ISSN: 0973-7510
E-ISSN: 2581-690X
This current study investigates the biological efficiency of essential oils extracted from Bistorta affinis and Malcolmia cabulica on human and foodborne pathogens as well as against insects and termites. The oils were obtained by steam distillation using a Clevenger-type system and analyzed for their constituents using GC-MS. Twenty compounds were identified, wherein carvacrol and thymol were the main constituents in both plants. Essential oils exhibited significant activity against all tested foodborne bacteria, fungi, and other pathogens. In addition, essential oils from both plants have shown promising activity against all tested insects, which is a positive sign of being used as an insect repellent. In contrast, no activity was observed against termites. The bioactivities are mainly due to carvacrol and thymol’s levels in the essential oils that known for their effectiveness against foodborne pathogens and pests. The present study constitutes a basis for further investigation and development of bioactive compounds in Bistorta affinis and Malcolmia cabulica.
Carvacrol, Thymol, GC-MS, Biological Activities, Insecticidal Activity, Antifungal Activity
Essential oil (EO) is a complex phytochemicals in volatile liquids characterized by their intense aroma. EO has rare color and is soluble in both lipids and organic solvents. It is present in different parts of the plant and synthesized in all its organs, like stems, leaves, fruits, flowers, buds, roots, bark or wood, seeds, and twigs. EO is stored in secretaries, cavities, canals, epidermic cells, or glandular trichomes.1 Medicinal plants contain EO, extracted by the secondary metabolism of a complex mixture of volatile molecules containing 20-60 components.2 EO has many food technological applications, including phyto-preparations, cosmetics, fragrances, and folk medicine.3 EO from medicinal plants exhibited bactericidal, virucidal, and fungicidal activities and has several constituents responsible for the growth inhibition of many pathogenic microorganisms like bacteria, viruses, fungi, and protozoa.4 EO’s use is a promising approach to combat the growth of many foodborne pathogens and multiple drug-resistant microorganisms. There are wide applications of EO in food safety and preservation to increase its shelf-life5 and limit the use of synthetic additives.6 Besides, the EO obtained from different plant species is known for repellent properties against many insect species, which are considered more environmentally friendly compatible pesticides and exhibit ovicidal and larvicidal activities.7 The invention of botanical insecticides derived from plants has limited the use of synthetic insecticides, and their demand has increased currently due to their safety.
Bistorta affinis is a perennial herb that belongs to the family Polygonaceae and is commonly called the Himalayan fleece flower. This family encompasses various medicinal plants known for many bioactivities because of many phytochemical constituents.8 Several phenolics were identified in the genus Polygonum; some of them proved remarkable biological activities.9 Phytochemical studies on this family revealed many bioactive chemicals like alkaloids, tannins, sapogenins, flavonoids, unsaturated sterols, and anthraquinones.10 B. affinis has also been used traditionally due to its numerous medicinal values. It is used in the traditional medicine of Tibetan as antipyretic and anti-inflammatory. It treats fever, tonsillitis, cough, and cold in Nepal’s traditional medicine.11 This plant’s rhizome helps prepare tea in Pakistan and treats abdominal pain, flu, back pain, expels worms, lung disorders, and fever.12
Malcolmia cabulica is an annual or biennial herb that belongs to the family Brassicaceae (Cruciferae). It is used in the traditional medicine of Lakki Marwat in Pakistan for animal weight loss, carminative, and galactagogue.13 The family Brassicaceae is known for its effectiveness in treating cancer and diabetes. In addition, it has been proven for significant antibacterial, antifungal, and anti-rheumatic properties. Moreover, the family Brassicaceae has been reported for its intense insecticidal action.14 This family also incorporates plants mainly used for edible or ornamental purposes; thus, they are vital economic plants. Owing to the sharp flavor of sulfur metabolites, these plants are often called the “mustard” plant family.
The present study aims to identify EO’s volatile constituents from B. affinis and M. cabulica using GC-MS and evaluate their antibacterial, antifungal, insecticidal, and anti-termite activities.
Collection of plants
Bistorta affinis was collected from Deosai, Gilgit Baltistan division, and Malcolmia cabulica was harvested from Lakki Marwat district, Khyber Pakhtunkhwa (KPK), Pakistan. The plants were identified by Mr. Ghulam Jelani, Department of Botany, University of Peshawar, KPK, Pakistan.
Isolation of EO
The EO of air-dried plants was extracted by hydrodistillation with a Clevenger type system for 3 h according to the standard procedure recommended in the British Pharmacopoeia.15 Then, the EO was dried with the help of anhydrous sodium sulfate and stored at a temperature of 4°C in a sealed vial until analysis.
Gas chromatography-mass spectrometry (GC-MS analysis)
EO components were identified using Gas Chromatography-Mass Spectrometry (GC-MS).16,17 The analysis was performed using the Perkin Elmer-Auto XL GC and Perkin Elmer TurboMass mass spectrometry. PE-5ms (5% phenyl-95% methylpolysiloxane) column (20×0.18 mm Ø with 0.18-µm film thickness) was used with helium at 0.5 mL/min as the carrier gas. GC oven temperature was kept at 45°C for 2 min and programmed to 240°C at a rate of 6°C/min and kept constant at 240°C for 5 min. The split ratio was adjusted to 1:100, while the injection volume was 0.1 μL. Electron impact mass spectrometry (EI-MS) was taken at 70 eV ionization energy. The mass range was from m./z. 35 to 350 amu. The MSD Chem-Station was used as operating software. Retention indices were calculated using retention times of n-alkanes (C8-C24) that were injected after the EO at the same conditions. Library research was performed using NIST and Wiley’s GC-MS Library of EO. A computerized integrator calculated the relative percentage amount of separated compounds from an ion chromatogram.
Antibacterial activity
Antibacterial activity of EO was tested against Salmonella typhi, Escherichia coli, Klebsella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis, Proteus mirabilis, and Serratia marcescens according to Nisar et al.18 Eighteen hours of the test organism’s culture was transferred to the nutrient broth and then to sterile nutrient agar plates to make a bacterial lawn. After 30 min, wells were dug in plates using a sterile 6 mm borer. Finally, 100 µL of EO were loaded to their respective labeled wells. Amoxicillin was used as a positive control. The zone of inhibition was measured (mm) compared to positive control.
Antifungal activity
The antifungal activity of EO has been carried out against Alternaria solanni, Aspergillus niger, Paecilomyces fulvus, Penicillium pallidum, Fusarium oxysporum, Rhizopus stolonifer, and Triticum harzianum.19 Sabouraud Dextrose Agar (SDA) media was used to refresh the fungal test isolates in Petri plates. First, 4 mL of SDA media was introduced into the test tubes to make slants. After autoclaving, when the temperature was about 50°C, 66.6 µL of EO were introduced into respective test tubes. Next, the seven-day-old fungal culture was introduced into the labeled test tubes and incubated at 25±1°C for 7 days in a growth chamber. Tubes supplemented with Miconazole served as a positive control. The results were taken on day 7 by measuring the linear growth on the slanted test tubes compared to the positive control.
Insecticidal activity
The EO was evaluated for possible insecticidal traits against three insect species: Tribolium castaneum, Rhyzopertha dominica, and Callosobruchus analis, according to a standard protocol.20 One mL of EO was introduced into the Petri plates using a micropipette having blotting paper at the base. The next day, 10 active and healthy insects (having the same age and size) were released to each plate. The standard drug (Permethrin, 393.17 µg/cm2) served as a positive control. The plates were incubated in the growth chamber at 27°C for 24 h with 50% relative humidity. The number of survived insects was counted for calculation, and the mortality (%) was determined. Results were the mean of three different experiments.
Anti-termite activity
The anti-termite effect was evaluated against test termite Heterotermes indicola, according to Salihah et al.21 The EO at a concentration of 1 mL of both plant species was introduced into the Petri plates having blotting paper at the base. Twenty-five healthy termites were released to each Petri dish using a clean brush and noticed their percent mortality after 24 h. All the experiments were performed in triplicate, and the average number of termites killed each day was noted.
Chemical composition of EO
The flowers of B. affinis yielded 0.3% (v/w), and M. cabulica yielded 0.2 % (v/w) of yellowish oil with an aromatic odor. Twenty different chemical components, comprising 99.1% of the total EO, were identified in the flowers of B. affinis and M. cabulica. The identified components and their percentage are given in Table 1, wherein the components are listed in order of their elution on the HP-5MS column. The results of the current GC-MS examination of B. affinis and M. cabulica EO extracted from flowers displayed 20 different chemical components, wherein carvacrol and thymol were the major constituents in both essential oils. Carvacrol (35.5%) and thymol (28.8%) were detected in the EO of B. affinis, while carvacrol (31.0%) and thymol (30.5%) were detected in the EO of M. cabulica.
Table (1):
Composition of EO from B. affinis and M. cabulica.
| No | Compound | M.W. | Molecular formula | Retention time (min) | (% concentration) | RIExp | RILit | ||
|---|---|---|---|---|---|---|---|---|---|
| B. affinis | M. cabulica | ||||||||
| 1 | 3-Octanone | 128 | C8H16O | 6.940 | 0.21 | 0.15 | 965 | 984 | |
| 2 | p-Cymene | 134 | C10H14 | 8.189 | 2.15 | 2.92 | 1007 | 1015 | |
| 3 | α-Pinene | 136 | C10H16 | 5.800 | 3.20 | 3.72 | 930 | 936 | |
| 4 | β-Pinene | 136 | C10H16 | 7.2 10 | 1.32 | 1.28 | 966 | 978 | |
| 5 | Terpinolene | 136 | C10H16 | 7.960 | 0.43 | 0.30 | 1079 | 1086 | |
| 6 | α-Terpinene | 136 | C10H16 | 9.389 | 1.85 | 1.75 | 1010 | 1017 | |
| 7 | Camphene | 136.2 | C10H16 | 32.710 | 3.05 | 3.18 | 940 | 950 | |
| 8 | Limonene | 137 | C10H17 | 32.700 | 2.38 | 1.01 | 1018 | 1025 | |
| 9 | Thymol | 150 | C10H14O | 18.750 | 28.89 | 30.56 | 1258 | 1267 | |
| 10 | Carvacrol | 150 | C10H14O | 30.035 | 35.54 | 31.05 | 1264 | 1278 | |
| 11 | Durenol | 150 | C10H14O | 19.195 | 3.00 | 2.89 | 956 | 983 | |
| 12 | Linalool | 154 | C10H18O | 10.850 | 4.89 | 4.15 | 1083 | 1086 | |
| 13 | 𝛿 -Terpineol | 154 | C10H18O | 13.950 | 0.24 | 0.23 | 1148 | 1164 | |
| 14 | α-Terpineol | 154 | C10H18O | 14.525 | 0.38 | 0.38 | 1175 | 1189 | |
| 15 | O-Methylthymol | 164 | C11H16O | 16.699 | 0.45 | 0.33 | 1214 | 1234 | |
| 16 | Thymol acetate | 192 | C12H16O2 | 22.001 | 0.78 | 0.65 | 1342 | 1356 | |
| 17 | Caryophyllene | 204 | C15H24 | 23.892 | 1.09 | 1.66 | 1565 | 1578 | |
| 18 | Alloaromadendrene | 204 | C15H24 | 24.673 | 0.31 | 0.29 | 1459 | 1464 | |
| 19 | Viridiflorene | 204 | C15H24 | 26.904 | 0.15 | 0.11 | 1492 | 1494 | |
| 20 | Isospathulenol | 220 | C15H24O | 30.240 | 0.71 | 0.56 | 1625 | 1633 | |
| Total | 91.02 | 87.17 | 23,496 | 23,717 | |||||
Earlier reports mentioned that phenolic constituents like thymol, carvacrol, and eugenol in many medicinal plants’ EO showed good antibacterial activity against several foodborne pathogens.22 Thymol, carvacrol, terpinen-4-ol terpinen were reported earlier as major chemical constituents in the EO obtained from Origanum species. Carvacrol has a strong aroma that acts as a fumigant and is known for strong acaricidal and insecticidal effects against medical, agricultural, and stored-product pests. On the other hand, it is highly lethal to the adults of pulse beetles, mites, rice weevils, cigarette beetles, and the nymphs of termites.23
The second major component in B. affinis and in M. cabulica was linalool (4.89%, and 4.15%, respectively). The occurrence of linalool in the EO has pleasant, fragrant, refreshing, and sweet characteristics known for good antibacterial effect.24 Linalool found in the EO of both plants suggests that these oils might have a significant antibiotic effect and might also be used in food systems to control the contamination of bacteria.25 Other workers have also documented the presence of linalool in Rosa abyssinica (12.7%),26 R. centifolia (6.9%),27 and R. centifolia (0.22%).28
The major monoterpene of hydrocarbon nature is a-pinene, which is known for significant bacteriostatic and bactericidal activities.29 The presence of α-pinene in both plants’ EO might help in the manufacturing of many antibacterial formulations. A few monoterpenes were detected in the EO of both plants, which are widely utilized in food, spices, flavors, condiments, beverages, cosmetics, and pharmaceutical industries.30 Therefore, it could be concluded that this EO might be essential for good human health. The obtained EO of both plants may be used by industries as flavor and fragrance and may be commercialized for the local people’s income generation. The remaining components detected in both plants’ EO were less than 1%, while the components like β-myrcene, germacrene D, and cis-beta-ocimene were not observed in EO.
Antibacterial activity
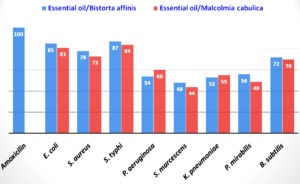
The EO derived from plants has been used for thousands of years in pharmaceuticals, natural therapies, food preservation, and alternative medicine.31,32 There are novel antimicrobial components found in the EO of plants,33 having a potential effect against many bacterial pathogens. The present investigation of antibacterial studies revealed that both plants’ EO inhibited bacterial isolates’ growth, but their effectiveness varied. The result of the antibacterial activity is given in Figure 1, and the values of MIC50 and MBC are given in Table 2. The EO of B. affinis showed maximum inhibition against S. typhi (85.7% with MIC50= 6.4 mg/mL and MBC=13.6 mg/mL), followed by E. coli (82.2% with MIC50= 3.9 mg/mL and MBC=8.6 mg/mL), S. aureus (80% with MIC50= 3.7 mg/mL and MBC=7.9 mg/mL), B. subtilis (65.5% with MIC50= 2.3 mg/mL and MBC=5.0 mg/mL), P. mirabilis (48.6% with MIC50= 6.9 mg/mL and MBC=2.3 mg/mL), K. pneumonia (48.1% with MIC50= 2.0 mg/mL and MBC=4.3 mg/mL), P. aeruginosa (47.7% with MIC50= 2.3 mg/mL and MBC=5.0 mg/mL), and S. marcescens (45.5% with MIC50= 4.2 mg/mL and MBC=9.4 mg/mL). The EO of M. cabulica showed the highest inhibition against S. typhi (81.4% with MIC50= 5.2 mg/mL and MBC=11.1 mg/mL), followed by S. aureus (79.1% with MIC50= 3.0 mg/mL and MBC=6.2 mg/ml), K. pneumonia (76.1% with MIC50= 2.8 mg/mL and MBC=5.9 mg/mL), P. aeruginosa (75.7% with MIC50= 2.9 mg/mL and MBC=6.4 mg/mL), E. coli (70.0% with MIC50= 4.3 mg/mL and MBC=8.8 mg/mL), and S. marcescens (65.5% with MIC50= 2.3 mg/mL and MBC=4.9 mg/mL). EO moderately inhibited the growth of B. subtilis (48.3% with MIC50= 2.6 mg/mL and MBC=5.7 mg/mL) and P. mirabilis (40.6% with MIC50= 2.5 mg/mL and MBC=5.6 mg/mL).
Table (2):
MIC50 and MBC values of EO from B. affinis and M. cabulica against tested bacteria.
| Bacteria Names | Essential oil of B. affinis | Essential oil of M. cabulica | ||
|---|---|---|---|---|
| MIC50 | MBC | MIC50 | MBC | |
| E.coli | 3.9 | 8.6 | 4.3 | 8.8 |
| S. aureus | 3.7 | 7.9 | 3.0 | 6.2 |
| S. typhi | 6.4 | 13.6 | 5.2 | 11.1 |
| P. aeruginosa | 2.3 | 5.0 | 2.9 | 6.4 |
| S. marcescens | 4.2 | 9.4 | 2.3 | 4.9 |
| K. pneumoniae | 2.0 | 4.3 | 2.8 | 5.9 |
| P. mirabilis | 3.2 | 6.9 | 2.5 | 5.6 |
| B. subtilis | 2.3 | 5.0 | 2.6 | 5.7 |
Antifungal Activity
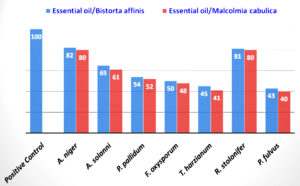
The main bioactive components found in EO have been widely used against moulds. The EO extracted from citrus, basil, lemongrass, fennel, oregano, thyme, and rosemary is known for its extensive antifungal activity against various fungal pathogens.34 Therefore, the EO of both plants were evaluated for antifungal activity, and their results are depicted in Figure 2. The EO of B. affinis showed the maximum inhibitory activity (80%) against A. niger and R. stolonifer, good inhibitory activity against A. solanni (62%), moderate inhibitory activity against P. pallidum (53%) and F. oxysporum (50%), low inhibitory activity against T. harzianum (42%) and P. fulvus (40%). On the other hand, the EO of M. cabulica showed the maximum inhibitory activity (79%) against A. niger and R. stolonifer, good inhibitory activity against A. solanni (60%), moderate inhibitory activity against P. pallidum (51%), and F. oxysporum (50%), and low inhibitory activity against T. harzianum (40%) and P. fulvus (38%).
Insecticidal activity
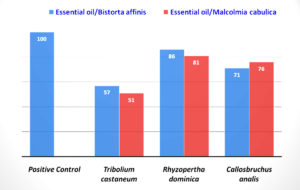
Synthetic insecticides have a toxic and harmful effect on humans and the environment. To prevail over this toxic effect, plants’ natural insecticides should replace these harmful insecticides.20 The insecticidal effect of EO against test insects Tribolium castaneum, Rhyzopertha dominica, and Callosobruchus analis were evaluated (Figure 3). The EO of B. affinis showed maximum activity against R. dominica (84.2%), good activity against C. analis (71.0%), and low activity showed against T. castaneum (48.3%). In the case of M. cabulica, significant insecticidal activity showed by the EO against R. dominica (80.3%), good activity against C. analis (78.0%), and low activity showed against T. Castaneum (45.7%).
Antitermite activity
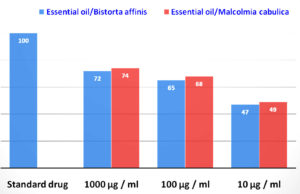
Termites help aerate the soil and act as decomposers that decompose the principal constituent of wood (i.e., cellulose). In another way, they become economic pests causing a tremendous economic loss when they start destroying wood and wooden products of human homes, forests, and other commercial products.35 The EO from both plant species showed significant activities against H. indicola, and the experiment was extended for three days. Three concentrations, such as 1000, 100, and 10 µg/mL of both plant species EO, were checked in the experiment (Figure 4). The EO of B. affinis killed the average percent of termites (73%) at 1000 µg/mL, (63%) at 100 µg/mL, and (48%) at 10 µg/mL, respectively. In M. cabulica, high percent mortality (74%) showed by EO at a concentration of 1000 µg/mL, 68% at 100 µg/mL, and 50% at 10 µg/mL, respectively.
A wide variety of volatile constituents in the EO include aliphatic and aromatic compounds, phenolics, terpenoids, and terpenes that might have bactericidal, fungicidal, and virucidal efficacy. The EO derived from plants directly targets the pathogenic microorganism cell membrane, which causes an increase in permeability, leakage of essential intracellular components, and finally disrupts cellular respiration and enzyme organization of microorganisms. Moreover, the EO also possesses a cytotoxic effect on living cells, depending on their concentration and type. The results of the current study suggested that the EO extracted from B. affinis and M. cabulica might be used as a natural alternative source due to its significant antimicrobial efficacy and help in discovering and synthesizing novel drugs. Furthermore, the detected fumigant effect of EO revealed that it contains active biological vapours having potential insect and termite repellent activity. Thus, the prospect of employing these natural fumigants for controlling insects and termites in stored products may be valuable for further exploration.
ACKNOWLEDGMENTS
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work.
CONFLICT OF INTEREST
The author declares no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was funded by Deanship of Scientific Research at Umm Al-Qura University with Grant Code 22UQU4350073DSR09.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Bozin B, Mimica-Dukic N, Simin N, Anackov G. Characterization of the volatile composition of essential oils of some Lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J Agric Food Chem. 2006;54(5): 1822-1828.
Crossref - Abd Wahab NZ, Ja’afar NSA, Ismail SB. Evaluation of Antibacterial Activity of Essential Oils of Melaleuca cajuputi Powell. J Pure Appl Microbiol. 2022;16(1):549-556.
Crossref - Ambade S, Deshpande N, Abhyankar P. Effect of Lemongrass Essential Oil based Mouthwash against Microflora Associated with Dental Plaque. J Pure Appl Microbiol. 2022;16(1):174-181.
Crossref - Sonam, Kumari A, Kumar V, et al. Antimicrobial Potential and Chemical Profiling of Leaves Essential Oil of Mentha Species Growing under North-West Himalaya Conditions. J Pure Appl Microbiol. 2021;15(4):2229-2243.
Crossref - Tongnuanchan P, Benjakul S. Essential oils: extraction, bioactivities, and their uses for food preservation. J Food Sci. 2014;79(7):R1231-R49.
Crossref - Mulyaningsih S, Sporer F, Zimmermann S, Reichling J, Wink M. Synergistic properties of the terpenoids aromadendrene and 1, 8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine. 2010;17(13):1061-1066.
Crossref - Cetin H, Erler F, Yanikoglu A. Larvicidal activity of a botanical natural product, AkseBio2, against Culex pipiens. Fitoterapia. 2004;75(7-8):724-728.
Crossref - Gong ZF, Yang GL, Yan ZT, Xie JS. Survey of chemical constituents and bioactivity of Polygonum L. plants. Chinese Trad Herb Drugs. 2002;33, 82-84.
- Fu YX, He XR, Li JC, Liu XX. Study on effective composition analysis and anti-bacterial effects of herb Polygonum perfoliatum L. Prog Vet Med. 2008;29:45-49.
- Qaiser M. Polygonaceae. In: Flora of Pakistan. Eds. Ali, SI. and Qaisar, M. Department of Botany, Karachi University and Missouri Botanical Garden, St. Louis, Missouri, USA. 2001;205:76-111.
- Bano A, Ahmad M, Hadda TB, et al. Quantitative ethnomedicinal study of plants used in the skardu valley at high altitude of Karakoram-Himalayan range, Pakistan. J Ethnobiol Ethnomed. 2014;10:43.
Crossref - Rajbhandari M, Mentel R, Jha PK, et al. Antiviral activity of some plants used in Nepalese traditional medicine. Evid Based Complement Alternat Med. 2009;6(4):517-522.
Crossref - Ullah S, Khan MR, Shah NA, Shah SA, Majid M, Farooq MA. Ethnomedicinal plant use value in the Lakki Marwat District of Pakistan. J Ethnopharmacol. 2014;158(Part A):412-422.
Crossref - Esmaeili A, Moaf L and Rezazadeh S. Volatile Compounds of Essential Oil Malcolmia africana (L.) R. Br. Grown in Iran. J Essential Oil Bear Plant. 2014;17(4):664-669.
Crossref - British Pharmacopoeia. HMSO Publication, London. 1988;2:A137-A138.
- Swigar AA, Silverstein RM. Monoterpenes. Infrared, Mass,1H-NMR, 13C-NMR Spectra and Kovats Indices, Aldrich Chemical Company Inc., Wisconsin. 1981.
- Erdemgil FZ, Ilhan S, Korkmaz F, et al. Chemical composition and biological activity of the essential oil of Perovskia atriplicifolia. from Pakistan. Pharmaceutical Biology. 2007;45(4):324-331.
Crossref - Nisar M, Khan I, Ahmad B, Ali I, Ahmad W, Choudhary MI. Antifungal and antibacterial activities of Taxus wallichiana Zucc. J Enzyme Inhib Med Chem. 2008;23(2):256-260.
Crossref - Ahmad B, Ali N, Bashir S, Choudhary MI, Azam S, Khan I. Parasiticidal, antifungal and antibacterial activities of Onosma griffithii Vatke. Afr J Biotechnol. 2009;8(19).
- Ahn YJ, Kim GH, Cho KY. Bioassay system for insecticidal compounds. In Proceedings of the third symposium on the biochemical methodology for the research and development of the bioactive substances, held at Seoul, Republic of Korea. 1995:495-506.
- Salihah Z, Khatoon R, Khan A, Alamzeb SA. A termite trap, NIFATERMAP, for capturing large number of field population of Heterotermes indicola. Proc Pakistan Cong Zool. 1993:395-400.
- Kim J, Marshall MR, Wei C. Antibacterial activity of some essential oils components against five foodborne pathogens. J Agric Food Chem. 1995;43(11):2839-2845.
Crossref - Isman MB. Plant essential oils for pest and disease management. Crop Protection. 2000;19(8-10):603-608.
Crossref - Zaks A, Davidovich-Rikanati R, Bar E, Inbar M, Lewinsohn E. Biosynthesis of linalyl acetate and other terpenes in lemon mint (Mentha aquatica var. citrata, Lamiaceae) glandular trichomes. Israel J Pl Sci. 2008;56(3):233-244.
Crossref - Fisher K, Phillips CA. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J Appl Microbiol. 2006;101(6):1232-1240.
Crossref - Al-Rehaily AJ, Al-Howiriny TA, Bizzo HR. Essential oil of Rosa abyssinica R. Br. from Saudi Arabia. J Essential Oil Res. 2003;15(5):344-345.
Crossref - Mumtaz W, Mukhtar H, Anwar F, Nadeem R. Extraction and characterization of essential oil of Rosa Gruss-an-teplitz. Asian J Chem. 2007;19(2):949-953.
- Shabbir MK, Nadeem R, Mukhtar H, Anwar F, Mumtaz MW. Physico-chemical analysis and determination of various chemical constituents of essential oil in Rosa centifolia. Pak J Bot. 2009;41(2):615-620.
- Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12(4):564-582.
Crossref - Bhat S, Maheshwari P, Kumar S, Kumar A. Mentha species: in vitro regeneration and genetic transformation. Molecular Biology Today. 2002;3(1):11-23.
- Jones FA. Herbs-useful plants. Their role in history and today. Eur J Gastroenterol Hepatol. 1996;8(12):1227-1231.
Crossref - Lis-Balchin M, Deans SG. Bioactivity of selected plant essential oils against Listeria monocytogenes. J Appl Microbiol. 1997;82(6):759-762.
Crossref - Mitscher LA, Drake S, Gollapudi SR, Okwute SK. A modern look at folkloric use of anti-infective agents. J Nat Prod. 1987;50(6):1025-1040.
Crossref - Kivanc M, Akgul A, Dogan A. Inhibitory and stimulatory effects of cumin, oregano and their essential oils on growth and acid production of Lactobacillus plantarum and Leuconostoc mesenteroides. Int J Food Microbiol. 1991;13(1):81-85.
Crossref - Suszkiw J. The Formosan termite: A formidable foe!. Agricultural Research. 1998;46(10):4.
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.