Theobroma cacao L.). Food Chem, 2008; 106: 967-975.
- Bentil, J.A., Dzogbefia, V.P., Alemawor, F. Enhancement of the nutritive value of cocoa (Theobroma cacao) bean shells for use as feed for animals through a two-stage solid state fermentation with Pleurotus ostreatus and Aspergillus niger. Int J Appl Microbiol Biotechnol Res, 2015; 3: 20-30.
- Botelho, P.B., Galasso, M., Dias, V., Mandriolo, M., Lobato, L.P., Rodriguez-Estrada, M.T., Castro, I.A. Oxidative stability of functional phytosterol-enriched dark chocolate. Food Sci Technol, 2014; 55: 444-451.
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods – A review. Int J Food Microbiol, 2004; 94: 223-253.
- Calatayud, M., López-de-Dicastillo, C., López-Carballo, G., Vélez, D., Muñoz, P., Gavara, R. Active films based on cocoa extract with antioxidant, antimicrobial and biological applications. Food Chem, 2013; 139: 51-58.
- Caudle, A.G., Gu, Y., Bell, L.N. Improved analysis of theobromine and caffeine in chocolate food products formulated with cocoa powder. Food Res Int, 2001; 34: 599-603.
- Cushnie, T.P., Lamb, A.J. Antimicrobial activity of flavonoids. Int J Antimicrob Agents, 2005; 26: 343-356.
- Das, K., Tiwari, R.K.S., Shrivastava, D.K. Techniques for evaluation of medicinal plant products as antimicrobial agent: Current methods and future trends. J Med Plants Res, 2010; 4: 104-111.
- Dillinger, T.L., Baringa, P., Escarcega, S., Jimenez, M., Salazar Lowe, D., Grivetti, L.E. Food of the gods: cure for humanity? A cultural history of the medicinal and ritual use of chocolate. J Nutr, 2000; 130: 2057S-2072S.
- Dzogbefia, V.P., Buamah, R., Oldman, J.H. The controlled fermentation of cocoa (Theobroma cacao L.) using yeasts: Enzymatic process and associated physico-chemical changes in cocoa sweatings. Food Biotechnol, 1999; 13: 1-12.
- Erickson, J.A., Weissberger, W., Keeney, P.G. Tocopherols in the unsaponifiable fraction of cocoa lipids. J Food Sci, 1973; 38: 1158-1161.
- Fedeli, E., Lanzani, A., Capella, P., Jacini, G. Triterpene alcohols and sterols of vegetable oils. J Am Oil Chem Soc, 1966; 43: 254-256.
- Fernandes, P., Cabral, J.M.S. Phytosterols: Applications and recovery methods. Bioresour Technol, 2007; 98: 2335-2350.
- Fowler, M.S. Cocoa beans: from tree to factory, In: Beckett, S.T (ed) Industrial chocolate manufacture and use, 4th ed. Blackwell Science, Oxford, 2009; pp. 10-47
- Gálvez, S.L., Loiseau, G., Paredes, J.L., Barel, M., Guiraud, J.P. Study of the microflora and biochemistry of cocoa fermentation in the Dominican Republic. Int J Food Microbiol, 2007; 114: 124-130.
- Ganeswari, I., Khairul Bariah, S., Amizi, M.A., Sim, K.Y. Effects of different fermentation approaches on the microbiological and physicochemical changes during cocoa bean fermentation. Int Food Res J, 2014; 22: 70-76.
- Hii, C.L., Law, C.L., Suzannah, S., Misnawi, Cloke, M. Polyphenols in cocoa (Theobroma cacao L.). Asian J Food Agro-Industry, 2009; 2: 702-722.
- Hubert, J., Berger, M., Nepveu, F., Paul, F., Daydé, J. Effects of fermentation on the phytochemical composition and antioxidant properties of soy germ. Food Chem, 2008; 109: 709-721.
- Jahurul, M.H.A., Zaidul, I.S.M., Norulaini, N.A.N., Sahena, F., Jinap, S., Azmir, J., Sharif, K.M., Mohd Omar, A.K. Cocoa butter fats and possibilities of substitutions in food products concerning cocoa varieties, alternatives sources, extraction methods, composition, and characteristics. J Food Eng, 2013; 117: 467-476.
- Jesperson, L., Nielsen, D.S., Hønholt, S., Jakobsen, M. Occurrence and diversity of yeasts involved in fermentation of West African cocoa beans. FEMS Yeast Res, 2005; 5: 441-453.
- Kim, J.Y., Kim, J.Y., Shim, J.S., Lee, C.Y., Lee, K.W., Lee, H.J. Cacao phytochemical: Recent advances in molecular mechanisms on health. Critical Review. Food Sci Nutr, 2014; 54: 1458-1472.
- Knight, I. Chocolate and cocoa. Health and Nutrition (1st ed.) Blackwell Science, Oxford, UK, 1999.
- Lengyel, J., Rimarcik, J., Vaganek, A., Fedor, J., Lukes, V., Klein, E. Oxidation of sterols: energetics of C-H and O-H bond cleavage. Food Chem, 2012; 133: 1435-1440.
- Lima, L.J.R., Helena Almeida, M., Rob Nout, M.J., Zwietering, M.H. Theobroma cacao L., “The Foods of Gods”: Quality determinants of commercial cocoa beans, with particulars reference to the impact of fermentation. Critical Review. Food Sci Nutr, 2011; 51: 731-761.
- Lim, T.K. Edible Medicinal and Non-medicinal Plants. Volume 3, Springer, Netherlands, 2012.
- Maleyki, M.J.A., Ismail, A. Antioxidant properties of cocoa powder. J Food Biochem, 2008; 34:111-128.
- Mhd-Jalil, A.M., Ismail, A. Polyphenols in cacao and cocoa products: Is there a link between antioxidant properties and health? Molecules, 2008; 13: 2190-2219.
- Newell, D.G., Koopmans, M., Verhoef, L., Duizer, E., Aidara-Kane, A., Sprong, H., Opsteegh, M., Langelaar, M., Threfall, J., Scheutz, F., Giessen, J.V.D., Kruse, H. Food-borne diseases – The challenges of 20 years ago still persist while new ones continue to emerge. Int J Food Microbiol, 2010; 139: 3-15.
- Nielsen, D.S. The microbiology of Ghananian cocoa fermentations. Dissertation, University of Copenhagen, 2006.
- Nsor-Atindana, J., Zhong, F., Mothibe, K.J., Bangoura, M.L., Lagnika, C. Quantification of total polyphenolic content and antimicrobial activity of cocoa (Theobroma cacao L.) bean shells. Pak J Nutr, 2012; 11: 574-579.
- Ouattara, D.H., Ouattara, H.G., Goualie, B.G., Kouame, L.M., Niamke, S.L. Biochemical and functional properties of lactic acid bacteria isolated from Ivorian cocoa fermenting beans. J Appl Biosci, 2014; 77: 6489-6499.
- Ouattara, H.G., Koffi, B.L., Karau, G.T., Sangaré, A., Niamke, S.L., Diopoh, J.K. Implication of Bacillus sp. in the production of pectinolytic enzymes during cocoa fermentation. World J Microbiol Biotechnol, 2008; 24: 1753-1760.
- Panganiban, C.A., Reyes, R.B., Agojo, I., Armedilla, R., Consul, J.Z., Dagli, H.F., Esteban, L. Antibacterial activity of cacao (Theobroma cacao L.) pulp crude extract against selected bacterial isolates. Int J Sci Clin Lab, 2012; 1: 32-44.
- Paoletti, R., Andrea, P., Conti, A., Visioli, F. Chocolate and Health. In Colombo, M. L., Pinorini-Godly, M. T. And Paoletti, R (eds.) Botany and Pharmacognosy of Cacao Tree Springer-Verlag, Italia, 2012; pp. 41-63.
- Papalexandratou, Z., Vranckena, G., De Bruyne, K., Vandamme, P., De Vuyst, L. Spontaneous organic cocoa bean box fermentation in Brazil are characterized by a restricted species diversity of lactic acid bacteria and acetic acid bacteria. Food Microbiol, 2011; 28: 1326-1338.
- Papalexandratou, Z., Camu, N., Falony, G., Vuyst, L.D. Comparison of the bacterial species diversity of spontaneous cocoa bean fermentations carried out at selected farms in Ivory Coast and Brazil. Food Microbiol, 2011; 28: 964-973.
- Rukayadi, Y., Shim, J.S., Hwang, J.K. Screening of Thai medicinal plants for anticandidal activity. Mycoses, 2008; 51: 308-12.
- Rusconi, M., Conti, A. Theobroma cacao L., the Food of Gods: A scientific approach beyond myths and claims. Pharmacol Res, 2010; 61: 5-13.
- Saltini, R., Akkerman, R., Frosch, S. Optimizing chocolate production through traceability: A review of the influence of farming practices on cocoa bean quality. Food Control, 2013; 29: 167-187.
- Sanchez, J., Guiraud, J.P., Galzy, P. A study of the polygalacturonase activity of several yeast strains isolated from cocoa. Appl Microbiol Biotechnol, 1984; 20: 262-267.
- Schwan, R.F., Wheals, A.E. The microbiology of cocoa fermentation and its role in chocolate quality. Critical Review. Food Sci Nutr, 2004; 44: 205-221.
- Singh, G., Kumar, P., Jindal, K. Antibacterial potential of sterols of some medicinal plants. Int J Pharm Sci, 2012; 4: 159-162.
- Smullen, J., Koutsou, G.A., Foster, H.A., Zumbé, A., Storey, D.M. The antibacterial activity of plant extracts contaning polyphenols against Streptococcus mutans. Caries Res, 2007; 41: 342-349.
- Steinberg, F.M., Bearden, M.M., Keen, C.L. Cocoa and chocolate flavonoids: Implications for cardiovascular health. J Am Dietetic Assoc, 2003; 2: 215-223.
- Thompson, G.R., Grundy, S.M. History and development of plant sterol and stanol esters for cholesterol-lowering purposes. Am J Cardiol, 2005; 96: 3-9.
- Thompson, S.S., Miller, K.B., Lopez, A., Camu, N. Cocoa and coffee, In: Doyle, M.P., Buchanan, R.L. (Eds.), Food Microbiology: Fundamentals and Frontiers, 4th ed. ASM Press,Washington, DC, 2013; pp. 881–889.
- Voigt, S., Biehl, B., Syed Wazir, S.K. The major seed proteins of Theobroma cacao L. Food Chem, 1993; 47: 145-151.
- Zyzelewicz, D., Krysiak, W., Budryn, G., Oracz, J., Nebesny, E. Tocopherols in cocoa butter obtained from cocoa bean roasted in different forms and under various process parameters. Food Res Int, 2014; 63: 390-399.
© The Author(s) 2016. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


