ISSN: 0973-7510
E-ISSN: 2581-690X
Drought stress is the primary constraint on plant-based food production, particularly paddy production. Several studies have examined plant resistance to heat and osmotic pressure. This study aimed to isolate bacteria with plant growth-promoting properties that could tolerate high temperatures and improve paddy growth during drought. Five isolates with a high optical density value (OD600) at 30% PEG 6000 (equivalent to -1.03MPa) and able to grow at high temperatures were identified based on the 16S rRNA gene sequences as Achromobacter spanius UKM UR10, Bacillus pumillus UKM UR11, Bacillus cereus UKM R66, and Bacillus altitudinis UKM RB11, which were isolated from the root, where as Bacillus sp. UKM S8 was isolated from rhizosphere soil. These isolates exhibited 1-amino cyclopropane-1-carboxylate (ACC) deaminase activities ranging between 1.01 and 1.12 mmol α-ketobutyrate mg-1 protein h-1, which degraded ACC to α-ketobutyrate and ammonia. Other plant growth promoters assessed include indole acetic acid (IAA; concentration between 9.69 and 13.15µg/ml) and phosphate solubilization (concentrations between 31.74 and 51.30 mg/l) production. Subsequently, the selected plant growth-promoting rhizobacteria (PGPR) were incorporated as a consortium and inoculated on paddy seeds, thus increasing total chlorophyll, proline, and soluble sugar content in paddy subjected to drought-stress conditions. Paddy yield components and performances, such as panicle number, spikelet number, dry grain weight, number of leaves, stalk length, and root length increased significantly. This isolated PGPR exhibits heat resistance, promotes plant growth, and can serve as an inoculant for paddy plants under drought conditions.
Plant Growth Promoter, Drought Stress, ACC Deaminase Enzyme, Phosphate-solubilizing Bacteria
Paddy (Oryza sativa) fields require consistent irrigation system to contribute to high-yield performance. Drought is where water sources are limited due to low rainfall and high temperatures. Water supply depends on rainfall in paddy-growing areas without irrigation infrastructure (e.g., upland paddy). Climate change leads to abiotic stresses, such as drought, which affect the availability and transport of nutrients into the soil. When water supply is inadequate, the nutrient uptake of paddy is reduced. Global climate change projections for the Southeast Asian subregion indicate that annual temperatures will rise by approximately 0.4°C to 1.3°C by 2030.1 The Malaysian Meteorological Department (MET) indicated that the temperature in some peninsula regions ranged from 34°C to 36°C in 2019. Farmers in Malaysia, particularly in Kelantan, are concerned about the unpredictability of the weather because they are experiencing water supply issues to irrigate paddy fields. The current water supply in Kelantan (the peninsula’s east coast) is insufficient to irrigate 24, 374 hectares of paddy cultivation.
PGPRs are microbes that have several beneficial effects on plant growth and can minimize abiotic stress on plants. Carmen et al.2 indicate that PGPR and some rhizobacterial strains alleviate biotic and abiotic stress in several plant species. Beneficial microbes in the rhizosphere stimulate plant growth and development through various direct and indirect mechanisms. The biological activity of ACC deaminase (ACCd), indole acetate acid production, nitrogen fixation, phosphate solubilization, and phytohormone production are some of the important mechanisms by which PGPR protects plants from diseases and abiotic stresses.
ACC deaminase cleaves the plant precursor ethylene, ACC, into ammonia and α-ketobutyrate.3 Ethylene is an important moderator of plant growth and healthy plant development and a critical feature in plant responses to various stresses.4 By reducing ACC levels in plants, ACC-producing bacteria can lower plant ethylene levels.5
PGPR acts as a plant growth promoter, including biological nitrogen fixation (BNF). These bacteria facilitate the conversion of atmospheric nitrogen (N2) to ammonia (NH3) via the enzyme nitrogenase.6 Nitrogen-fixing bacteria are essential for the nitrogen source needed for plant growth and production. Many researchers have screened and selected novel and effective PGPR to be included in established agroecological integrated cropping practices.7 Phosphorus (P) is the second most crucial element for plant growth after nitrogen (N). More than 80% of P becomes immobile and cannot be used by plants due to the absorption, precipitation, or conversion of P to organic forms.8 Moreover, researchers have also observed that many P-soluble microorganisms (PSMs), particularly bacteria, fungi, and actinomycetes, reside in the plant rhizosphere and play an essential role in a bound phosphate solution that makes them available to plants.9 Production of plant phytohormones such as auxin, is an important feature of PGPR to enhance plant growth. The most active auxin for plant growth and development is IAA. Various plant species inoculated with IAA-producing bacteria promote root growth and formation of lateral and hairy roots besides increasing water and nutrients uptake.10
Isolated PGPRs in this study showed plant-promoting abilities to survive at elevated temperatures. PGPRs with high-temperature resistance may benefit plant survival and growth in soils under temperature stress. The ability of PGPR to perform biological activities at high temperatures is a distinguishing feature of inoculants suitable for use in paddy crops under drought conditions. As a result, PGPR has been offered as an alternative source to help paddy crops cope with drought by increasing soil nutrient uptake. This study aimed to isolate PGPRs that could tolerate high temperatures and drought and determine their ability to improve paddy production under drought conditions. In addition, the differences in inoculated and non-inoculated paddy yield components and performance were also monitored under drought conditions compared to non-stress conditions. As the drought continues, this study developed alternative methods to introduce plant growth-promoting rhizobacteria (PGPR) as a biofertilizer inoculant.
Collecting samples and isolating PGPR
The bacterial strain was isolated from paddy rhizosphere in two locations: in Alor Setar, Kedah (5°57’24.7″N, 100°22’03.2″E) and upland paddy areas in Bau, Sarawak (1°292 11.63 N, 110°002 55.33 E). The soil and paddy samples were stored at 10°C in a cold room. Paddy roots were thoroughly rinsed under running tap water to remove adhering soil. Approximately 10 g of soil and cleaned paddy roots were added to 90 ml of sterile distilled water, after which samples were shaken for a few minutes to homogenize them. Each sample underwent serial dilution of 10-7 and 50µl of each dilution was pipetted onto a nutrient agar plate (NA). After cultivating at an optimum temperature of 37°C11 for 24 hours, a single colony was selected and streaked onto new sterile NA plates to grow at 50°C. Only isolates that can grow at 50°C were selected. The pure cultures were then immersed in 30% glycerol and stored at 80°C as pure culture stock.
Identification of the isolates
The selected PGPR is amplified through the colony-PCR method. Genomic DNA samples for each PGPR were taken directly from pure cultures on the LB agar plates. Amplification of the 16S ribosomal DNA gene (16S rDNA) on the PGPR bacterial genome DNA was performed using forward primer 27f (5′ AGAGTTTGATCMTGGCTCAG 3′) and reverse primer 1492r (5′ GGTTACCTTGTTACGACTT 3′). The 16S ribosomal DNA gene (16S rDNA) was amplified using DreamTaq Polymerase (Thermo Fisher Scientific, U.S.). The obtained PCR products were then analyzed through 1% agarose gel electrophoresis. Sequencing was performed to obtain nucleotide sequence data encoding the 16S ribosomal subunit for use in Basic Local Alignment Search Tool (nucleotide) analysis, BLASTN, at the National Centre for Biotechnology Information, NCBI (https://www.ncbi.nlm.nih.gov/Blast.cgi) to identify the isolated PGPR species. All the 16S rRNA sequence data have been deposited into the NCBI GenBank database under the following accession numbers: Achromobacter spaniusUKM UR10 (MZ221547 ), Bacillus pumillus UKM UR11 (MZ221209 ), Bacillus sp. UKM S8 (MZ221185), Bacillus cereus UKM R66 (MZ221607), and Bacillus altitudinis UKM RB11 (MZ221599 ).
Drought assay
In an incubator shaker, PGPR isolates were cultured in an Erlenmeyer flask containing 20 ml of nutrient solution (N.B.) and grown for 24 hours at 37°C. A 1% v/v inoculum with a homogeneous cell density (108 CFU/ml) was inoculated into a nutrient solution containing 30% PEG -6000 (corresponding to the osmotic pressure of -1.03 MPa). This nutrient solution was cultured in an incubator for 24 hours. The bacterial growth in 30% PEG-6000 solution and without PEG was measured using a spectrophotometer at OD600. The nutrient solution was used as an empty control solution, whereas the nutrient solution containing the DH5ב strain was a negative control.
ACC deaminase-producing bacteria
Isolates were grown in a minimal solution of Dworkin and Foster12 containing ACC and incubated for 24 hours at 28°C. The bacterial growth was measured by using a spectrophotometer at OD600.
ACC deaminase enzyme assay
Isolates were grown in 5 ml tryptic soy broth (Oxoid, UK) at 28°C until they reached the stationary phase. Cells were centrifuged and washed twice with 0.1 M Tris-HCl (pH 7.5) to induce ACCd activity and mixed with 2 ml of a minimal D.F. solution containing 3 mM ACC. Thereafter, the culture was incubated at 28 °C in a shaking incubator for 36 to 72 hours. The activity of the ACC deamination enzymes was determined by measuring the production of α-ketobutyrate and ammonia. Both were produced by ACC cleavage deaminated by ACC enzymes.13 The induced bacterial cells were collected by centrifugation at 10,000 rpm for 10 minutes, washed twice with 0.1 M Tris-HCl (pH 7.5), and resuspended in 200µl 0.1 M Tris-HCl (pH 8.5). Cells were ‘labilized’ by adding 5% toluene (v/v) and rapid vortexing for 30 seconds. Approximately 50µl of the labilized cell suspension was incubated at 28°C for 30 minutes with 5µl of 0.3 M ACC in an Eppendorf tube. The negative control solution consisted of 50µl of ‘labilized’ cells without ACC, whereas the ‘blank’ solution consisted of 50µl of 0.1 M Tris-HCl (pH 8.5) with 5µl of 0.3 M ACC. After uniformly mixing the sample with 500µl of 0.56 N HCl solution using a vortex, the supernatant was removed by centrifugation at 12,000 rpm for 5 minutes. Afterward, approximately 500µl of the aliquoted supernatant was transferred to a glass test tube and mixed with 400µl of 0.56 N HCl solution and 150µl of DNF solution (0.1 g of 2,4-dinitrophenylhydrazine in 100 ml of 2 N HCl), after which the mixture was incubated at 28°C for 30 minutes. Thereafter, 1 ml of NaOH 2N was added to the sample before absorbance was measured at 540nm.
Indole acetic acid (IAA)
PGPR was inoculated into a 0.1% L-tryptophan-containing Luria Bertani solution (10 g NaCl, 10 g peptone, 5 g yeast extract, and 1 L distilled water). After 48 hours of inoculation at 28°C ± 2°C, the growing bacterial cultures were centrifuged and 1 ml of the supernatant was mixed with 1 ml of Salkowski reagent; resulting in the development of a pink coloured solution, which indicate the synthesis of IAA.14 Bacterial growth was determined by OD530 measurement after 30 minutes in the dark at room temperature. IAA concentrations produced by PGPR were calculated using a formula derived from the IAA standard curve (not shown).
Phosphate solubilization
The bacterial isolates were cultured in a Pikovskaya medium (10.0 g glucose, 5.0 g Ca3(PO4)2, 0.5 g yeast extract, 0.1 g MgSO4.7H2O, 0.2 g KCl, 0.2 g NaCl, and 0.5 g (NH4) 2SO4, as well as the trace elements of FeSO4 and MnSO4)to determine their ability to dissolve phosphate.15 Before autoclaving, the pH of the solution was adjusted to 7.0. The bacterial isolates were cultured in Pikovskaya solution (80 ml), whereas the non-inoculated medium served as a control. The flasks were shaken at 170 rpm for up to one week at 37°C. The pH of the culture medium was measured after 24 hours. Phosphate content was determined spectrophotometrically at OD880 using the standard method of Murphy and Riley. 16
Pot trial in the glasshouse
The experiment conducted in a glasshouse at the Faculty of Science and Technology, Universiti Kebangsaan Malaysia. Pots measuring 11 cm in diameter were filled with sterilized alluvium-type soil (approximately 180 g per pot), which is normally used for paddy cultivation in Malaysia. This soil was obtained from a paddy cultivation area at Tanjung Karang, Selangor, Malaysia. Paddy seed variety MR219 were obtained from MARDI Genebank, Seberang Perai. Paddy seeds that require inoculation were placed in a PGPR culture solution with a population density of 107 CFU/ml or an optical density reading (OD600) of 1.0 following surface sterilization. Meanwhile, non-inoculated seeds are placed in sterile distilled water. Afterward, the seeds were incubated at a temperature of 28oC in an incubator shaker at 130 rpm. After one week of inoculation and germination, seedlings were transferred to pots. PGPR bacteria were also inoculated into the soil in pots one week after sowing. Paddy plants were watered every days. No watering is given to paddy subjected to drought stress for a week.
Total chlorophyll content
Arnon’s17 technique was used to determine the content of photosynthetic pigments (chlorophyll a and b). Leaf samples (0.1 g) were extracted in a 10ml dimethyl sulfoxide (DMSO) solution and heated at 65°C. Leaf extract was measured at OD645 and OD663 using a microplate reader (BioTek Synergy H1).
Proline content
Leaf proline content was determined using the method of Bates et al.18 Leaf samples (0.2 g) were homogenized in 10 ml of a 3% (w/v) sulfosalicylic acid solution. The supernatant was transferred to a universal bottle and mixed with glacial acetic acid and ninhydrin reagent solution in equal volume ratios. The samples were incubated at 100°C for 1 hour. Afterward, the universal bottle is placed on ice to stop the reaction. Thereafter, 3 ml of toluene was mixed and shaken to produce two liquid layers and allowed to stand for a few minutes. Readings were taken at OD520 for analysis using a microplate reader.
Statistical analysis
The collected data are subjected to statistical analysis using the ANOVA method. Significant differences between treatments means were compared at p< 0.05 using GraphPad Prism 9 software.
Identification of the isolates
Five cultures were selected from 35 isolates that could survive at high temperatures (data not shown). These isolates were sequenced and classified into two genera: Bacillus (four isolates) and Achromobacter (one isolate). These isolates were identified as Achromobacter spanius UKM UR10, Bacillus pumillus UKM UR11, Bacillus sp. UKM S8, Bacillus cereus UKM R66, and Bacillus altitudinis UKM RB11.
Drought assay
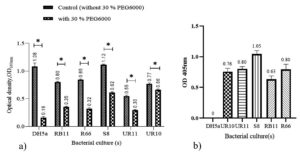
PEG 6000 was used as it can mimic drought stress level. The high osmotic pressure outside the bacterial cell causes fluid to diffuse out of the cell, thus resulting in lysis due to the destruction of microbial cell walls. Five isolates labelled UR10, UR11, S8, RB11, and R66 isolated from paddy rhizosphere and soil were characterized for their potential as plant growth promoters. These five isolates exhibited PGPR features through various mechanisms, including IAA production, ACCd production, nitrogen fixation, and phosphate solubilization. Additionally, these five isolates could survive at elevated temperatures of up to 50°C and in dry conditions (by osmotic pressure), i.e., at 30% PEG 6000, corresponding to an osmotic value of -1.03 MPa. According to Michel and Kaufmann,19 isolates that can survive at a density of -1.03 MPa are drought-tolerant. In Figure 1a, RB11, R66, UR11 and S8 showed lower growth in the 30% PEG solution compared to that of the control solution (without 30 % PEG6000) with a difference gap of approximately two-fold. E.coli strain DH5a was used as a control bacteria.DH5a was used because it is a bacterial model often used for metabolism studies and does not have PGPR characteristics, which is safe to use in the laboratory.20 UR10 showed a higher OD reading, which is 0.6 in the 30% PEG 6000 solution, which offers a small gap compared to the control
Figure 1b shows that after 24 hours of incubation in minimal Dworkin and Foster (DF) solutions containing ACC substrate, all cultures exhibited growth greater than 0.6 at OD405. Bacteria grown in ACC medium can use plant ACC as a carbon and nitrogen source.
Figure 1. Culture growth in two different medium solutions. a) bacterial growth in 30% PEG solution and control (no PEG) and b) bacterial growth in D.F. solutions containing ACC substrates. Bar graphs represent the mean (± S.D.). Values with an asterisk (*) are significantly different (p ≤ 0.05)
ACC deaminase activity
The action of the ACCd was evaluated by catalyzing the deamination reaction with a single nitrogen source in a minimum D.F. salt solution (ACC). The ACC deaminase enzyme activity isolated from PGPR ranged from 1.01 to 1.12 mmol-1 mg protein-1 hr, as shown in Table. UR10 produced the most significant ACC deaminase enzyme activity, followed by R66 with1.12 mmol-1mg protein-1 h and 1.11 mmol-1 mg protein-1 h, respectively. The absorption measured the ACCd activity at OD540 as presented in Table.
Table:
The ACC deaminase enzyme activity
Isolates |
ACC deaminase activities (mmol/mg/hour)* |
|---|---|
Achromobacter spanius UKM UR10 |
1.12 ± 0.015a |
Bacillus pumillus UKM UR11 |
1.08 ± 0.004bc |
Bacillus sp. UKM S8 |
1.01 ± 0.006a |
Bacillus altitudinis UKM RB11 |
1.04 ± 0.007a |
Bacillus cereus UKM R66 |
1.11 ± 0.004b |
*Values with different letters are significantly different (p ≤ 0.05)
IAA production
IAA is also reported to play an important role in plant adaptation to drought stress.21 Plants’ IAA production decreases under drought stress, rendering plant growth and development infertile. Figure 2a shows that RB11 produced the highest IAA (13.23 ug/ml in 10 ml culture solution), followed by R66 with 13.08 ug/ml IAA in 10 ml of culture solution. Conversely, S8 had the lowest IAA concentration of 9.71 ug/ml in a 10 ml culture solution. In this experiment, 0.1% L-tryptophan was added as a precursor for IAA activity.
Phosphate solubilization
Phosphorus, besides nitrogen, is the second most important macronutrient that plants need. The mechanism of phosphate solubilization is the combined result of a decrease in pH and the production of organic acids.22 During the 24-hour incubation period, the pH of the Pikovskaya solution decreased in most isolates except UR10 compared with the controls. Pikovskaya solution inoculated with R66 showed a 23% decrease in pH from 7.46 to 5.71 compared to the control pH, followed by UR11 (21%), RB11 (19%), and S8 (8.6%) in that order (data not shown). The pH of the bacteria isolated in the Pikovskaya solution decreased in this study, consistent with the pH decrease that Perez et al.23 observed during phosphate solubilization. Figure 2b shows a significant difference in soluble phosphate concentration (p < 0.05) over 24 hours. RB11 and UR11 had the highest phosphate concentrations (51.07 and 50.49 mg/ml, respectively), whereas UR10 produced the lowest concentration at 32.25 mg/ml soluble phosphate (P).
Figure 2. Characterization for PGPR traits. a) Production of indole acetic acid and b) concentration of soluble phosphate produced by bacterial isolates. Bars represent the mean (± S.D.).
Effects of PGPR on total chlorophyll, proline and total soluble sugar
The paddy plants were subjected to individual inoculation with PGPR cultures before being treated with the PGPR consortium. These treatments were done to assess the efficacy of these PGPR strains in enhancing the growth and survival of paddy plants under drought conditions. The findings related to the overall chlorophyll levels and proline concentration in paddy leaves subjected to drought stress and properly watered are presented in Figure 3.
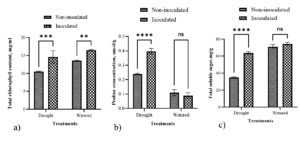
In drought conditions, paddy inoculated with individual PGPR cultures had significantly higher chlorophyll levels than the negative control (Figure 3a). The chlorophyll content in the leaves of paddy treated with UR11 and S8 cultures were 40.22 mg/g and 39.67 mg/g, respectively. Their chlorophyll contents were significantly difference compared to those of the negative control. Meanwhile, the chlorophyll levels in paddy plants treated with RB11, R66, and UR10 cultures were 32.26 mg/g, 35.55 mg/g, and 26.59 mg/g, respectively. This value increased significantly compared to the negative control but was slightly lower than the positive control.
Individual PGPR treatments (UR10, RB11, S8, R66, and UR11) significantly increased proline production in paddy compared to both controls (positive and negative), as shown in Figure 3b. The positive control, which received consistent watering, exhibited a low proline content. All inoculated paddy showed a higher proline content compared to the negative control. The paddy samples inoculated with strain R66 showed the highest proline content relative to treatments involving other strains.
Figure 3. Effects of individual PGPR cultures on paddy physiology. a) Total chlorophyll content and b) proline concentrations in paddy leaves treated under drought and well-watered conditions. Bars represent the mean (± S.D.). Values with an asterisk (*) are significantly different (p ≤ 0.05).
Under drought conditions, total chlorophyll content differed significantly in paddy treated with the consortium (Figure 4a). Inoculation of paddy is more significant compared to non-inoculated paddy under drought conditions. Likewise, in well-watered conditions, the total chlorophyll content in inoculated paddy is more significant than in non-inoculated paddy.
Figure 4b presents the proline content of paddy leaves. Inoculated paddy had a significant effect on drought conditions compared to non-inoculated paddy. These treatments demonstrated that PGPR inoculation could increase proline concentrations in paddy leaves. Under drought conditions, the inoculated paddy’s proline content was 0.39 umol/g compared to the non-inoculated paddy at 0.24 umol/g. On the contrary, under well-watered conditions, the inoculated and non-inoculated paddy had low proline contents at 0.11 umol/g and 0.08 umol/g, respectively.
Figure 4c shows the total soluble sugar (TSS) content in both inoculated and non-inoculated paddy under drought stress and well-watered conditions. There is a significant difference in TSS content between inoculated and non-inoculated paddy under drought conditions. In inoculated paddy, TSS content is two-fold higher compared with non-inoculated paddy. The increase in TSS content indicates the presence of PGPR inoculants, which effectively protect paddy leaves under drought stress. Compared to well-watered paddy, PGPR inoculation did not significantly increase TSS content.
Figure 4. Effects of PGPR consortiums on paddy physiology. a) Total chlorophyll content, b) proline concentrations and c) total soluble sugar content in paddy leaves treated under drought and well-watered conditions. Bars represent the mean (± S.D.). Values with an asterisk (*) are significantly different (p ≤ 0.05).
Effects of PGPR consortiums on paddy yield components and performances
Figure 5a-5c shows the effects of mixed PGPR inoculation on paddy yield components as determined by panicle, spikelet, and grain weight. Paddy treated with PGPR and under drought stress significantly affected panicle number, spikelet number, and paddy grain weight. Paddy subjected to drought stress showed a significant difference in the spikelet number in both inoculated and non-inoculated treatments and paddy under well-watered conditions. However, the panicle number in treated paddy (inoculated and non-inoculated) showed no significant difference under drought conditions. The dry grain weight is also affected by drought stress. Inoculated paddy under drought stress is significantly higher than non-inoculated dry grain weight. Under well-watered conditions, the dry grain weight (inoculated and non-inoculated) is higher than paddy under drought stress. The inoculated paddy was significantly higher than the non-inoculated paddy under well-watered conditions.
Figure 5. The effect of mixed PGPR inoculation on paddy components. a) spikelets number, b) panicle number and c) the weight of dry grain in drought and well-watered conditions. Bars represent the mean (± S.D.). Values with an asterisk (*) are significantly different (p ≤ 0.05).
In this study, standard agronomic practices using regular fertilization (including pesticides and fungicides) was not used to minimize the effect of the fertilizer on the inoculant. As a result, we used only a subset of the data to determine the paddy yield component and performance. As a result, paddy performance (as shown in Figures 6a-6c) is represented by stalk length, number of leaves, and root length. The number of paddy leaves showed a significant difference in inoculated paddy compared with non-inoculated paddy under drought conditions, whereas the stalk length and root length showed no significant difference. In well-watered conditions, there were no significant differences in inoculated and non-inoculated paddy performances.
Drought-tolerant strains are the main characteristics of the initial screening for strain selection, although they are not a feature of PGPR. PEG 6000 solution is introduced in the growth medium to assess the water shortage tolerance level because it exerts an osmotic effect on the bacterial cells cultured in the solution.
In this present study, the five isolates (UR10, UR11, S8, RB11, and R66) could survive at osmotic pressure in the presence of PEG 6000 and are heat resistant based on their ability to grow at 50°C.24 PEG with a higher molecular weight (4000 to 8000) is commonly used in physiological experiments to induce drought stress in nutrient solution cultures.25 Wang et al.26 reported that three out of ten isolated PGPR strains could live in a 30% PEG 6000 solution with optical density (OD) readings obtained not exceeding 0.2 after 25 hours. Based on the stated characteristics, it was suggested that five isolates may tolerate dry and drought conditions based on this assay.
The next screening is the ability to produce the enzyme ACCd. ACC is often extracted from plant roots, which is subsequently taken up and hydrolyzed by soil microorganisms through the secretion of the enzyme ACCd. The presence of bacteria producing ACCd is significant in controlling ethylene levels in plants because plants cannot produce the enzyme ACCd as it is only produced by microorganisms.27 In this study, all five isolates with high ACCd activity (between 1.01 to 1.12 mmol α-ketobutyrate mg-1 hr-1) should effectively reduce plant ethylene levels. Mayak et al.28 reported that Achromobacter piechaudii and Variovorax paradoxus producing ACCd for tomatoes and peas have made both plants tolerant to drought stress. In addition, Arshad et al.29 reported that strains of Pseudomonas spp. with ACCd activity could partially reduced the effect of drought stress on peas.
IAA plays a vital role in plant development and it supplies additional support to the plant host in adverse conditions such as drought. When Salkowski’s reagent is added to the solution containing the culture, a pinkish colour change indicates indole production. It has been reported that the colourimetric method is the simplest and has been used for a long time to detect indole acetic acid produced by plants and microorganisms.30 In this study, the highest IAA concentration was produced by RB11, followed by R66, which was 13.15 ± 0.02 and 13.05 ± 0.01µg ml-1, respectively in nutrient solution supplemented with 0.1% tryptophan. Both RB11 and R66 were isolated from roots. Khalid et al.31 described that isolates from roots were more efficient auxin producers compared to isolates from soil. Besides, it also clarified that the IAA pathway among the cultures was tryptophan-dependent. Bacterial synthesis of IAA differs among species and strains. In addition, culture conditions, growth rate, and substrate availability are all affected. The production of indole acetic acid by bacteria in the rhizosphere depends on the availability of precursors in the root exudate. Li et al.32 defined L-tryptophan as a primary precursor for bacteria’s indole acetic acid biosynthesis pathway.
Most of the P in the soil is present in mineral form and is insoluble.33 Therefore, PGPR can provide phosphate to plants by dissolving both organic and inorganic phosphate in the soil.34 Most soil bacteria convert insoluble phosphorus and phosphates into soluble forms by producing low molecular weight organic acids such as gluconic and citric acid. Phosphate (P)-solubilizing bacterial growth assays are performed in the Pikovskaya solution by measuring the pH value. This method refers to one of the mechanisms of phosphate dissolution: a decrease in pH. The mechanism of phosphate solubilization is the combined result of a reduction in pH due to the production of organic acids and phosphate solubilizing enzymes such as acid phosphatase, phytase and phosphonates.35 In this study, the pH values of bacterial isolates R66, UR11, RB11 and S8 in Pikovskaya’s solution decreased between pH 5.8 and 6.8. Flatian et al.36 conducted the same test, which also experienced a decrease in pH during the phosphate solubilization analysis by some phosphate solubilizing bacteria after incubation for a week. In contrast, the increased pH of the UR10 isolates is likely due to the bacteria utilizing the organic acids produced in the solution or forming alkaline compounds.33
After evaluating their specific properties as PGPR, the efficacy of five inoculants on paddy growth under drought-stress conditions was tested in glasshouses. The effects of PGPR inoculants in paddy was determined by measuring the chlorophyll, proline, and total soluble sugar concentrations of paddy leaves. The chlorophyll content for each treatment indicated that the level of tolerance of paddy to drought stress is different. A decrease in chlorophyll content is a common condition observed under drought stress.37 Paddy treated with the PGPR consortium and subjected to drought stress conditions showed a higher total chlorophyll content than untreated paddy under the same conditions. Zhang et al.38 also reported an increase in total chlorophyll content in Glycyrrhiza uralensis plants treated with PGPR B. pumillus under drought conditions compared to untreated ones. The total chlorophyll content in the paddy leaves demonstrates the effectivess of the PGPR consortium in maintaining chlorophyll content under drought conditions.
Proline is an essential amino acid in all plants, even when not stressed. Bacteria accumulate proline in response to hypo-osmolarity by synthesizing or taking it from the environmental media. In this study, paddy treated with the PGPR consortium showed increased proline under drought conditions. The PGPR consortium reduces the effects of drought stress in paddy plants by accumulating proline, which increases plant growth.39 Because of that, the proline content of paddy leaves in drought conditions is higher than in normal conditions. However, proline is present only in trace amounts and increases under stress. Under well-watered conditions, the lower proline content is consistent with the proline’s function as an osmolyte, which is present only when the plant is stressed. In addition, proline accumulation contributes to maintaining osmotic adjustment in plant cells. It supports water movement into leaves and protects the macromolecular organization and cell membranes during water scarcity.40 Gusain et al.39 showed that paddy treated with PGPR bacteria had higher leaf proline content at all drought stress levels (regular, moderate, or severe). This study also showed an increase in the proline content of paddy treated with PGPR consortium by 52% compared with paddy without PGPR under drought conditions. In addition, Wang et al.41 reported that treatment of cucumber plants (Cucumis sativa L.) with a mixture of three PGPR strains (Bacillus cereus AR156, Bacillus subtilis SM21 and Serratia sp. XY21) increased leaf proline content three to fourfold compared to that of the untreated control.
Glucose induces stomatal closure and increases plant adaptability under drought stress.42 In addition, soluble sugars maintain leaf water content and osmotic adjustment of plants under drought stress. In general, sugars act as osmoprotectants and stabilize membranes under abiotic stress. According to Harsh et al.,43 higher total soluble sugars and other osmolytes can potentially increase plant tolerance to abiotic stress. Soluble sugars help plants suffering drought stress maintain their water content in the leaves and the osmotic adjustment. According to Xu et al.,44 drought stress significantly increased the concentration of soluble sugars in paddy roots and leaves. In this study, paddy treated with the PGPR consortium showed a higher soluble sugar concentration in drought conditions than paddy without PGPR. A study conducted by Batool et al.45 on potato plants treated with PGPR B. subtilis HAS31 also showed an increase in the amount of soluble sugar by 42% and potatoes without PGPR increased by 36% in drought conditions. According to Van den Ende and Valluru,46 sugar functions as a substrate or signal for changes due to drought stress and is a scavenger of reactive oxygen species(ROS) at high and low concentrations.
The results of this study showed an increase in the number of spikelets, panicles and weight of dry grain treated with PGPR consortium in drought conditions compared to non-treated paddy. Similarly, under well-watered conditions, paddy treated with PGPR increased spikelets, panicles, and dry grain weight. These results demonstrate that the interaction between PGPR and drought stress strongly influences paddy yield components. In this study, the effect of paddy with PGPR consortium treatment showed high paddy production (stalk length, no. of leaves and root length) compared to paddy without PGPR under drought stress. The PGPR consortium increases plant nutrient uptake, produces phytohormones and stimulates the plant’s immune system. Microbial consortia provide significant advantages where there are PGPR characteristics between strains that other strains may not have to act as plant growth promoters under stress conditions.47 The increase in growth may occur by providing nutrients that can be assimilated well (e.g., P, iron (Fe), and N) by each strain of the PGPR consortium.48 These observations substantiate that the presence of the PGPR consortium can protect plants by bringing positive changes at physiological and morphological levels under drought-stress conditions.
Paddy inoculated with PGPR gave benefits to plants by reducing the reactive oxygen species effect under drought conditions. These isolates (UR10, UR11, S8, RB11, and R66) are better than others because they can live in temperatures as high as 50°C. Thermophilic PGPR and drought resistance have not been reported as frequently as an inoculum to enhance paddy growth under drought conditions. In this study, the main things that make PGPR cultures unique are their ability to grow under osmotic pressure, their production of the ACCd enzyme and IAA, and their ability to dissolve P. Moreover, they also induced drought tolerance by producing proline and total soluble sugar. Therefore, due to their ability to build and employ such mechanisms, these PGPR cultures are the most promising for use as PGPR inoculants to promote plant development under drought stress and high temperatures. The rice planted with the chosen cultures as part of the PGPR consortium exhibited significant variations in the levels of total chlorophyll, proline, and soluble sugar. Paddy yield components and performance also showed some significant changes. Generally, this PGPR consortium is ideal as an inoculant in biofertilizers. Along with promoting plant growth, it can also assist plants in coping with drought stress.
ACKNOWLEDGMENTS
The authors would like to thank the Malaysian Agricultural Research and Development Institute (MARDI) for sponsoring the scholarship.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
The author would like to express their gratitude to the 12th Malaysia Plan Development Project (RMK12–PRP515) for funding the publication.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Tangang F, Supari S, Chung JX, et al. Future changes in annual precipitation extremes over Southeast Asia under global warming of 2 C. APN Science Bulletin. 2018;8(1).
Crossref - Carmen CA, Patricia P, Ruben B, Victoria SM. Plantrhizobacteria interaction and drought stress tolerance in plants. Drought Stress Tolerance in Plants. Physio Biochem. 2016;1.
Crossref - Singh RP, Shelke GM, Kumar A, Jha PN. Biochemistry and genetics of ACC deaminase: A weapon to “stress ethylene” produced in plants. Front Microbiol. 2015;6:937.
Crossref - Iqbal N, Khan NA, Ferrante A, Trivellini A, Francini A, Khan MIR. Role of ethylene in plant growth, development and senescence: interactions with other phytohormones. Front Plant Sci. 2017;8:475.
Crossref - Gamalero E, Glick BR. Bacterial modulation of plant ethylene levels. Plant Physiol. 2015;169(1):13-221.
Crossref - Backer R, Rokem JS, Ilangumaran G, Lamont J, Praslickova D, Ricci E, Subramanian S, Smith DL. Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Frontiers in Plant Science. 2018:1473.
Crossref - Islam MR, Garcia SC, Horadagoda A. Effects of irrigation and rates and timing of nitrogen fertilizer on dry matter yield, proportions of plant fractions of maize and nutritive value and in vitro gas production characteristics of whole crop maize silage. Anim Feed Sci Technol. 2012;172(3-4):125-135.
Crossref - Johan PD, Ahmed OH, Omar L, Hasbullah NA. Phosphorus transformation in soils following co-application of charcoal and wood ash. Agronomy. 2021;11(10):2010.
Crossref - Tian J, Ge F, Zhang D, Deng S, Liu X. Roles of phosphate solubilizing microorganisms from managing soil phosphorus deficiency to mediating biogeochemical P cycle. Biology. 2021;10(2):158.
Crossref - Hussain MJ, Abbas Y, Nazli N, et al. Root Cultures, a Boon for the Production of Valuable Compounds: A Comparative Review. Plants (Basel). 2022;11(3):439.
Crossref - Gutierrez C, Somoskovi A, Natarajan K, Bell D. Need for better adherence to optimal incubation temperature for quality laboratory diagnostics and antibiotic resistance monitoring. Afr J Lab Med. 2018;7(2):789.
Crossref - Dworkin M, Foster J. Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol. 1958;75(5):592-603.
Crossref - Penrose DM, Glick BR. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant. 2003;118(1):10-15.
Crossref - Gordon SA, Weber RP. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951;26(1):192-195.
Crossref - Pikovskaya RI. Mobilization of phosphorus in soil connection with the vital activity of some microbial species microbiol. Scispace 1948;17:362-370.
- Murphy J, Riley JP. A Modified single solution method for the determination of phosphate in natural waters. Analyt Chim Act. 1962;27:31-36.
Crossref - Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949;24(1):1-15.
Crossref - Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205-207.
Crossref - Michel BE, Kaufmann MR. The osmotic potential of polyethylene glycol 6000. Plant Physio. 1973;51(5):914- 916.
Crossref - Olchanheski LR, Dourado MN, Beltrame F, et al. Mechanisms of tolerance and high degradation capacity of the herbicide mesotrione by Escherichia coli strain DH5-a. PloS One. 2014;9(6):e99960.
Crossref - Defez R, Andreozzi A, Dickinson M, Charlton A, Tadini L, Pesaresi P, Bianco C. Improved drought stress response in alfalfa plants nodulated by an IAA over-producing Rhizobium strain. Frontiers in Microbiology. 2017;8:2466.
Crossref - Chang CH, Yang SS. Thermo-tolerant phosphate-solubilizing microbes for multi-functional biofertilizer preparation. Bioresour Technol. 2009;100(4):1648-58.
Crossref - Perez E, Sulbaran M, Ball MM, Yarzabal LA. Isolation and characterization of mineral phosphate-solubilizing bacteria naturally colonizing a limonitic crust in the south-eastern Venezuelan region. Soil Biol Biochem. 2007;39(11):2905-2914.
Crossref - Russell AD. Lethal effects of heat on bacterial physiology and structure. Sci Prog. 2003;86(1/2):115- 137.
Crossref - Nahar S, Lakshminarayana RV, Lingaraj S, Bhaben T. Antioxidant protection mechanisms reveal significant response in drought-induced oxidative stress in some traditional paddy of Assam, India. Paddy Science. 2018;25(4):185-196.
Crossref - Wang C, Guo Y, Wang C, et al. Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting Rhizobacterium strains. PLoS ONE. 2012;7(12):e52565.
Crossref - Glick B. Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microb Lett. 2005;251(1):1-7.
Crossref - Mayak S, Tirosh T, Glick BR. Plant growth-promoting bacteria that confer resistance to water stress in tomato and pepper. Plant Sci. 2004;166(2):525-530.
- Arshad M, Shaharoona B, Mahmood T. Inoculation with Pseudomonas spp. containing ACC-deaminase partially eliminates the effects of drought stress on growth, yield, and ripening of pea (Pisum sativum L.). Pedosphere. 2008;18(5):611-620.
Crossref - Patten CL, Glick BR. Bacterial biosynthesis of Indole-3- Acetic acid. Canadian J Microbiol. 1996;42(3):207-220.
Crossref - Khalid A, Tahir S, Arshad M, Zahir ZA. Relative efficiency of rhizobacteria for auxin biosynthesis in rhizosphere and non-rhizosphere soils. Soil Research. 2004;42(8):921-6. Crossref
- Li M, Guo R, Yu F, et al. Indole-3-Acetic acid biosynthesis pathways in the plant-beneficial Bacterium arthrobacter pascens ZZ21. Int J Mol Sci. 2018;19(2):443.
Crossref - Kalayu G. Phosphate Solubilizing Microorganisms: Promising Approach as Biofertilizers. Int J of Agro. 2019;4917256.
Crossref - Bechtaoui N, Raklami A, Tahiri A-I, et al. Characterization of plant growth promoting rhizobacteria and their benefits on growth and phosphate nutrition of faba bean and wheat. Bio Open. 2019;8(7):bio043968.
Crossref - Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA. Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus. 2013;2:1-14.
Crossref - Flatian AN, Anas Sutandi A, Ishak. The ability of some microbes to solubilize the hardly soluble phosphorous and potassium from various sources in vitro. IOP Conference Series: Earth and Environmental Science. 2021;648(1):012143.
Crossref - Zhuang J, Wang Y, Chi Y, et al. Drought stress strengthens the link between chlorophyll fluorescence parameters and photosynthetic traits. Peer J. 2020;8:e10046.
Crossref - Zhang Z, Yin L, Li X, et al. Analyses of the complete genome sequence of the strain Bacillus pumilus zb201701 isolated from rhizosphere soil of maize under drought and salt stress. Microbes and Environ. 2019;34(3):310-315.
Crossref - Gusain YS, Singh US, Sharma AK. Bacterial mediated amelioration of drought stress in drought tolerant and susceptible cultivars of paddy (Oryza sativa L). Afr J Biotech. 2015;14(9):764-773.
Crossref - Ma Y, Dias MC, Freitas H. Drought and salinity stress responses and microbe-induced tolerance in plants. Front Plant Sci. 2020;11:591911.
Crossref - Wang S, Ouyang L, Ju X, Zhang L, Zhang Q, Li Y. Survey of plant drought-resistance promoting bacteria from populus euphratica tree living in arid area. Indian J Microbiol. 2014;54(4):419-426.
Crossref - Osakabe Y, Osakabe K, Shinozaki K, Tran L-SP. Response of plants to water stress. Front Plant Sci. 2014;5:86.
Crossref - Harsh A, Sharma YK, Joshi U, et al. Effect of short-term heat stress on total sugars, proline and some antioxidant enzymes in moth bean (Vigna aconitifolia). Annals of Agric Sci. 2016;61(1):57-64 .
Crossref - Xu W, Cui K, Xu A, Nie L, Huang J, Peng S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in paddy seedlings. Acta Physiol Planta. 2015;37(2):1-11.
Crossref - Batool T, Ali S, Seleiman MF, et al. Plant growth promoting Rhizobacteria alleviates drought stress in potato in response to suppressive oxidative stress and antioxidant enzymes activities. Sci Rep. 2020;10(1):16975.
Crossref - Van den Ende W, Valluru R. Sucrose, sucrosyl oligosaccharides, and oxidative stress: scavenging and salvaging. J Exp Bot. 2009;60(1):9-18 .
Crossref - Woo SL, Pepe O. Microbial consortia: promising probiotics as plant biostimulants for sustainable agriculture. Front Plant Sci. 2018;9:1801.
Crossref - Finney DM, Kaye JP. Functional diversity in cover crop polycultures increases multifunctionality of an agricultural system. J App Eco. 2017;54(2):509-517.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.