ISSN: 0973-7510
E-ISSN: 2581-690X
The water quality including toxic gas parameters and Vibrio density is a serious problem in intensive shrimp culture. Yucca schidigera extract and Bacillus sp. are considered as a practical solution to improve the water quality. The aim of our study was to determine the effect of combination of Yucca schidigera extract and Bacillus spp. on total ammonia nitrogen (TAN) and nitrite contents, Vibrio count, and the growth performance of white leg shrimp (Penaeus vannamei). Shrimp (PL12) were assigned to seven treatments (triplicates) including treatments with combination of yucca extract and single strain of Bacillus (AY, B2Y, B3Y), combination of yucca extract and Bacillus consortium (ABBY), yucca extract only (Y), Bacillus consortium (ABB), and control treatment (C). Vibrio count was carried out by the spread plate technique every 7 days. TAN and nitrite contents were determined by using sodium nitroprusside, and sulphanilic acid with N-(1-naphthyl) ethylenediamine, respectively, at 24 hours and 168 hours after adding Bacillus strains and yucca extract. The shrimp growth parameters include the final biomass, final body weight (FBW, g), weight gain rate (WGR, %), survival rate (SR, %), and feed conversion ratio (FCR) were recorded after 5 weeks. The results showed that the water quality (0.073 – 0.179 ppm of TAN, 0.187 – 0.934 ppm of nitrite, and Vibrio count of 2.552 – 3.038 log CFU/ml) of tanks treated with combination of yucca extract and single strain of Bacillus (AY, B2Y, B3Y) or Bacillus consortium (ABBY), and Bacillus consortium (ABB) was significantly controlled compared to the control and yucca extract only treatments. The ABBY treatment most significantly improved the shrimp growth parameters (FBW, biomass, WGR, FCR, and SR – 0.424 ± 0.010 g, 40.202 ± 1.510 g, 14048.902 ± 328.756 %, 1.100 ± 0.040, and 94.667 ± 1.856 %, respectively) compared to the other treatments. Overall, our study concluded that the combination of yucca extract and Bacillus consortium could control water quality (0.036 – 0.105 ppm of TAN, 0.187 – 0.604 ppm of nitrite, and Vibrio count of 2.553 – 2.624 log CFU/ml), reduce 46.49% of FCR and significantly increase the growth performance (approximately 92.73% of FWB, 163.19% of biomass, 93.93% of WGR, 46.49% of FCR) of P. vannamei (PL12) with density of 100 individuals/100L.
Bacillus consortium, Yucca schidigera, Ammonia Nitrite Removal, Yucca Extract, Penaeus vannamei, Shrimp Growth Performance
Ammonia and nitrite are major limiting factors in aquaculture systems.1-3 The process of decomposition of organic compounds in the pond and from the excretory system of aquatic animals along with excess feed will produce ammonia and nitrite, when the pond accumulates a certain amount of ammonia and nitrite, the aquatic animals will be stressed and even die.4 Feed control and daily water changes have been successfully applied to maintain acceptable ammonia and nitrite levels in the culture of Litopenaeus vannamei.4 However, this method is extremely expensive, increases production costs, consumes labor, affects the environment and can introduce pathogens into the culture system.3 Therefore, controlling ammonia and nitrite with an eco-friendly biological solution can be an effective alternative to large aquaculture systems.
Probiotics are also an effective solution to maintain water quality, and promote growth performance of shrimp.5,6 Bacillus strains (Bacillus subtilis, Bacillus flexus, Bacillus licheniformis, Bacillus firmus, and Bacillus megaterium) are commonly used in aquaculture to improve water quality, especially in the treatment of ammonia and nitrite.7,8 In addition, Bacillus sp. also has the ability to control pathogenic bacteria, including Vibrio sp. cause disease in shrimp, without causing side effects, this can replace antibiotics and bactericidal chemicals in aquaculture.7 Bacillus consortium including Bacillus flexus QG-3, Bacillus flexus NS-4, and Bacillus licheniformis XCG-6 (proportion 5:5:4) was effectively resistant to Vibrio spp. with a inhibition ratio of approximately 90% after 144 hours in white leg shrimp (L. vannamei) culture water.9 The bacterial consortium including Rhodopseudomonas palustris SUP-2, Bacillus subtilis SUP-3, and Bacillus firmus SUP-1 reduced the total Vibrio density by approximately 68% in the trials of white shrimp Penaeus vannamei.6 Bacillus subtilis L10 and Bacillus subtilis G1 (proportion 1:1) decreased total Vibrio count in culture water of white shrimp L. vannamei by approximately 82 – 91% after 8 weeks of the experiment.10
One of the medicinal plants with useful uses in aquaculture is Yucca schidigera which possesses many beneficial properties for aquatic animals. The deserts of Mexico and Southwestern United States are the sources of Yucca schidigera plant.11 In many studies, the growth performance and physiological status of aquatic animals were significantly improved when Yucca schidigera extract was added to the diet or applied directly to the aquatic environment.11-13 Wang et al. studied the growth parameters of mirror carp (Cyprinus carpio) when Yucca schidigera extract was added to the diet at concentrations of 0, 200, or 400 mg/kg for 8 weeks. The results showed that the diet containing 400 mg/kg of yucca extract significantly improved the final body weight and weight gain rate compared to the control treatment.14 Baei et al. investigated the feed efficiency and growth of olive flounder P. olivaceus with the diet containing yucca extract (1.5 g/kg diet). The results showed that there was no significant differences between the diet supplemented with yucca extract and the control group.15 Abdel-Tawwab et al. added yucca extract and/or yeast Saccharomyces cerevisiae to the culture water of Nile tilapia at a dose of 1 g/m3 for 8 weeks. At the end of the experiment, the treatment with the addition of yucca extract and yeast showed the highest growth of Nile tilapia.12 In another study, Hernandez-Acosta et al. investigated the effects of Yucca schidigera and Quillaja saponaria (NTF) extracts (0, 0.25, 0.50, 1.00 and 2.00 g/kg diet) on growth parameters of white shrimp Litopenaeus vannamei. After 40 days, the diets supplemented with 1.00 and 2.00 g/kg of NTF increased the weight and decreased FCR of shrimp compared to the control group.16 Yucca extract improves protein metabolism leading to increased feed utilization of aquatic animals.17 Furthermore, Yucca schidigera extract, which contains antibacterial compounds including 5b-spirostan-3b-ol, sarsasapogenin, and smilagenin, is considered antibacterial agent, enhancing the immune system for aquatic animals.14,18,19 One of the most important functions of yucca extract is to improve water quality. Yucca extract has the ability to remove ammonia as well as reduce the adverse effects of ammonia on the health of aquatic animals due to possessing significant content of saponins and resveratrol, and can be used as a liquid or powdered supplement.11,20 Castillo-Vargasmachuca et al. investigated the effect of Yucca schidigera extract on the water quality of Pacific Red Snapper (Lutjanus peru) culture. The results showed that yucca extract at a dose of 0.75 mg/L (0.30 ± 0.04 ppm of N-NH4) could reduce approximately 0.6 mg/L of N-NH4 compared to the control group (0.90 ± 0.05 ppm of N-NH4).21 Santacruz-Reyes & Chien investigated the reduction of ammonia pollution from effluent of shrimp farm by using Yucca schidigera extract. The results showed that yucca extract at a concentration of 18 mg/L reduced 71 – 72% and 86 – 87% at 6 and 24 hours, respectively.22 Fayed et al. demonstrated that yucca liquid extract with a concentration of 0.75 ml/L reduced the TAN content from 0.8000 ppm (day 0) to 0.0754 ppm (day 45) in the culture water of European seabass juveniles (Dicentrarchus labrax).23 Moreover, Yucca schidigera extract has the ability to remove nitrite in aquaculture.4 Yucca extract is one of the effective alternatives in water quality control. Therefore, yucca extract becomes an ingredient in many commercial products for aquaculture, particularly in intensive systems.
Abdel-Tawwab et al. studied the effects of Yucca schidigera extract and yeast Saccharomyces cerevisiae on growth performance of Nile tilapia Oreochromis niloticus. The results showed that 1 g/L of both yucca extract and yeast significantly reduced ammonia concentrations in the pond water (0.046 – 0.055 mg/L) compared to that of the control ponds (0.124 mg/L).12 Biernasiak et al. investigated the impact of the combination of probiotics and yucca extract to the performance and faecal microflora of broiler chicken. This study suggested that the formula containing in one kg with Lactobacillus spp. (4.0 x 1010 CFU), Saccharomyces cerevisiae (4.0 x 106 CFU) and 50 grams of Yucca schidigera extract decreased Clostridium density in broilers’ faeces, although there is no significant differences of FBW, FCR, BWG among treatments. This formula may be an effective alternative to antibiotic growth promoters used in poultry breeding.24 Similar results were reported by Benamirouche et al., the diet supplemented with Pediococcus acidilactici MA18/5M (109 CFU/kg), Saccharomyces cerevisiae type boulardii CNCM I-1077 (109 CFU/kg), and Yucca schidigera extract was supplemented to the drinking water at a dose of 1L/1000L may successfully replace antibiotic in broiler production with the functions of health preservation and meat quality improvement.25 Especially, Abdo et al. studied the improvement of water quality, histopathology, antioxidant, and innate immunity by the combination of Bacillus species and Yucca schidigera extract in Nile tilapia culture with acute ammonia exposure. The results showed that the addition of Bacillus species and yucca extract mixture to the culture water of Nile tilapia significantly controlled the ammonia concentration, restored the pH and recovered the genes relating to inflammatory and immune at the control level.26 However, the option of combining yucca extract and Bacillus spp. (CYB) for improvement of water quality and aquatic animal’s growth performance has not been widely studied. Therefore, this study was conducted to determine the effects of combining Yucca schidigera extract and Bacillus spp. on ammonia and nitrite concentration, Vibrio count in Penaeus vannamei shrimp culture water. The shrimp growth parameters in 35 days of experiment in intensive tanks were also determined.
Material
Animals
3000 white leg shrimp postlarvae (PL12 – Penaeus vannamei), approximately 0.003 g/individual, were provided by Viet Uc Seafood JSC (Vietnam).
Bacillus Isolates
Bacillus sp. strains AQ1, BIO2, and BAL3 were isolated from sludge of Can Gio Mangrove Forest, southeast of Ho Chi Minh city, Vietnam in our previous study. The sterile vials were used for collecting the sludge samples from at least 10 cm of depth. The samples were stored in an ice box at 4°C, then transferred to the laboratory. The samples were plated onto NA media (Nutrient Agar) by spread plate method after being diluted with saline. Bacillus isolates were obtained and screened for ability of NH3 and NO2 removal. Bacillus sp. AQ1, BIO2, and BAL3 have a best ability to reduce NH3 and NO2 among isolates. These Bacillus strains were stored at –20°C Nutrient Broth (Himedia, India) containing 15% glycerol (v/v).27
Bacillus preparation
Bacillus strains (AQ1, BIO2, and BAL3) were grown in Nutrient Broth (Himedia, India) with shaking incubator at 37°C for 24 h and centrifuged at 6000 rpm for 20 min, the supernatants were discarded. The sterile saline solution (9‰ NaCl) was used for re-suspending and washing three times the bacterial pellet. A spectrophotometer was used for measuring the density of cell suspensions at 600 nm that correlated to colony–forming units (CFU) using a spread plate technique. The bacterial suspension of Bacillus strains were stored at 4°C until use or immediately applied to the rearing tanks.
Yucca Schidigera Extract
Yucca schidigera extract powder, which was used in this study, originates in XiAnRainbow Biotech Co., Ltd. – No. 4, South of Tianhu Road, Leping City, Jiangxi Province, China.
Experimental Design
The experiment was conducted at biotechnology department of Lien Hiep Phat Sci-Tech Co., Ltd (Ho Chi Minh city, Vietnam) and lasted for 35 days with 7 treatments, every treatment was triplicated. Total tank for shrimp culture is 21. Before the beginning of the trial, the shrimp were adapted to the rearing conditions for 3 days in a circular plastic tank (1000 L). The acclimatization conditions were set up similar to those in the hatchery including temperature (29°C), salinity (10%), DO (5 ppm), pH (7.8), and kH – Alkalinity (140 ppm). In this period, shrimp were fed daily with an industrial feed at 3% body weight. After adaptation, 100 shrimp were randomly reared in each 100L glass tank. The water parameters of experimental tanks were adjusted within appropriate range of white leg shrimp, as well as similar to the acclimatization stage, including kH – Alkalinity (140 – 160 ppm), salinity (10%), pH (7.8 – 8.2), temperature (28 – 30°C) and DO (4 – 6 ppm). The experimental tanks weren’t changed water during the trials. The tanks were treated with Bacillus spp. and yucca extract according to the following treatments:
- AY treatment: Bacillus sp. AQ1 and yucca extract.
- B2Y treatment: Bacillus sp. BIO2 and yucca extract.
- B3Y treatment: Bacillus sp. BAL3 and yucca extract.
- ABBY treatment: Bacillus consortium (AQ1, BIO2, BAL3) and yucca extract.
- ABB treatment: Bacillus consortium (AQ1, BIO2, BAL3).
- Y treatment: yucca extract only.
- Control treatment: No addition of yucca extract and Bacillus spp.
The dosages used in the treatments were 1.5 × 104 CFU/ml (Bacillus spp.),28 and 1 mg/L (Yucca extract).12,29 Bacillus spp. and yucca extract were added to the tanks every 7 days during the experiment. Each Bacillus strain was prepared in NB at 37oC for 24 hours. The method described in section 2.1.3 which was used for making a bacterial stock at 5 x 108 CFU/ml of each Bacillus strain. On the first day and every 7 days of the experiment, the bacterial stocks and yucca extract were adding into the 100L glass tanks of 6 treatments with 3 ml of AQ1 stock and 100 mg of yucca extract, 3 ml of BIO2 stock and 100 mg of yucca extract, 3 ml of BAL3 stock and 100 mg yucca extract, 1 ml of each bacterial stock (AQ1, BIO2, and BAL3) and 100 mg yucca extract, 1 ml of each bacterial stock (AQ1, BIO2, and BAL3) and none of yucca extract, and only 100 mg yucca extract to generate the final dosages in the culture water of these treatments containing 1.5 x 104 CFU/ml of Bacillus sp. AQ1 and 1 mg/L of yucca extract (AY), 1.5 x 104 CFU/ml of Bacillus sp. BIO2 and 1 mg/L of yucca extract (B2Y), 1.5 x 104 CFU/ml of Bacillus sp. BAL3 and 1 mg/L of yucca extract (B3Y), 1.5 x 104 CFU/ml of Bacillus sp. AQ1, BIO2, BAL3 and 1 mg/L of yucca extract (ABBY), 1.5 x 104 CFU/ml of Bacillus sp. AQ1, BIO2, BAL3 (ABB), and 1 mg/L of yucca extract (Y), respectively. The tanks of control treatment were not supplemented with Bacillus strains and yucca extract.
Feeding
Shrimp postlarvae were fed with industrial feed (Tomboy Aquafeed JSC) four times a day with 5 – 10% of body weight (8:00 a.m.; 12:00 p.m.; 4:00 p.m., and 8:00 p.m.).30,31
Chemical assays
Ammonia determination
Ammonia concentration was measured by APHA 4500 NH3 F – Phenate method. 0.2 ml of phenol solution (11.1 % v/v), 0.2 ml of sodium nitroprusside (0.5 % w/v ), 0.5 ml of oxydizing solution (Alkaline citrate and NaClO 5% – ratio 4:1) were added into the tube contained 5 ml of sample. UV/VIS Spectrophotometer (LLG – uniSPEC 2, Germany) was used for measuring this solution at 640 nm absorbance after incubation at least 1 hour in dark.32
Nitrite determination
Nitrite concentration was determined using APHA 9245 B – Multiple tube method. 1 ml of sulfanilic acid (1% v/v), 1 ml of N-(1-Naphthyl)-ethylenediamine dihydroclorua (0.1 % w/v) were added into the tube contained 5 ml of sample. UV/VIS Spectrophotometer (LLG – uniSPEC 2, Germany) was used for measuring this solution at 540 nm absorbance after incubation at least 30 minutes in dark.33
Determination of Bacillus and Vibrio density
Water samples were collected every 7 days during the experiment. The enumeration of bacteria was carried out by the spread plate technique. Saline (0.9% NaCl) was used to dilute the water samples for determining total plate count (TPC) on trypticase soy agar (TSA), Vibrio count (VC) on thiosulfate citrate bile salt sucrose agar (TCBS), Bacillus sp. (BS) on nutrient agar (NA) via spore count.34,35
Sampling procedure
15 ml of water sample was collected at a depth of 20 cm of each tank in a clear sterile glass tube with screw–cap (Pyrex, USA) by plastic Pasteur pipette for ammonia and nitrite determination (24 and 168 hours), and Bacillus and Vibrio density (168 hours) after adding Bacillus strains and yucca extract. After the experimental period (35 days), all shrimp in the experimental tanks of the treatments were harvested by a hand net (0.3 m in a diameter and 0.2 cm mesh size), weighed, and counted to determine survival rate (SR, %), the final biomass, the final body weight (FBW, g), weight gain rate (WGR, %), and feed conversion ratio (FCR).4,12
Weight gain rate (WGR, %) = ([Average final body weight (g) – Average initial weight (g)])/(Average initial weight) × 100
Feed conversion ratio (FCR) = (Feed intake (g))/(Alive weight gain (g))
Survival rate (SR, %) = (The number of shrimp at the end of the test)/(The number of shrimp at the beginning of the test) × 100
Statistical analysis
One-way analysis of variance (ANOVA) was used to analyze all data of experiments that determine the statistically significant differences among the treatments (Duncan’s test) by Statgraphics Centurion XV software.
Effect of CYB on ammonia and nitrite content of culture water
The obtained results showed that CYB was effective in controlling TAN and nitrite in shrimp culture water (Figure 1 and 2). The TAN concentrations of ABB, AY, B2Y, and B3Y treatments increased in the first week, then gradually decreased from the second week until the end of the experiment. The TAN content in the water of the Y treatment was well controlled within 3 weeks (D0 – D22), and increased in the remaining 2 weeks. At the end of the experiment, the TAN concentration in the water treated with ABBY, ABB, AY, B2Y and B3Y had no statistical difference and was significantly lower than compared to the treatment supplemented with only yucca extract (Y) and the control with ppm values of 0.073 ± 0.011a, 0.179 ± 0.011a, 0.079 ± 0.015a, 0.093 ± 0.018a, 0.080 ± 0.014a, 0.708 ± 0.050b, and 5,820 ± 0.107c, respectively.
The nitrite concentration in the water treated with only yucca extract at all sampling times was significantly lower than the control but higher than the treatments supplemented with Bacillus strains. The ABBY treatment showed the most effective control of nitrite content compared to the other treatments throughout the experiment. The AY, B2Y, and B3Y treatments needed 4 weeks to stabilize nitrite content in shrimp culture water. The ABB and Y treatments did not stably control the nitrite content of the water during the trial. At the end of the experiment, the lowest content of nitrite was found in the ABBY, AY, B2Y, and B3Y which was significantly different compared to the treatment supplemented with Bacillus consortium (ABB), only yucca extract (Y), and the control with ppm values of 0.187 ± 0.015a, 0.247 ± 0.025a, 0.242 ± 0.016a, 0.241 ± 0.017a, 0.934 ± 0.034b, 2.298 ± 0.114c, and 6.719 ± 0.576d, respectively.
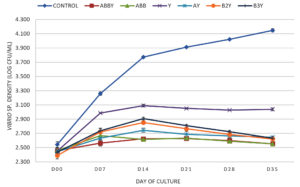
Effect of CYB on total Vibrio count in culture water
The initial TPC among the treatments was not statistically different, the density fluctuated in the range of 4.061 – 4.158 log CFU/ml. The significant differences of TPC were obtained at weeks 1 to 5, especially at week 5, where TPC of ABBY, ABB, AY, B2Y, and B3Y treatments was significantly higher compared to the others with log CFU/mL values of 5.331 ± 0.019b (Control), 5.771 ± 0.009c (ABBY), 5.767 ± 0.007c (ABB), 5.282 ± 0.018a (Y), 5.773 ± 0.011c (AY), 5.763 ± 0.005c (B2Y), and 5.781 ± 0.007c (B3Y). The results showed that the treatments supplemented with Bacillus strains had a shorter time to increase TPC compared to the control and the treatment only supplemented with yucca extract (Figure 3).
Figure 4 showed that the Bacillus density of the control and Y treatment had no statistical difference, only slightly increased after 2 weeks and almost unchanged until the end of the experiment. However, the Bacillus density of the remaining treatments increased obviously after the first week, and there was no statistical difference among treatments during the experimental period. The ABBY, ABB, AY, B2Y, and B3Y treatments had the highest Bacillus counts at the end of the experiment which was significantly different from the Bacillus counts of the control and Y treatment, with log CFU/ml values of 4.040 ± 0.022b, 4.023 ± 0.008b, 4.038 ± 0.016b, 4.010 ± 0.005b, 4.028 ± 0.009b, 2.564 ± 0.017a and 2.605 ± 0.013a, respectively. Thus, strains of Bacillus sp. AQ1, BIO2, BAL3 all grow and maintain high density in shrimp culture water.
The initial Vibrio count among the treatments had log CFU/ml values in the range of 2.389 – 2.542, that were not significantly different. After week 1, Vibrio count tended to increase in all treatments. In the treatments supplemented with Bacillus and yucca extract, the Vibrio density was stably controlled from week 2 – 5. At the end of the experiment, the ABBY and ABB treatments had the lowest total Vibrio count which was significantly different from the total Vibrio count of the other treatments with log CFU/ml values of 2.553 ± 0.014a (ABBY), 2.552 ± 0.022a (ABB), 3.038 ± 0.019c (Y), 2.645 ± 0.014b (AY), 2.621 ± 0.007b (B2Y), 2.631 ± 0.018b (B3Y), and 4.146 ± 0.022d (Control).
Effect of CYB on the growth performance of shrimp
Bacillus strains and yucca extracts had a positive effect on the growth parameters of shrimp (Table). The final body weight, biomass, weight gain rate, survival rate, and FCR of the treatments supplemented with Bacillus strains and yucca extract were all significantly better than the control treatment.
Table :
Effect of CYB on growth parameters of shrimp.
Items |
Control |
ABBY |
ABB |
Y |
AY |
B2Y |
B3Y |
|---|---|---|---|---|---|---|---|
FBW (g) |
0.220 ± 0.001a |
0.424 ± 0.010e |
0.335 ± 0.008d |
0.270 ± 0.004bc |
0.344 ± 0.007d |
0.290 ± 0.006c |
0.267 ± 0.008b |
Biomass (g) |
15.275 ± 0.294a |
40.202 ± 1.510e |
30.628 ± 0.956d |
23.922 ± 0.403b |
32.249 ± 0.580d |
27.091 ± 0.857c |
24.348 ± 0.520b |
WGR (%) |
7244.434 ± 20.521 a |
14048.902 ± 328.756e |
10718.952 ± 252.471d |
8895.695 ± 148.333bc |
11377.005 ± 219.665d |
9570.604 ± 185.069c |
8791.164 ± 271.370b |
SR (%) |
40.333 ± 8.819 a |
94.667 ± 1.856b |
94.333 ± 0.882b |
88.667 ± 1.453b |
93.667 ± 0.333b |
93.333 ± 1.202b |
91.333 ± 0.882b |
FCR |
2.056 ± 0.034c |
1.100 ± 0.040a |
1.265 ± 0.038b |
1.317 ± 0.022b |
1.089 ± 0.020a |
1.231 ± 0.039b |
1.294 ± 0.027b |
The FCR of shrimp from ABBY and AY treatments were significantly lower (1.100 ± 0.040a, and 1.089 ± 0.020a, respectively) than that of shrimp from the other treatments with values of 2.056 ± 0.034c (Control), 1.265 ± 0.038b (ABB), 1.317 ± 0.022b (Y), 1.231 ± 0.039b (B2Y), 1.294 ± 0.027b (B3Y). The survival rate of the treatments supplemented with Bacillus strains and yucca extract was not significantly different ranging from 88 to 94%. The weight gain rate (WGR) of ABBY treatment was the highest and was significantly different from all other treatments. In the treatment only adding yucca extract, WGR was higher than that of the control but lower than that of all other treatments. FWB and biomass of ABBY treatment were significantly higher than that of the other treatments (Table).
Effect of CYB on ammonia and nitrite content of culture water
The combination of two different agents including Bacillus sp. and Yucca schidigera extract in controlling water quality and improving growth parameters of P. vannamei shrimp has not yet been studied. The results of this study showed that the combination of Bacillus spp. and yucca extract were significantly better than either single agent. Treatment of ABBY, AY, B2Y, and B3Y with 1 mg/L of yucca extract and 1.5 x 104 CFU/ml of Bacillus spp. demonstrated the ability to effectively reduce TAN and nitrite significantly compared to using yucca extract only (1 mg/L – Y) or Bacillus consortium (1.5 x 104 CFU/ml – ABB). This result is similar to the study of Abdel-Tawwab et al., yucca extract (1 mg/L) combined with yeast Saccharomyces cerevisiae (1 mg/L) was effective in reducing ammonia in rearing Nile tilapia O. niloticus.12 Novriadi et al. demonstrated that the addition of Sapotan PowderTM which contains oak – yucca extract with 19.2 mg/L in the water from a pacific white shrimp culture system resulted in the lowest contents of TAN and nitrite with ppm values of 0.0165 ± 0.0058 and 0.2656 ± 0.0486, respectively. In this research the addition of CYB containing 1 mg/L of yucca extract resulted in the lowest concentrations of TAN and nitrite with ppm values of 0.073 ± 0.011 and 0.187 ± 0.015, respectively. This proved that combining yucca extract with Bacillus spp. reduces the cost of TAN and nitrite control. Dawood et al. suggested that Yucca schidigera extract contains the steroidal saponin and glycocomponent fraction that can bind ammonia or convert ammonia into nitrite and nitrate.18 On the other hand, Bacillus sp. was considered as one of the probiotic strains commonly used for controlling ammonia and nitrite in aquaculture.2 Bacillus spp. likely possess the hao gene encoding the enzyme hydroxylamine oxidoreductase, and nxrB gene encoding the enzyme nitrite oxidoreductase, which is involved in the oxidation of ammonia and nitrite.36 This made the reduction of ammonia and nitrite more powerful when the yucca extract was combined with Bacillus strains.
Effect of CYB on total Vibrio count in culture water
In the present study, Bacillus counts of ABBY, AY, B2Y, and B3Y treatments were much higher than those of the control and the treatment with yucca extract only. This shows that the Bacillus strains added to the shrimp tank grew well until the end of the experiment. In addition, the tanks were supplemented with yucca and Bacillus spp. will control Vibrio sp. better than the addition of yucca extract. The ABBY and ABB treatments had a Bacillus consortium (AQ1, BIO2, and BAL3) have outstanding Vibrio control. Bacillus sp. can control pathogenic bacteria in ponds with antibacterial compounds and quorum-quenching enzymes.37 Bacillus species produce more than 800 different peptide antibiotics, which are antibacterial compounds that effectively against bacteria [38]. Some antibacterial peptides derived from Bacillus spp. including bacitracin (Bacillus megaterium, Bacillus subtilis, and Bacillus licheniformis), lichenin (Bacillus licheniformis), megacin (Bacillus megaterium), coagulin (Bacillus coagulans), polyfermenticin (Bacillus polyfermenticus), cerein (Bacillus cereus), thuricins, tochicin, kurstakin, entomocin and bacthuricin (Bacillus thuringiensis).39-41 The chemical signal molecules (autoinducers) were produced and released from quorum sensing bacteria in response to change the cell populatin density.42 While there are many types of signaling molecules produced by bacteria, Autoinducer-1 (AHLs – N-acyl homoserine lactones) derived from gram-negative bacteria is perhaps the most interesting.43 Marine Vibrio is a common genus of bacteria that produces AHLs signaling molecules.44 Quorum quenching implies degradation of autoinducer signaling molecules by the enzymes AHL lactonase, AHL acylase, and AHL oxidoreductase.45 Bacillus species (Bacillus cereus, Bacillus mycoides, and Bacillus thuringiensis) are AHL-degrading bacteria that produce AHL lactonase enzymes, which hydrolyze the lactone ring of AHLs to acylated homoserine.46,47 Bacillus cereus LNE7 was shown to degrade the signaling molecule (AHL N-3-hydroxybutanoyl- -homoserine lactone) of Vibrio campbellii.48 Bacillus thuringiensis and Bacillus cereus isolated from barramundi fish Lates calcarifer identified as AHL-degrading bacteria which produce AHL lactonase enzymes (AiiA group). These two strains are resistant to Vibrio haveryi and Vibrio alginolyticus through degradation of two signaling molecules N-[(RS)-3-Hydroxybutyryl]- -homoserine lactone and N-(3-Oxodecanoyl)- -homoserine lactone.43 Bacillus licheniformis DAHB1 was determined to inhibit Vibrio parahaemolyticus DAHP1-GFP through quorum quenching with the enzyme AHL lactonase.49 Furthermore, Bacillus strains have the ability to prevent the dominance of other microorganisms including pathogenic microorganisms, helping to maintain a stable microbiome in ponds.8 Previous studies also reported that the consortium of microorganisms and the Bacillus consortium were able to antagonize or inhibit very effectively Vibrio spp. in shrimp culture.6,9 Additionally, Surawut et al. suggested that the Bacillus consortium (Bacillus subtilis, Bacillus licheniformis, and Bacillus megaterium) could control severity level of AHPND disease caused by Vibrio parahaemolyticus in white shrimp Litopenaeus vannamei.50 In another study, Zhang et al. reported that the combination of Bacillus consortium (Bacillus subtilis and Bacillus licheniformis) and isomaltooligosaccharide (IMO) added to the diet at 108 CFU/g and 0.2%, respectively, reduced the total Vibrio density in the shrimp gut and improved survival rate of shrimp challenged with Vibrio alginolyticus.51 The correlation between the TPC and total Vibrio count among treatments (Figures 3 and 5) showed that Vibrio sp. in the control treatment was the predominant bacterial strain with 4.146 ± 0.022d log CFU/ml (approximately 1,504 x 104 CFU/ml) while the total Vibrio count exceeding 104 CFU/ml of pond water can cause diseases in shrimp.52
Effect of CYB on the growth performance of shrimp
Nitrite and TAN concentrations in the water of the control treatment increased over time compared to the other treatments (Figure 1 and 2). This is probably the reason why shrimp growth parameters (FBW, biomass, WGR, and FCR) of the control treatment were much worse than that of the other treatments, especially the survival rate with 40,333 ± 8.819a % (Table). In contrast, the ABBY treatment with the combination of Bacillus consortium and the yucca extract showed better growth parameters than the other treatments. This is similar to the study of Abdel-Tawwab et al. when the addition of a combination of Saccharomyces cerevisiae and yucca to the pond water increased the growth parameters of Nile tilapia Oreochromis niloticus better than using either yucca extract or yeast Saccharomyces cerevisiae.12 Bacillus cereus and Pediococcus acidilactici probiotics, which were added to ponds water, improved FCR and weight gain of white leg shrimp (Penaeus vannamei).53 In addition, Barman et al. also tested the consortium of Rhodopseudomonas palustris SUP-2, Bacillus subtilis SUP-3, and Bacillus firmus SUP-1 in the culture of white shrimp Penaeus vannamei, the results showed that the bacterial consortium significantly promoted the shrimp growth parameter.37 Previous studies have suggested that extracellular enzymes biosynthesized from Bacillus spp. such as protease, lipase and carbohydrolase promote better shrimp growth and improve pond water quality.5,9 Besides, yucca extract in the previous study has also shown the ability to promote the growth of white leg shrimp higher than the control with 9.76% (FBW – Final body weight), 9.72% (PWG – Percentage weight gain), and 20.81% (Biomass).4 Although the mechanism of yucca extract’s growth-promoting is still unclear, steroidal saponins and other bioactive compounds of Yucca schidigera may cause the cell membrane structure of the animal’s gastrointestinal epithelium changes in a positive way,54 helping to better absorb nutrients.4,31 Some other theories suggest that yucca extract stimulates animal biosynthesis of digestive enzymes, enhances metabolism, and increases nutrient absorption.4,55
Bacillus consortium combined with yucca extract efficiently improved the water quality by controlling TAN and nitrite concentrations, and significantly inhibited Vibrio spp that enhances surviral rate of shrimp. This combination also effectively enhanced the FBW, biomass, WGR, and FCR of Penaeus vannamei shrimp that was better than either Bacillus sp. or yucca extract.
The present study showed that the combination of yucca extract (1 mg/L) and Bacillus consortium (1.5 x 104 CFU/ml) could effectively reduce 46.49% of FCR, promote shrimp growth parameters higher than compared to the control with approximately 92.73% (FBW), 163.19% (Biomass), and 93.93% (WGR), exhibit efficient control of Vibrio sp., ammonia, and nitrite with values of 2.553 log CFU/ml, 0.073 ppm, 0.187 ppm compared to control with value of 4.146 log CFU/ml, 5.820 ppm, 6.719 ppm, respectively, in the white leg shrimp rearing water. This combination as water additives was suitable for adding to the rearing water of P. vannamei (PL12) with density of 100 individuals/100L.
ACKNOWLEDGMENTS
The authors would like to thank Lien Hiep Phat Science Technology Company Limited for providing necessary facilities to carry out the research work. Authors also acknowledge Ho Chi Minh City University of Technology (HCMUT), VNU-HCM for supporting this study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
TDP designed the experiments, analyzed the data and wrote the manuscript. TDP, PTTD, BTHL and TVM performed the experiment and collected the data. NTH performed supervision and revised the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
This research is funded by Ho Chi Minh City University of Technology, Vietnam National University-Ho Chi Minh City (VNU-HCM).
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Frias-Espericueta MG, Harfush-Melendez M, Paez-Osuna F. Effects of ammonia on mortality and feeding of postlarvae shrimp Litopenaeus vannamei. Bull Environ Contam Toxicol. 2000;65(1):98-103.
Crossref - Hlordzi V, Kuebutornye FKA, Afriyie G, et al. The use of Bacillus species in maintenance of water quality in aquaculture: A review. Aquaculture Reports. 2020;18:100503.
Crossref - Rostami F, Davoodi R, Nafisi Bahabadi M, Salehi F, Nooryazdan H. Effects of ammonia on growth and molting of Litopenaeus vannamei postlarvae reared under two salinity levels. J Appl Aquac. 2019;31(4):309-321.
Crossref - Novriadi R, Albasri H, Wahyudi AE, Fadhilah R, Ali A, Trullas C. Effects of the addition of oak (Quercus robur L.) and yucca (Yucca schidigera) on the water quality and growth performance of pacific white shrimp (Litopenaeus vannamei) cultured intensively in concrete tanks. Journal of the World Aquaculture Society. 2022;53(5):984-994.
Crossref - Chen L, Lv C, Li B, et al. Effects of Bacillus velezensis Supplementation on the Growth Performance, Immune Responses, and Intestine Microbiota of Litopenaeus vannamei. Front Mar Sci. 2021;8:744281.
Crossref - Barman P, Raut S, Sen SK, Shaikh U, Bandyopadhyay P, Mohapatra PKD. Effect of a three-component bacterial consortium in white shrimp farming for growth, survival and water quality management. Acta Biologica Szegediensis. 2017;61(1):35-44. https://abs.bibl.u-szeged.hu/index.php/abs/article/view/2911
- Kuebutornye FKA, Abarike ED, Lu Y. A review on the application of Bacillus as probiotics in aquaculture. Fish Shellfish Immunol. 2019;87:820-828.
Crossref - Soltani M, Ghosh K, Hoseinifar SH, et al. Genus Bacillus, promising probiotics in aquaculture: Aquatic animal origin, bio-active components, bioremediation and efficacy in fish and shellfish. Reviews in Fisheries Science & Aquaculture. 2019;27(3):331-379.
Crossref - Ren W, Wu H, Guo C, et al. Multi-Strain Tropical Bacillus spp. as a Potential Probiotic Biocontrol Agent for Large-Scale Enhancement of Mariculture Water Quality. Front Microbiol. 2021;12:699378.
Crossref - Zokaeifar H, Babaei N, Saad CR, Kamarudin MS, Sijam K, Balcazar JL. Administration of Bacillus subtilis strains in the rearing water enhances the water quality, growth performance, immune response, and resistance against Vibrio harveyi infection in juvenile white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2014;36(1):68-74.
Crossref - Adegbeye MJ, Elghandour MMMY, Monroy JC, et al. Potential influence of Yucca extract as feed additive on greenhouse gases emission for a cleaner livestock and aquaculture farming – A review. Journal of Cleaner Production. 2019;239:118074.
Crossref - Abdel-Tawwab M, Mounes HAM, Shady SHH, Ahmed KM. Effects of yucca, Yucca schidigera, extract and/or yeast, Saccharomyces cerevisiae, as water additives on growth, biochemical, and antioxidants/oxidant biomarkers of Nile tilapia, Oreochromis niloticus. Aquaculture. 2021;533:736122.
Crossref - Elbialy ZI, Rizk M, Al Hawary II, et al. Yucca schidigera extract mediated the growth performance, hepato renal function, antioxidative status and histopathological alterations in Nile tilapia (Oreochromis niloticus) exposed to hypoxia stress. Aquaculture Research. 2020;52(5):1965-1976.
Crossref - Wang L, Wu D, Fan Z, et al. Effect of Yucca schidigera extract on the growth performance, intestinal antioxidant status, immune response, and tight junctions of mirror carp (Cyprinus carpio). Fish Shellfish Immunol. 2020;103:211-219.
Crossref - Bae J, Hamidoghli A, Won S, et al. Evaluation of seven different functional feed additives in a low fish meal diet for olive flounder, Paralichthys olivaceus. Aquaculture. 2020;525:735333.
Crossref - Acosta MH, Salazar GJG, Saenz FMG, et al. The effects of Yucca schidigera and Quillaja saponaria on growth performance and enzymes activities of juvenile shrimp Litopenaeus vannamei cultured in low salinity water. Lat Am J Aquat Res. 2016;44(1):121-128.
Crossref - Preetham E, Amitha K, Sreeja L, Caterina F, Angeles EM, Einar R. Herbal Immunomodulators in Aquaculture. Reviews in Fisheries Science & Aquaculture. 2020;29(1):33-57.
Crossref - Dawood MAO, Gewaily MS, Monier MN, Younis EM, Van Doan H, Sewilam H. The regulatory roles of yucca extract on the growth rate, hepato-renal function, histopathological alterations, and immune-related genes in common carp exposed with acute ammonia stress. Aquaculture. 2021;534:736287.
Crossref - Killeen GF, Madigan CA, Connolly CR, et al. Antimicrobial Saponins of Yucca schidigera and the Implications of Their in Vitro Properties for Their in Vivo Impact. J Agric Food Chem. 1998;46:3178-3186.
Crossref - Fleck JD, Betti AH, da Silva FP, et al. Saponins from Quillaja saponaria and Quillaja brasiliensis: Particular Chemical Characteristics and Biological Activities. Molecules. 2019;24(1):171.
Crossref - Castillo-Vargasmachuca S, Ponce-Palafox J, Arredondo-Figueroa J, et al. The effect of Yucca schidigera liquid extract on water quality and survival of Pacific Red Snapper Lutjanus peru during acclimatization. Arch Med Vet. 2015;47(1):107-109.
Crossref - Santacruz-Reyes RA, Chien YH. The potential of Yucca schidigera extract to reduce the ammonia pollution from shrimp farming. Bioresour Technol. 2012;113:311-314.
Crossref - Fayed WMA, Khalil RH, Sallam GR, Mansour AT, Elkhayat BK, Omar EA. Estimating the effective level of Yucca schidigera extract for improvement of the survival, haematological parameters, immunological responses and Water quality of European seabass juveniles (Dicentrarchus labrax). Aquaculture Reports. 2019;15:100208.
Crossref - Biernasiak J, Zli|ewska K, Libudzisz Z, Smulikowska S. Effect Of Dietary Probiotic Containing Lactobacillus Bacteria, Yeast And Yucca Extract On The Performance And Faecal Microflora Of Broiler Chickens. Pol J Food Nutr Sci. 2007;57(4(A)):19-25. http://journal.pan.olsztyn.pl/effect-of-dietary-probiotic-containing-lactobacillus-bacteria-yeast-and-yucca-extract,98777,0,2.html
- Benamirouche K, Baazize-Ammi D, Hezil N, Djezzar R, Nia A, Guetarni D. Effect of probiotics and Yucca schidigera extract supplementation on broiler meat quality. Acta Sci Anim Sci. 2019;42(1):e48066.
Crossref - Abdo SE, El-Nahas AF, Abdelmenam S, et al. The synergetic effect of Bacillus species and Yucca shidigera extract on water quality, histopathology, antioxidant, and innate immunity in response to acute ammonia exposure in Nile tilapia. Fish Shellfish Immunol. 2022;128:123-135.
Crossref - Phuc TD, Huong NT, Thong DH, Tung CV, Dan PTT, Phuong TTM. Investigation of Ability to Remove NH3 and NO2 By Combination of Yucca Schidigera Extract and Bacillus Strains. IOSR J Biotechnol Biochem. 2021;7(4):01-17.
Crossref - Kewcharoen W, Srisapoome P. Probiotic effects of Bacillus spp. from Pacific white shrimp (Litopenaeus vannamei) on water quality and shrimp growth, immune responses, and resistance to Vibrio parahaemolyticus (AHPND strains). Fish Shellfish Immunol. 2019;94:175-189.
Crossref - Reham AA, Mounes HAM, Ahmed KM. Use of humic acid and Yucca extract as a benefactor on water quality and their impact on some hematological and histological parameters of Oreochromis niloticus. Egyptian Journal of Aquatic Biology and Fisheries. 2018;22(5):447-460.
Crossref - Tacon AGJ, Jory D, Nunes A. Shrimp feed management: issues and perspectives. presented at: On-farm feeding and feed management in aquaculture workshop; 13-15 September, 2010 2013; Manila, Philippines. https://www.fao.org/fishery/docs/CDrom/T583/root/18.pdf
- Yang Q-h, Tan B-p, Dong X-h, Chi S-y, Liu H-y. Effects of different levels of Yucca schidigera extract on the growth and nonspecific immunity of Pacific white shrimp (Litopenaeus vannamei) and on culture water quality. Aquaculture. 2015;439:39-44.
Crossref - Weatherburn MW. Phenol-Hypochlorite Reaction for Determination of Ammonia. Analytical Chemistry. 1967;39(8):971-974.
Crossref - Nerdy N, De Lux Putra E. Spectrophotometric Method for Determination of Nitrite and Nitrate Levels in Broccoli and Cauliflower with Different Fertilization Treatment. Oriental Journal of Chemistry. 2018;34(6):2983-2991.
Crossref - Dabade DS, Wolkers-Rooijackers JC, Azokpota P, et al. Bacterial concentration and diversity in fresh tropical shrimps (Penaeus notialis) and the surrounding brackish waters and sediment. Int J Food Microbiol. 2016;218:96-104.
Crossref - Marwiyah UC, Mahasri G, Ratnasari RE, Wiradana PA. Total plate count and identification of vibrio in pacific white shrimp (Litophenaeus vannamei) from ponds and in those exposed to immunogenic protein membrane Zoothamnium penaei. IOP Conference Series: Earth and Environmental Science. 2019;236:012087.
Crossref - Mendoza LFD, Quimi Mujica JG, Risco Cunayque JM, et al. Assessment of Heterotrophic Nitrification Capacity in Bacillus spp. and its Potential Application in the Removal of Nitrogen from Aquaculture Water. J Pure Appl Microbiol. 2019;13(4):1893-1908.
Crossref - Jayaprakashvel M, Subramani R. Implications of Quorum Sensing and Quorum Quenching in Aquaculture Health Management. Implication of Quorum Sensing and Biofilm Formation in Medicine, Agriculture and Food Industry. 2019:299-312.
Crossref - Saxena S. Microbes in Production of Fine Chemicals (Antibiotics, Drugs, Vitamins, and Amino Acids). Appl Microbiol. 2015:83-120.
Crossref - Al-Thubiani ASA, Maher YA, Fathi A, et al. Identification and characterization of a novel antimicrobial peptide compound produced by Bacillus megaterium strain isolated from oral microflora. Saudi Pharm J. 2018;26(8):1089-1097.
Crossref - Francis D. Antimicrobials from Microbes. Bioresources and Bioprocess in Biotechnology. 2017:291-326.
Crossref - Kim JC, Jeon B. Novel adjuvant strategy to potentiate bacitracin against MDR MRSA. J Antimicrob Chemother. 2016;71(5):1260-1263.
Crossref - Huang J, Shi Y, Zeng G, et al. Acyl-homoserine lactone-based quorum sensing and quorum quenching hold promise to determine the performance of biological wastewater treatments: An overview. Chemosphere. 2016;157:137-151.
Crossref - Ghanei-Motlagh R, Mohammadian T, Gharibi D, et al. Quorum Quenching Properties and Probiotic Potentials of Intestinal Associated Bacteria in Asian Sea Bass Lates calcarifer. Mar Drugs. 2019;18(1).
Crossref - Purohit AA, Johansen JA, Hansen H, et al. Presence of acyl-homoserine lactones in 57 members of the Vibrionaceae family. J Appl Microbiol. 2013;115(3):835-847.
Crossref - Chen F, Gao Y, Chen X, Yu Z, Li X. Quorum quenching enzymes and their application in degrading signal molecules to block quorum sensing-dependent infection. Int J Mol Sci. 2013;14(9):17477-500.
Crossref - Fast W, Tipton PA. The enzymes of bacterial census and censorship. Trends Biochem Sci. 2012;37(1):7-14.
Crossref - Noorashikin MN, Karim M, Daud HM, Natrah FMI Screening and identification of quorum sensing degraders from live feed Artemia. J Environ Biol. 2016;37:811-816. http://www.jeb.co.in/index.php?page=abstract&issue=201607_jul16_spl&number=21
- Pande GS, Natrah FM, Flandez AV, et al. Isolation of AHL-degrading bacteria from micro-algal cultures and their impact on algal growth and on virulence of Vibrio campbellii to prawn larvae. Appl Microbiol Biotechnol. 2015;99(24):10805-13.
Crossref - Vinoj G, Vaseeharan B, Thomas S, Spiers AJ, Shanthi S. Quorum-quenching activity of the AHL-lactonase from Bacillus licheniformis DAHB1 inhibits Vibrio biofilm formation in vitro and reduces shrimp intestinal colonisation and mortality. Mar Biotechnol (NY). 2014;16(6):707-715.
Crossref - Surawut S, Suntara K, Puckdee W, et al. Decreased severity of acute hepatopancreatic necrosis disease in white shrimp (Litopenaeus vannamei) by mixed culture of Bacillus subtilis, Bacillus licheniformis and Bacillus megaterium. Trop Life Sci Res. 2023;34(1):85-98.
Crossref - Zhang Q, Tan B, Mai K, et al. Dietary administration of Bacillus (B. licheniformis and B. subtilis) and isomaltooligosaccharide influences the intestinal microflora, immunological parameters and resistance against Vibrio alginolyticus in shrimp, Penaeus japonicus (Decapoda: Penaeidae). Aquaculture Research. 2011;42(7):943-952.
Crossref - Supono, Wardiyanto, Harpeni E, Khotimah AH, Ningtyas A. Identification of Vibrio sp. as cause of white feces diseases in white shrimp Penaeus vannamei and handling with herbal ingredients in East Lampung Regency, Indonesia. AACL Bioflu. 2019;12(2). http://www.bioflux.com.ro/docs/2019.417-425.pdf
- Khademzade O, Zakeri M, Haghi M, Mousavi SM. The effects of water additive Bacillus cereus and Pediococcus acidilactici on water quality, growth performances, economic benefits, immunohematology and bacterial flora of whiteleg shrimp (Penaeus vannamei Boone, 1931) reared in earthen ponds. Aquaculture Research. 2020;51(5):1759-1770.
Crossref - Goetsch A, Owens F. Effects of sarsaponin on digestion and passage rates in cattle fed medium to low concentrate. J Dairy Sci. 1985;68(9):2377-2384.
Crossref - Paray BA, El-Basuini MF, Alagawany M, Albeshr MF, Farah MA, Dawood MAO. Yucca schidigera Usage for Healthy Aquatic Animals: Potential Roles for Sustainability. Animals. 2021;11(1):93.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.