ISSN: 0973-7510
E-ISSN: 2581-690X
Antibiotic resistance is a universal problem in bacterial infections. Hence it gives high priority for new therapeutic methods to alternate conventional antibiotic treatment. Pigment production is one of the virulence characteristics of bacteria regulated by a quorum-sensing mechanism. Antiquorum sensing activities will not directly affect the viability of bacteria; however, they will reduce the pathogenicity of bacteria. Thereby it gives an adverse probability of the development of drug resistance. Aim of our study is to evaluate the activity of (+) usnic acid on pigment production in Pseudomonas aeruginosa MTCC 2453, Chromobacterium violaceum MTCC 2656 and Serratia marcescens MTCC 8708. (+) usnic acid eluted by column chromatography. Dimethyl sulphoxide (DMSO) was used as the solvent for (+) usnic acid. Antibacterial activity determined by Agar well diffusion and broth microdilution methods. Effect on pigment production was assessed by spectroscopy. (+) usnic acid showed accumulative inhibition as its concentration increases on pigment production by Pseudomonas aeruginosa MTCC 2453, Chromobacterium violaceum MTCC 2656 and Serratia marcescens MTCC 8708. The lowest concentrations of (+) usnic acid manifested 50% inhibition of pigment production was 122.67, 87.73 and 205.26 µg/ml respectively on above mentioned order in bacteria. The concentration of (+) usnic acid that showed pigment production inhibition did not hinder the growth of the bacteria, but it can reduce the virulence of the bacteria. This property can be used to resolve the drug resistance in bacteria. Further studies are required to check the action of (+) usnic acid on other virulence factors of the bacteria to prove the quorum quenching activity.
Bio pigments, Chromobacteria, Lichens, Natural compound, Quorum sensing, Quorum quenching
Various microorganisms such as bacteria and fungi release pigments as secondary metabolic end products such as alkaloids, essential oils, antibiotics, lectins, etc.1 These pigments play a vital role in achieving bacterial virulence and protecting from the host immune mechanisms and ultraviolet light.2 Certain bio pigments extracted from living organisms exhibit antibacterial activity and utilized as medicaments.3 The colour of pigments varies with different organisms, and the change in colour intensity affects the immune-modulatory properties of bacteria.4
Pseudomonas aeruginosa produces a bluish-green pigment known as pyocyanin, which inhibits the growth of other bacteria and becomes dominant in mixed infections.5 Almost 90-95% of strains of Pseudomonas aeruginosa produce pyocyanin which is one of the major virulence factors in it.6 Serratia sp. produces a red coloured pigment called prodigiosin, which shows antimicrobial, immunomodulating and anti-tumour properties.7 The violet-coloured pigment violacein produced by Chromobacterium violaceum has many biological activities against viruses, bacteria, and anticancer properties.8 High occurrence of resistance to various antibiotics is the significant challenges of most bacterial infections.9 Pigment production of bacteria is controlled by a process known as quorum sensing, analogous with biofilm formation and other virulence factors of bacteria.10 Quorum sensing is a microbial signal mechanism that responds to cell population density by gene regulation and is also responsible for the various physiological processes of bacteria.11
Lichens are the symbiotic association of different groups of organisms such as algae, fungi, and bacterial communities and are distributed worldwide.12 It exhibits unique chemical complexity and produces secondary metabolites termed lichen acids due to the presence of a broad spectrum of microorganisms in it.13 Lichen acids provide a wide variety of bio- medicinal values such as antibiotic, antiviral, antitumor, antiprotozoal, anti-inflammatory, analgesic, antipyretic and cytotoxic effects and also a defence against adverse environmental conditions such as ultraviolet rays and excessive dryness.14
Usnic acid is one of the most important lichen secondary metabolites among the hundreds of others.15 It is effective against various types of infections, particularly those affecting the throat, respiratory tract, mouth, skin and urinary tract and also has antioxidant and free radical-scavenging capabilities.16 It is a yellowish coloured pigment and its chemical nature is 2,6- diacetyl-7,9- dihydroxy- 8,9b- dimethyl- 1,3- (2H,9bH)- dibenzo- furandione; C18 H16 O7. The two relatively similar forms of usnic acid are (+) and (-) usnic acid based on the orientation of the methyl group present in the 9b position.17
The present study aimed to evaluate the effect of the (+) usnic acid on the pigment-producing capacity of three Gram-negative chromogenic bacteria by Pseudomonas aeruginosa MTCC 2453, Chromobacterium violaceum MTCC 2656 and Serratia marcescens MTCC 8708.
The study was conducted in the Department of Microbiology, Saveetha Medical College, Chennai, India.
Bacterial strains and culture conditions
Test strains Pseudomonas aeruginosa MTCC 2453, Chromobacterium violaceum MTCC 2656 and Serratia marcescens MTCC 8708 were purchased from Microbial type culture collection (MTCC), Chandigarh. Cultures acquired as freeze-dried forms and were regenerated by using nutrient broth and nutrient agar.
(+) usnic acid isolation
The lichen Roccella montagnei Bel. (Family: Roccellaceae) was collected from Kodikkarai forests, Nagappattinam district, Tamil Nadu (India). About 200 g of the shade-dried material was extracted with methanol in the cold. After 72 hours, the solvent was distilled off, and the methanolic extract so obtained was successively treated with petroleum ether and acetone. The latter fraction was column chromatographed on silica gel and eluted with solvents of increasing polarity. Elution of the column with benzene afforded shining yellow crystals with melting point of 196-198°C. The above compound was identified as (+) usnic acid.18
Antibacterial assay and determination of minimum inhibitory concentration (MIC)
Antibacterial activity determined by the agar well diffusion method to choose the antimicrobial activity of different concentration of (+) usnic acid (5, 25, 50 and 100 µg/ ml). 5 µl and 25 µl from 1:5 dilutions of 5 mg/ml stock solution and 10 µl and 20 µl directly from 5 mg/ ml stock solution of (+) usnic acid were added into the wells of 6 mm size to get a final concentration of 5, 25, 50 and 100 µg/ml respectively in dimethyl sulphoxide (DMSO), the solvent used. Ciprofloxacin (2 µg/ml) (Hi media Laboratories Ltd, Mumbai) was used as the standard drug. Bacterial suspensions were prepared and adjusted to McFarland standards (5×105 CFU/ml) to maintain the standard turbidity of the bacterial broth culture. Broth dilution method following Clinical Laboratory Standard Institute (2015) guidelines was used to determine the minimum inhibitory concentration.19 The minimum inhibitory concentration was the lowest concentration at which (+) usnic acid inhibited the bacterial growth after overnight incubation and the turbidity measured at 600 nm.20 The stock solution was prepared at a concentration of 5 mg/ml. Concentrations of (+) usnic acid used to determine minimum inhibitory concentration were 1250, 625, 312.5, 156.25, 78.125, 39.06 and 19.53 µg/ml. 0.5 McFarland standard was used to standardize the turbidity of the liquid culture of bacteria.
Effect of (+) usnic acid on pigment production
Pyocyanin extraction method
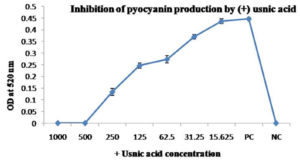
The test was done in Luria Bertani broth at 37°C for 48 hours.21 Different concentrations of the (+) usnic acid (1000, 500, 250, 125, 62.5, 31.25 and 15.63 µg/ml) from 5 mg/ml stock solution were used to check its effect pigment production in Pseudomonas aeruginosa MTCC 2453. Bacterial suspension without (+) usnic acid retained as a positive control to compare the result after the incubation period. Pyocyanin was extracted using 3 ml of chloroform and then with 1ml of 0.2 N HCl, and absorbance was measured using spectroscopic analysis at 520 nm (Biorad, smart spec 3000).
Quantification of violacein pigment
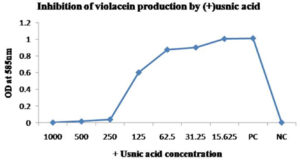
Chromobacterium violaceum MTCC 2656 was incubated at 30°C in Luria Bertani broth with varying concentrations of (+) usnic acid (1000, 500, 250, 125, 62.5, 31.25 and 15.63µg/ml) for 48 hours in a shaking incubator. The stock solution of (+) usnic acid made at a concentration of 5mg/ml. Broth culture without (+) usnic acid was the control. 1 ml culture from the overnight incubated broth was centrifuged at 8000 gravity for 5 minutes to precipitate the insoluble violacein. I ml of DMSO added after discarding the culture supernatant, and it was vortexed vigorously for 30 seconds and centrifuged at 8000 gravity for 5 minutes to remove the cells. Violacein in the supernatant was quantified by spectroscopic analysis at 585 nm22 (Biorad, smart spec 3000) and inhibition was calculated with respect to control.
Prodigiosin inhibition assay
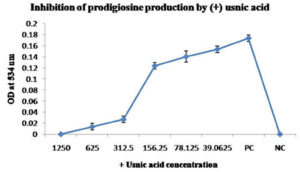
Serratia marcescens MTCC 8708 was cultivated in Luria Bertani broth with different concentrations of (+) usnic acid (1250, 625, 312.5, 156.25, 78.125, 39.0625 and 15.63 µg/ml) at 30°C for 48 hours. 5 mg/ ml stock solution made to prepare different concentrations of (+) usnic acid. Bacterial suspension without (+) usnic acid kept as a control to compare the pigment production. Cells separated by centrifugation at 10,000 rpm for 10 minutes after the incubation period. Pigment extracted from sediment by using 1 ml of acidified ethanol solution. The pigment concentration was measured at 534 nm by using a spectrophotometer23 (Biorad, smart spec 3000).
Statistical analysis
Data are presented as the mean ± standard deviation from experiments analysed in triplicate. Statistical analyses were performed using spearman’s rank correlation test to find the correlation between drug concentration and pigment concentration for bacteria. Here the hypotheses are as follows.
Ho: There is no significant correlation between drug concentration & pigment concentration.
H1: There is a significant correlation between drug concentration & pigment concentration the p-value is less than 0.05 was applied in all the tests.
Antibacterial activity and minimum inhibitory concentration of (+) usnic acid
Agar well diffusion assay showed no growth inhibition zone at any tested concentration of (+) usnic acid (5, 25, 50, 100 µg/ml). This states that used concentrations of (+) usnic acid do not affect the growth of selected bacteria.
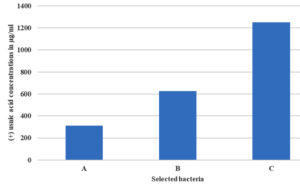
Minimum inhibitory concentrations of (+) usnic acid for Pseudomonas aeruginosa MTCC 2453, Chromobacterium violaceum MTCC 2656 and Serratia marcescens MTCC 8708 were 312.5, 625 and 1250 µg/ml, respectively (Fig. 1). In contrast, the minimum inhibitory concentration of the standard drug ciprofloxacin was 0.195, 0.063 and 0.125 µg/ ml, respectively.
Fig. 1. Minimum inhibitory concentration (MIC) of (+) usnic acid on Pseudomonas- aeruginosa MTCC 2453 (A), Chromobacterium violaceum MTCC 2656 (B) and Serratia marcescens MTCC 8708 (C).
Effect of (+) usnic acid on pigment production
The result of the study showed a concentration-dependent reduction of pigment production in Pseudomonas aeruginosa MTCC 2453, Chromobacterium violaceum MTCC 2656 and Serratia marcescens MTCC 8708 after treatment with (+) usnic acid by spectroscopic analysis at a suitable wavelength for each bacterium. Results have been shown in Fig. 2(A and B), Fig. 3(A, B and C), Fig. 4 (A and B) respectively. The concentration of (+) usnic acid is inversely proportional to the pigment production as the optical density value of pigment reduced when the concentration of (+) usnic acid increased. However, to consider the potency of (+) usnic acid, 50% inhibition of pigment formation was taken as a marker for the potency of the compound. Concentrations of (+) usnic acid for the inhibition of 50% of pigment production in Pseudomonas aeruginosa MTCC 2453, Chromobacterium violaceum MTCC 2656 and Serratia marcescens MTCC 8708 were 122.67, 87.73 and 205.26 µg/ml, respectively. The minimal concentrations of (+) usnic acid showing 50% inhibition of pigment production was 87.73 µg/ml for Chromobacterium violaceum MTCC 2656.
Fig. 2A. Effect of (+) usnic acid on the pyocyanin production of Pseudomonas aeruginosa MTCC 2453 cultures were grown in the presence of different concentrations (µg/ml) of (+) usnic acid.
Fig. 2B. 1000, 500, 250, 125, 62.5, 31.25 and 15.63 microgram per ml of (+) usnic acid from left to right. 8th tube is the positive control (Pseudomonas aeruginosa MTCC 2453 in Luria Bertani broth without (+) usnic acid). Lesser or no green colour typical of pyocyanin can be seen in experimental tubes as compared to positive control.
Fig. 3A. Effect of (+) usnic acid on the violacein production of Chromobacterium- violaceum MTCC 2656 cultures were grown in the presence of different concentrations (µg/ml) of (+) usnic acid.
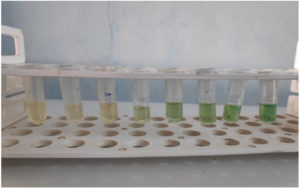
Fig. 3B. 1000, 500, 250, 125, 62.5, 31.25 and 15.63 microgram per ml of (+) usnic acid from right to left. 8th tube is the positive control (Chromobacterium violaceum MTCC 2656 in Luria Bertani broth without (+) usnic acid). Lesser or no violet colour typical of violacein can be seen in experimental tubes as compared to positive control.
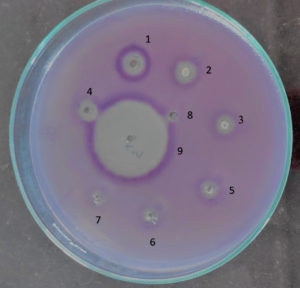
Fig. 3C. Inhibition of violacein production in Chromobacterium violaceum MTCC 2656 by (+) usnic acid indicated by clear zone around the well. (1) 1000 µg/ml, (2) 500 µg/ml, (3) 250 µg/ml, (4) 125 µg/ml, (5) 62.5 µg/ml, (6) 31.25 µg/ml, (7) 15.63 µg/ml (8) 7.81 µg/ml and (9) 2 µg/ml ciprofloxacin as standard antibacterial drug. The experiment was carried out on a Luria Bertani Agar overlaid with a lawn culture of Chromobacterium violaceum MTCC 2656, which was incubated overnight.
Fig. 4A. Effect of (+) usnic acid on the prodigiosin production of Serratia marcescens– MTCC 8708 cultures were grown in the presence of different concentrations (µg/ml) of (+) usnic acid.
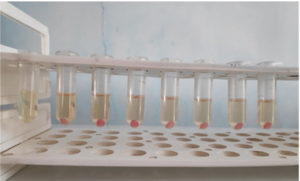
Fig. 4B. 1250, 625, 312.5, 156.25, 78.125, 39.0625 and 15.63 microgram per ml from left to right. 8th tube is the positive control (Serratia marcescens MTCC 8708 without (+) usnic acid). Lesser or no pink colour typical of prodigiosin can be seen in experimental tubes as compared to positive control.
Pseudomonas aeruginosa is an opportunistic pathogen continuing to exist and covering an extensive range of environments. It is strenuous to wipe them out because of the high level of antibiotic resistance due to diverse virulence factors controlled by the quorum sensing mechanism. Violet coloured pigment is a bioactive molecule produced by Chromobacterium violaceum and modulated by quorum sensing, widely accepted as a model organism in quorum sensing studies.24 Even though seldom involved in human infection, infections caused by them are generally lethal. Prodigiosine, the pigment produced by Serratia marcescens, is essential for its pathogenicity. It is under the regulation of the quorum sensing mechanism; hence, the obstruction of pigment production is possible to reduce pathogenicity.25 Therefore, quorum quenching activities gives ground for researchers focusing on an abiding plan to achieve a solution for drug resistance in pathogens by interfering with its quorum sensing mechanism.
Some studies correlated with the natural compounds having harmful antimicrobial effects proved its antivirulence activity by its action against bacterial biofilm formation.26 This study was done to check the ability of (+) usnic acid on pigment production of selected chromogenic bacteria. The various by-products are one of the pathogenicity factors of chromogenic bacteria restrained by the quorum-sensing mechanism. The inhibition of pigment production, thereby decreasing the infective nature of bacteria.
Like plants, lichens are also numerous sources of biologically active compounds. It can live in dreadful conditions due to its slow metabolism, production of specific bioactive molecules and so on. Also, it manifested its potent activity in combination with other therapeutic agents. The biological role of usnic acid varies from species to species and depends on its collection source. The higher minimum inhibitory concentration of usnic acid on Mycobacterium- aureum was eminent compared with the minimum inhibitory concentrations of the standard drugs used. Many studies are available to describe antimicrobial, cytotoxic or anti-inflammatory properties of (+) usnic acid, but their effect on quorum sensing property of bacteria is not studied yet. Hence, the inhibition of pigment leads to the destruction of quorum sensing and reduces the disease-causing ability of bacteria.
(+) Usnic acid has an inhibitory effect on pigment production without inhibiting the growth of the selected chromogenic bacteria. Several studies proved the adverse effects of different natural compounds on the pigment production of bacteria. It has been reported that 5-hydroxymethyl furfural has an inhibitory action on the violacein production by Chromobacterium violaceum and pyocyanin production by Pseudomonas aeruginosa.27 Similarly, another study noticed an expressive obstruction in violacein production in the presence of cinnamon, peppermint and lavender oil.28 (+) Usnic acid showed 50% of violacein inhibition at higher concentrations, such as 87.728 µg/ml. The previous report exists like the study of Amomumtsaoko, which inhibited 44.59% of violacein production at 4mg/ml.29 A study explained that 100 microgram/ ml curcumin and Centella Asiatica L30 limiting 89% and 50% of the violacein production, respectively.
A clinical study recorded that the concentration of 50 µM of eugenol has a remarkable effect in reducing pyocyanin production by Pseudomonas aeruginosa.31 Above 100 µg/ml of (+) usnic acid causes a 50% reduction of pyocyanin as observed in a study with Mangiferaindica L, which expressed a 69.7% reduction pyocyanin at 100 µg/ml. It was observed that three phenyls lactic acid repressed the synthesis of pyocyanin.32 Another report found a notable reduction in the pyocyanin on treatment with hordenin, 40% and 80% at 0.5 mg ml-1 and 1.0 mg ml-1 respectively.33 Clove oil caused remarkable inhibition in the production of pyocyanin varies from 37 – 75% at 0.2- 1.6 v/v concentrations.34 In contrast with the past, bacterial resistance to antibiotics is a universal problem. Consequently, there is an increasing demand for the latest anti-infective therapeutics, targeting bacterial behaviours such as quorum sensing instead of bacterial growth. This will reduce the drug resistance and undesirable side effects of certain antibiotics.35
A high concentration of (+) usnic acid (205.26 µg/ml) is required for a 50% reduction of prodigiosin in the present study. It has been already described the action of Anethumgraveolens on the decrease in the prodigiosin produced by Serratia marcescens in the level of 47, 64, 65, 66 and 71% respectively at concentrations of 64, 128, 256, 512 and 1024 µgML-1.36 This result was parallel with the conclusion of the study by N-nonanoylcyclopentylamide, which suppressed 87% of prodigiosin production AT 200µM.37
Usnic acid proved its activity against most Gram-positive organisms, and it has an inhibitory effect on the biofilm produced by gram-positive bacteria. An exciting characteristic showed by (+) usnic acid was its ability to change the morphology of the biofilm produced by Pseudomonas aeruginosa.38 In contrast, there was no antibacterial property at the lower concentration on this bacterium. This experiment established its ability to interfere with signalling molecules in quorum sensing of bacteria, which involves biofilm formation by its effect on morphology of biofilm. An identical type of biofilm morphology seen on the control polymer and the wild type Pseudomonas aeruginosa PA01 compared to biofilm in presence of (+) usnic acid.
In the present study, (+) usnic acid proved its inhibitory effects on the pigment-producing capacity of bacteria with the increasing concentrations. It has a vital role in this era due to the aspiring problem of antibiotic-resistant strains. Pigment production is one of the essential virulence characteristics of bacteria under the control of the quorum sensing mechanism. It has been noted that pigment inhibitory concentration of (+) usnic acid was below the minimum inhibitory concentration on each chosen bacterium. This conveys that pigment inhibitory concentration of (+) usnic acid does not affect bacterial growth. Here it ensures that the activity shown by (+) usnic acid is not on the ground of antibacterial activity.
P-value is less than 0.05. Hence, we reject the null hypothesis and conclude a high negative correlation between drug concentration & pigment concentration. The property of inhibitory activity on pigment production can make (+) usnic acid an antiquorum sensing agent. It manifested antibacterial activity at higher concentrations only. However, it may be possible to kill antibiotic-resistant bacteria while using it in association with the lower dose of standard antibiotic. Studies should be carried out on this basis, which can be positively incorporated with a reduction in the development of drug resistance. Further studies are required to test the effect of (+) usnic acid on other quorum sensing regulated virulence factors such as biofilm formation and impact on quorum sensing regulated genes to conclude its action as a quorum quenching agent.
ACKNOWLEDGMENTS
The authors are thankful to Dr Ethirajan Sukumar (Former Dean, Department of Research and Development, Saveetha Institute of Medical and Technical Sciences, Chennai), for helping in the materials and methods part.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
VR carried out the experiment and drafted the manuscript. BG and SC supervised the experiment and guided in drafting the manuscript. MM contributed in statistical analysis. All authors read and approved the final manuscript for publication.
FUNDING
None.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
All data sets generated or analysed during this study are included in the manuscript.
- Yokoyama A, Miki W. Composition and presumed biosynthetic pathway of carotenoids in the astaxanthin- producing bacterium Agrobacterium aurantiacum. FEMS Microbiol Lett. 1995;128(2):139-144.
Crossref - Liu GY, Nizet V. Color me bad: microbial pigments as virulence factors. Trends in Microbiol. 2009;17(9):406-413.
Crossref - Tuli HS, Chaudhary P, Beniwal V, Sharma AK. Microbial pigments as natural color sources: current trends and future perspectives. J Food Sci Technol. 2015;52(8):4669-4678.
Crossref - Liu GY, Essex A, Buchanan JT, Datta V, et al. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Exp Med. 2005;202(2):209-215.
Crossref - El- Fouly MZ, Sharaf AM, Shahin AA, El- Bialy HA, Omara AM. Biosynthesis of pyocyanin pigment by Pseudomonas aeruginosa. J Radiat Res Appl Sci. 2015;8(1):36-48.
- Mavrodi DV, Bonsall RF, Delaney SM, Soule MJ, Phillips G, Thomashow LS. Functional analysis of genes for biosynthesis of pyocyanin and phenazine 1- carboxamide from Pseudomonas aeruginosa PA01. J Bacteriol. 2001;183(21):6454-6465.
Crossref - Khanafari A, Assadi MM, Fakhr FA. Review of Prodigiosin, Pigmentation in Serratia- marcescens. OnLine Journal of Biological Sciences. 2006;6(1):1-3.
Crossref - Duran M, Ponezi AN, Faljoni- Alario A, Teixeira MF, Justo GZ, Duran N. Potential applications of violacein: a microbial pigment. Med Chem Res. 2012;21(7): 1524-1532.
Crossref - Martin I, Sawatzky P, Liu G, Mulvey MR. Antimicrobial resistance to Neisseria gonorrhoeae in Canada: 2009-2013. Can Commun Dis Rep. 2015;41(2):35-40.
Crossref - Rutherford ST, Bassler BL. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harbor Perspectives in Medicine. 2012;2:a012427.
Crossref - Zhou JW, Ruan LY, Chen HJ, et al. Inhibition of Quorum sensing and Virulence in Serratia marcescens by Hordenine. J Agric Food Chem. 2019;67(3):784-795.
Crossref - Galanty A, Pasko P, Podolak I. Enantioselective activity of usnic acid: A comprehensive review and future perspectives. Phytochem Rev. 2019;18(2):527-548.
Crossref - Ananthi R, Tinabaye A, Ganesan T, Selvaraj G, Arulmozhi S, Kumar S. Antimicrobial and anti- inflammatory activity of usnic acid and its acetyl derivative usnic acid diacetate. International Journal of Scientific and Engineering Research. 2016;7(8):39-44.
- Oran S, Sahin S, Sahinturk P, Ozturk S, Demir C. Antioxidant and Antimicrobial Potential and HPLC Analysis of Stictic and Usnic acids of Three Usnea species from Uludag Mountain (Bursa, Turkey). Iran J Pharm Res. 2016;15(2):527-535. PMCID: PMC5018281
- Macedo DCS, Almeida FJF, Wanderly MSO, et al. Usnic acid: from an ancient lichen derivative to promising biological and nanotechnology applications. Phytochem Rev. 2021;20(4):609-630.
Crossref - Hoffman AM, Mayer SG, Strobel GA, et al. Purification, identification and activity of phomodione, a furandione from an endophytic Phoma species. Phytochem. 2008;69(4):1049-1056.
Crossref - Sokolov DN, Zarubaev VV, Shtro AA, et al. Antiviral activity of (-)- and (+)- usnic acids and their derivatives against Influenza virus A (H1N1) 2009. Bioorg Med Chem Lett. 2012;22(23):7060-7064.
Crossref - Vijayakumar CS, Viswanathan S, Reddy MK, Parvathavarthini S, Kundu AB, Sukumar E. Anti- inflammatory activity of (+)- usnic acid. Fitoterapia. 2000;71(5):564- 566.
Crossref - Krochmal BK, Wicher RD. The minimum inhibitory concentration of Antibiotics: Methods, Interpretation, Clinical relevance. Pathogens. 2021;10:165.
Crossref - Nithyanand P, Shafreen RMB, Muthamil S, Pandian SK. Usnic acid inhibits biofilm formation and virulent morphological traits of Candida-albicans. Microbiological Research, 179;2015;20-28.
Crossref - Aybey A, Demirkan E. Inhibition of quorum sensing- controlled virulence factors in Pseudomonas aeruginosa by human serum paraoxonase. J Med Microbiol. 2016;65(2):105-113.
Crossref - Annapoorani A, Jabbar AKK, Musthafa SKS, Pandian SK, Ravi AV. Inhibition of Quorum sensing mediated virulence factors production in Urinary pathogen Serratia marcescens PS1 by Marine sponges. Indian J Microbiol. 2012;52(2):160-166.
Crossref - Zhu H, Sun SJ. Inhibition of bacterial quorum sensing regulated behaviors by Tremella- fuciformis extract. Curr Microbiol. 2008;57(5):418-422.
Crossref - Ghosh R, Tiwary BK, Kumar A, Chakraborty R. Guava Leaf Extract Inhibits Quorum sensing and Chromobacterium violaceum Induced Lysis of Human Hepatoma Cells: Whole Transcriptome Analysis Reveals Differential Gene Expression. PLOS ONE. 2014;9(9):e107703.
Crossref - Kothari V, Patel P, Joshi C, Mishra B, Dubey S, Mehta M. Sonic stimulation can affect production of Quorum Sensing Regulated Pigment in Serratia marcescense and Pseudomonas aeruginosa. Curr Trends in Biotechnol Pharm. 2017;11(2):122-129.
- Gonzalez JE, Keshavan ND.Messing with bacterial quorum sensing. Microbiol Mol Biol Rev. 2006;70(4):859-875.
Crossref - Rajkumari J, Borkotoky S, Reddy D, et al. Antiquorum sensing and anti- biofilm activity of 5- hydroxylmethylfurfural against Pseudomonas aeruginosa PA01: Insights from in vitro, in vivo and in silico studies. Microbiological Research. 2019;226:19-26.
Crossref - Khan MS, Zahin M, Hasan S, Husain FM, Ahmad I. Inhibition of quorum sensing regulated bacterial functions by plant essential oils with special reference to clove oil. Lett Appl Microbiol. 2009;49(3):354-360.
Crossref - Rahman MRT, Lou Z, Yu F, Wang P, Wang H. Anti- quorum sensing and anti- biofilm activity of Amommum tsao- ko (Amommum tsao- ko crevost et Lemarie) on food borne pathogens. Saudi J Biol Sci. 2017;24(2):324-330.
Crossref - Packiavathy IASV, Priya S, Pandian SK, Ravi AV. Inhibition of biofilm development of uropathogens by curcumin- An anti- quorum sensing agent from curcuma longa. Food Chem. 2014;148:453-460.
Crossref - Zhou L, Zheng H, Tang Y, Yu W, Gong Q. Eugenol inhibits quorum sensing at sub- inhibitory concentrations. Biotechnol Lett. 2013;35(4):631-637.
Crossref - Chatterjee M, D’morris S, Paul V, et al. Mechanistic understanding of phenyllactic acid mediated inhibition of quorum sensing and biofilm development in Pseudomonas aeruginosa. Appl Microbiol Biotechnol. 2017;101(22):8223-8236.
Crossref - Zhou JW, Luo HZ, Jiang H, Jian TK, Chen ZQ, Jia AQ. Hordenine: A novel quorum sensing Inhibitor and Antibiofilm agent against Pseudomonas aeruginosa. J Agric Food Chem. 2018;66(7):1620-1628.
Crossref - Hussain FM, Ahmad I, Asif M, Tahseen Q. Influence of clove oil on certain quorum sensing regulated functions and biofilm of Pseudomonas aeruginosa and Aeromonas- hydrophila. J Biosci. 2013;38(5):835-844.
Crossref - Varga Z, Szabo M, Kerenyi M, Molnar J. Interference in Quorum sensing signal transmission amongst Microbial species. Acta Microbiologica et Immunologica Hungarica. 2012;59(4):475-484.
Crossref - Salini R, Pandian SK. Interference of quorum sensing in urinary pathogen Serratia- marcescens by Anethum graveolens. FEMS Pathogens and Disease. 2015;73(6):ftv 038.
Crossref - Morohoshi T, Shiono T, Takidouchi K, et al. Inhibition of Quorum sensing in Serratia marcescens AS- 1 by synthetic Analogs of N- Acyl homoserine Lactone. Appl Environ Microbiol. 2007;73(20):6339-6344.
Crossref - Francolini I, Norris P, Piozzi A, Donelli G, Stoodley P. Usnic Acid, Natural Antimicrobial Agent Able to Inhibit Bacterial Biofilm Formation on Polymer surfaces. Antimicrob Agents Chemother. 2004;48(11):4360-4365.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.