ISSN: 0973-7510
E-ISSN: 2581-690X
The ecological success of microbes in consortium which was otherwise compatible in plate assay was evaluated in mung bean in this study. It was found that even though they show compatibility in plate assay, they may not always have synergistic effects when applied as consortium in the crop. Three isolates from rice rhizosphere (RI-3, RII-4 and RIII-4) were tested in single, dual and triple inoculants consortium in mungbean. Plants were raised in green house with eight treatments containing single, dual and triple inoculant consortium with appropriate control to check the ecological compatibility of three selected strains. The isolates were found enhancing seed germination, leaf emergence, 50% flowering, chlorophyll content, number of branches, number of pods per plant, number of seeds per plant, protein content and nutrient uptakeby plants as compared to control (untreated plants). Results were pronounced in sterilised soils than unsterlised soil. Higher seed yield was recorded in sterilized soil from dual inoculation of RI-3+RII-4 (10.21g/plant) and RI-3+RIII-4 (9.75g/plant) as compared to control (5.62g/plant) in sterilised soil. Though all three isolates were compatible, plants receiving triple inoculation produced 7.33g/plant of seed yield which was significantly higher than control but lower to dual inoculation. Dual inoculation found more ecological successful than triple. A more or less similar trend was observed in other parameters too. However, the effect of inoculation was clearer in sterile soil.
Ecological compatibility, Inoculation, Consortia, Plant growth promotion, Mungbean
Legumes have its own importance in nutrition, and have ecological significance too. It is rich in protein and fixes atmospheric nitrogen into soil (Beck and Roughley, 1987). One of the reasons for poor productivity of pulses is their cultivation low input agriculture. Thus, there is huge opportunity of fulfilling yield gap in legume crops. At the same time, awareness towards reducing use of agrochemicals is need of the hour. Thus, it is high time to have search for ecologically safe and sustainable way of crop production.
Effects of inoculation are proven from time immemorial. It is utilizing the enormous power of tiny microbes for substituting part of agrochemicals. The legumes specifically, are suitable example of inoculation in form of legume-Rhizobium symbiosis. There is an array of plant growth promoting rhizobacteria available with different capabilities for enhancing growth and yield of pulses. Nitrogen fixation of 50-55kg/ha by mung bean rhizobia was reported by Wani and Lee (1991). Bacillus subtilis inoculation with rock phosphate found enhancing phosphorus (P) nutrition of mung bean in P- deficient soils (Gaind and Gaur, 1991). Growth promotion in mung bean by plant growth promoting (PGP) rhizobacteria Enterobacter cloacae CAL3 was reported by Mayak et al. (2001). It was found enhancing plant yield and thus useful in reducing agrochemical load. Plant growth promoting rhizobacteria and rhizobia induces salt tolerance in mung bean by production of 1- amino cyclopropane -1- carboxy deaminase. This enzyme induces salt tolerance by reducing ethylene toxicity caused by high salt stress (Ahmad et al. 2011).
The effective use of these potential microbes in consortium can provide holistic approach in achieving high yield in low input. Consortium of microbes performs outstanding as compare to single organisms (Alagawadi and Gaur, 1992; Ahmad et al. 2011) and produces synergistic effects (Rather et al.2010).
This study was conducted to check the ecological compatibility of isolates so that a better consortial combination can be screened. This may help in proper nutrient management by bioagents to utilize synergistic effects of consortia combination for enhancing yield of legumes like mung bean.
Collection of rhizospheric soil samples
Soil samples from rhizosphere of rice (Oryza sativa L.) cv PR-118 and PRH-10 cultivated conventionally in a field at Directorate of Seed Research, Mau Nath Bhanjan, Uttar Pradesh were collected at different stages of growth i.e. seedling stage [37 days old after transplantation (RI Stage)]; booting stage [80 days old after transplantation (RII Stage)] and maturating stage [100 days old after transplantation (RIII Stage)]. These samples were processed in Molecular Biology Laboratory, ICAR-National Bureau of Agricultural Microorganisms (NBAIM), Mau Nath Bhanjan, Uttar Pradesh, India.
Isolation and maintenance of bacteria
Isolation of bacteria from rhizospheric soil was done by serial dilution plate count techniqueon different media like Yeast Mannitol Agar (YEMA), Pseudomonas Isolation agar (PIA), Actinomycetes isolation agar (AIA), Jenson’s media (JM), Pikovskaya’s Agar (PSB) and Nutrient Agar (NA) (All media were procured from HiMedia LTD., India) at 37±1°C for 24-48 hours.Total 101 rhizobacterial isolates were obtained from different cultivars and at different stages of growth. All isolates were maintained as per standard protocols.
In vitro screening of rhizobacteria for multiple plant growth promoting activities
All bacterial isolates were initially screened in vitro for their plant growth promoting (PGP) traits like P-solubilization, production of siderophore and ammonia. Twelve best isolates having multiple PGP traits were selected and further evaluated for quantitative assay for P-solubilization and indole acetic acid (IAA) production.Various in vitro PGP tests conducted were- siderophore production by CAS agar method and Universal Chemical Assay [CAS] (Schwyn and Neilands, 1987; Milagres et al. 1999); Nitrate reduction (Winter Version 2014); ammonia production (Cappucino and Sherman, 1992); qualitative and quantitative estimation of phosphate solublization (Nautiyal, 1999; Nautiyal and Mehta, 2001; Jackson, 1973) and IAA production (Loper and Schroth, 1986; Gorden and Weber, 1951) (Data not shown).
Compatibility among the selected isolates
Three best performing isolates in terms of multiple plant growth promoting traitsin vitro conditions viz., RI-3, RII-4 and RIII-4 were selected for evaluation as microbial bioinoculant for nutrient mobilization and PGP activity on mungbean in pot condition. Compatibility among all three isolates was tested to formulate consortia. The method described by Nikam et al. (2007) with slight modifications was used for in-vitro compatibility testing. Six mm size sterilized paper (whatman filter paper no.1) discs impregnated with bacterial suspension of RI-3, RII-4 and RIII-4. RI-3, RII-4 and RIII-4 of individual isolates were spread on Nutrient media plates the disc which is impregnated with bacterial suspension was placed on the petriplate. The Bacterial isolates were allowed to grow for 24 hrs at 37ºC. After incubation the zone of inhibition, if any was measured. All the isolates against each other were screened on this pattern. Suitable control (without bacterial isolates) was also taken.
Bacterial growth kinetics
Growth curves were drawn for all three selected isolates to determine optimum age of broth culture for inoculation. Broth culture of each microorganism kept under shaker @ 150 rpm at 37ºC and samples were drawn from it periodically at three hour intervals till 72 hour for determining microbial population. Appropriate dilutions of these samples were plated onto nutrient agar medium. All three isolates were found to reach to its maximum growth by 24 hrs of incubation. Thus cultures were inoculated 24 hrs before the constructing bioformulation.
Development of bioformulation
On the basis of grown curve drawn, the sterile broths of respective microorganisms were inoculated in such a way that it should reach to its maximum growth phase at the time of constituting consortium.
The formulation was developed with slight modification in a process as described by Amer and Utkhede (2000) using charcoal as carrierwith all aseptic precautions. RI-3, RII-4 and RIII-4 and consortia of RI-3 and RII-4 ; RI-3 and RIII-4; RII-4 and RIII-4 and consortia of RI-3, RII-4 and RIII-4 were grown individually in liquid nutrient broth for 24 hr as shaker culture in shaker incubator @ 150 rpm at 37ºC temperature. The carboxy methyl cellulose (CMC), carrier and bacterial suspension in broth (108 CFU/ml) were used in 1:25:15 ratio. The bioformulations were dried aseptically at room temperature. The materials were stored in sealed plastic bags at room temperature.
Seed coating with bioformulation
Seeds of mungbean (Vigna radiata L.) variety IPM 2-3 collected from Chandra Shekhar Azad University of Agriculture and Technology, Kanpur were coated with the bio formulationfor pot experiment.One gram of the respectiveinoculant formulation was added to 50 mung bean seeds moistened with 0.1 ml sterile distilled water in a sterile plastic bag. The control was seeds without any coating. The mixture was shaken gently until a fine film of bioformulation is coated around the seeds. The material was transferred at CSAUA&T, Kanpur for pot experiment. The following was the treatment detail-RI-3 (T1), RII-4(T2), RIII-4(T3), RI-3+ RII-4(T4), RI-3+ RIII-4(T5), RII-4+ RIII-4(T6) and triple inoculant consortia of RI-3RII-4+ RIII-4(T7) and control (T8).These treatments were taken to evaluate their effects on the growth, yield and nutrient uptake of mungbean.
The earthen pots had a size of 123 in diameter and capacity to hold 8 kg of soil (medium black and calcareous, pH 7.4, organic carbon 0.51%, total nitrogen content 0.2125 g kg”1, available phosphorus 20kg ha”1 and K2O 210 kg ha”1). There were a total of 8 treatments, two levels of sterilization (Unsterile and sterilized soil)each having 3 replications. Recommended dose of fertilizer was applied before sowing (NPK&S 20:40:20:20). Pots were kept in the glass house and watered at regular intervals. In each pot, eight seeds were sown at a depth of 5 cm. After germination, five seedlings were maintained in each pot. Observations recorded were-germination percent, plant height at different stages, number of branches/plant, 50% flowering , dry weight/plant (g), chlorophyll content (mg/g leaf) , number of clusters/plant,number of pods/cluster, number of pods/plant , number of seeds/pod , 1000-seed weight , N and P uptake in grains (%), protein content of grains (%), etc.
Statistical analysis
Statistical analysis of the data from green house investigation was done by using complete randomized design (CRD) and means were separated by DMRT (Little and Hills, 1978).All samples were analyzed with three replications for each analysis.Data was interpreted based on main treatment effect.
A right combination of plant growth promoting bacteria is very important for synergy in consortium. It was observed from the study that apparently compatible cultures from lab study did not always turn into synergistic combinations in mung bean. Few of the combinations were synergistic, but not all. The significance of the fact is searching ecologically compatible consortia to enhance the field performance of bioinoculants. This may help as additional precaution in reducing inconsistency of bioinoculants at field level.
Preparatory studies
The 101 isolates obtained from different stages of rice rhizosphere (Fig. 1) were screened in vitro for their plant growth promoting traits like P solubilization, ammonia and siderophore production. Forty eight bacteria isolates showed siderophore production, 56 isolates were seen to be ammonia producers and 36 were phosphate solubilizer (Data not shown).

Fig. 1. Isolates obtained from different cultivars and at different stages of growth
On the basis of multiple plant growth promoting activities 12 best isolates were selected and further evaluated for nitrate reduction, quantitative estimation of phosphate solubilization and indole acetic acid (IAA) production (Table 1). After in vitro PGP tests, three isolates viz., RI-3, RII-4 and RIII-4 which gave multiple PGP activities were selected for evaluation as microbial bioinoculant for nutrient mobilization and PGP activity studies in mungbean crop in pot condition.
Table (1):
In vitro Plant growth promoting traits of three best performing isolates from rice rhizosphere
| S. No | Isolates | Nitrate reduction | Ammonia production | Siderophore production | Phosphate solublization Concentration of P | IAA production Concentration of IAA) | |
|---|---|---|---|---|---|---|---|
| 12 DAI (µg/ml) | 20 DAI (µg/ml) | 5DAI (µg/ml | |||||
| 1. | RI-3 | + | + | + | 44.17±1.93 | 87.92±0.19 | 5856.47±1.15 |
| 2. | RII-4 | + | + | + | 20.32±0.19 | 88.36±0.19 | 2564.99±1.15 |
| 3. | RIII-4 | + | + | + | 20.54±0.19 | 98.13±0.14 | 1043.13±1.15 |
Initial growth and development in pot trial
In pots with sterilised soil, highest germination percent was observed from two dual inoculant consortia RI-3+ RII-4 (T4, 100%) and RII-4 +RIII-4 (T6, 100%) which was significantly higher as compare to control (Fig. 2). All dual inoculant consortia had given higher germination in compare to their single inoculation; but triple inoculant consortia gave lower germination (T7, 66.67%). Almost similar trends were obtained from non-sterilised soil. Dual inoculation was found ecologically compatible and given good germination by working in synergy in both sterilised and non-sterilised soil.
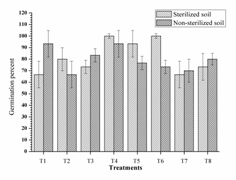
Fig. 2. Germination percent of mung bean as affected by inoculation
These results are similar to Couillerot et al. (2013); the incompatibility of triple inoculants in vivo should be taken care before formulating consortium. Similarly, single inoculant of RIII-4 and dual inoculation of RI-3+ RII-4 were faster in 50% seed emergence than other treatments in non-sterilised soils, however, the this difference was non-significant in sterilised soils. The triple inoculation performed poor than single and dual inoculation (Fig. 3). These results supports the work of Couillerot et al. (2013) in which consortia of Azospirillum, Pseudomonas fluorescens and three Glomus sp. were tested on the basis of transcription of auxin synthesis gene ipdC (phytostimulation), secondary metabolites and biomass production on maize. Remarkably, triple inoculation with Azospirillum spp. had performed lower than dual inoculation of Pseudomonas fluorescens and Glomus sp.
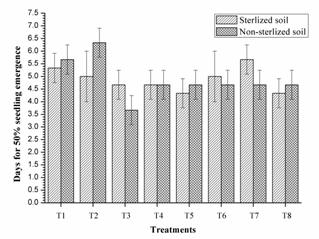
Fig. 3. Days for 50% seedling emergenceof mung bean as affected by inoculation
Plant height
Significantly better plant height on 15th day was recorded from single and dual inoculant consortium as compare to triple inoculation and control (Table 2). As plant grown older by 60 day, higher plant height was recorded fromdual inoculationRI-3+ RII-4 and RII-4 + RIII-4 in sterilised soils and RI-3+ RIII-4 in non-sterilised soils. It may be due to plant growth promoting activity and synergy which helps the plants for higher nutrient uptake by the microbial isolates (Fan et al. 2011).
Table (2):
Effect of different bioinoculant treatments on plant height (cm) at different days intervals after sowing in mung bean
| Treatments | 15 days | 30 days | 45 days | 60 days | ||||
|---|---|---|---|---|---|---|---|---|
| Sterilized soil | Non-sterilized soil | Sterilized soil | Non-sterilized soil | Sterilized soil | Non-sterilized soil | Sterilized soil | Non-sterilized soil | |
| T1 (RI-3) | 5.40ab | 5.03c | 7.70a | 7.97ab | 11.80abc | 10.32bc | 15.90cd | 12.63cde |
| T2 (RII-4) | 5.67a | 5.10bc | 8.10a | 7.10abc | 10.42cde | 9.78cd | 17.47abc | 12.47de |
| T3 (RIII-4) | 4.93c | 5.47ab | 8.00a | 8.03ab | 12.05ab | 10.47bc | 18.13ab | 12.20de |
| T4 (RI-3+ RII-4) | 5.00bc | 5.83a | 7.10ab | 8.33a | 11.22a-d | 10.65bc | 18.70a | 14.67bc |
| T5 (RI-3+ RIII-4) | 4.27d | 4.77c | 5.83b | 6.60bc | 10.88b-e | 12.50a | 15.27d | 19.60a |
| T6(RII-4+ RIII-4) | 4.80c | 4.87c | 6.83ab | 6.17c | 12.52a | 11.17b | 18.17ab | 15.40b |
| T7(RI-3+RII-4+RIII-4) | 4.20d | 4.97c | 6.90ab | 6.93abc | 9.82de | 9.68cd | 16.53bcd | 14.20bcd |
| T8(Control) | 4.10d | 4.83c | 5.90b | 6.13c | 9.43e | 9.25d | 12.97e | 11.03e |
| SEm± | 0.135398 | 0.134265 | 0.444255 | 0.453687 | 0.486807 | 0.325583 | 0.574102 | 0.658381 |
Chlorophyll content and 50% flowering
Highest chlorophyll mg/g leaf (53.80, 53.30 and 51.73) was observed from RIII-4, RI-3 and RI-3+ RIII-4 respectively along with other single and dual inoculation. Dual inoculation of RII-4+ RIII-4 recorded high chlorophyll content in non-sterilised soils (Fig. 4) and faster 50% flowering (41 days) in sterilised soil (Fig. 5). However, the difference was non-significant as compare to triple inoculation. This may be due to plant growth promoting activity of isolates and nitrogen fixation. These results are similar to Kang et al. (2009) and Shehata et al. (2010).
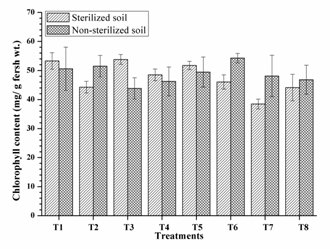
Fig. 4. Chlorophyll content of mung bean as affected by inoculation
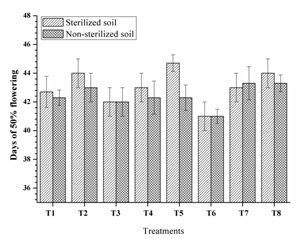
Fig. 5. Days of 50% flowering of mung bean as affected by inoculation
Nutrient uptake
Inoculation of RIII-4 (T3, 3.89%) has given significantly higher nitrogen content in grains followed by RI-3 + RII-4 (T4, 3.84%); the triple inoculation was found relatively poor than these two treatment combinations (T7, 3.71%) in sterilised soil. Similar was observed from unsterilized soil (Table 3). Protein content in grains (obtained from N content using universal factor of 6.25) was highest from RIII-4 (T3, 24.31%) followed by RI-3 + RII-4 (T4, 24%), the triple inoculation (T7) was significantly lower (23.21%) in sterilised soil with same trend in unsterilized soil (Table 3).
Significantly higher phosphorus content was recorded from RIII-4 (T3, 0.98%) and RI-3 + RII-4 (T4, 0.95%); in comparison to that, triple inoculation gave lower P- content (0.83%) in sterilised soil. This was also evident in unsterilized soil (Table 3).
Table (3):
Effect of different bioinoculant treatments on Nutrient uptake in mung bean
| Treatments | N content of grains | P content of grains | Protein content in grains | |||
|---|---|---|---|---|---|---|
| Sterilized soil | Non-sterilized soil | Sterilized soil | Non-sterilized soil | Sterilized soil | Non-sterilized soil | |
| T1 (RI-3) | 3.76d | 3.75b | 0.88de | 0.86d | 23.50d | 23.42b |
| T2 (RII-4) | 3.82bc | 3.78b | 0.90cd | 0.89c | 23.90bc | 23.63b |
| T3 (RIII-4) | 3.89a | 3.83a | 0.98a | 0.95a | 24.31a | 23.92a |
| T4 (RI-3+ RII-4) | 3.84b | 3.84a | 0.95b | 0.92b | 24.00b | 23.98a |
| T5 (RI-3+ RIII-4) | 3.81bc | 3.75b | 0.92c | 0.91b | 23.83bc | 23.46b |
| T6(RII-4+ RIII-4) | 3.79cd | 3.74bc | 0.89cde | 0.85d | 23.67cd | 23.35bc |
| T7(RI-3+RII-4+RIII-4) | 3.7e | 3.68d | 0.83f | 0.82e | 23.21e | 23.02d |
| T8(Control) | 3.69e | 3.70cd | 0.86e | 0.85d | 23.04e | 23.13cd |
| SEm± | 0.012241 | 0.014278 | 0.009621 | 0.006151 | 0.076191 | 0.089304 |
This might be due to higher P- solubilization and/or nitrogen fixation by the isolates. Healthy plant growth due to secretion of plant hormones by inoculants may also be a reason of profuse root growth and thus resulted in higher nutrient uptake and high protein content in seeds (Ahmed et al. 2005; Balemi et al. 2007). The synergy might not been worked out in triple inoculation because same microbes performed well in single and dual inoculation. This may be suggested that triple inoculant combination was not as successful as dual (T5) and using ecologically successful combination might be one of the reasons of improved nutrient uptake.
Yield and yield parameters
Different yield parameters were tested for the efficiency of isolates in single and consortium (Table 4). There are few critical observations in yield parameters; first, the effect of inoculation was observed mainly in sterilised soil; second, dual inoculation of RI-3+ RII-4 has given higher yield parameters (10.21 g seed yield/plant as compare to 5.62 g/plant in control, Table 4); third, there is effect of triple inoculation but lower than dual inoculation. These results signify the work by Sachin and Mishra (2009) whereenhancement in yield was a combined result of various direct-indirect effects of nitrogen fixation, phosphorus solubilization, production of auxin, cytokinin, gibberaline, other plant growth stimulators and anti-pathogenic compounds by the inoculated microbes, individually and in synergy. Beneficial microorganisms interacts synergistically in consortium by providing nutrients and/or stimulators and other biochemical activities, this in turn provides beneficial effects in plant yield parameters (Brahmaprakash and Sahu, 2012; Lavanya et al. 2015).
Table (4):
Effect of different bioinoculant treatments on yield and yield parameters in mung bean
| Treatments | Number of clusters per plant | Number of pods per cluster | Number of pods per plant | Number of seeds per pod | Number of seeds per plant | Seed yield (g per plant) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sterilized soil | Non-sterilized soil | Sterilized soil | Non-sterilized soil | Sterilized soil | Non-sterilized soil | Sterilized soil | Non-sterilized soil | Sterilized soil | Non-sterilized soil | Sterilized soil | Non-sterilized soil | |
| T1 (RI-3) | 3.90b | 3.77c | 3.86bc | 3.75b | 15.06cd | 14.14c | 9.57cd | 9.90a | 143.46bcd | 139.84bc | 6.87b | 6.56bc |
| T2 (RII-4) | 3.80b | 3.90c | 3.76bc | 3.93ab | 14.29d | 15.27c | 8.39d | 9.30ab | 120.10cd | 141.76bc | 6.54bc | 7.30ab |
| T3 (RIII-4) | 4.17ab | 3.93bc | 4.18ab | 3.87ab | 17.40b | 15.23c | 9.43cd | 9.33ab | 164.49b | 141.72bc | 7.75b | 6.56bc |
| T4 (RI-3+ RII-4) | 4.73a | 4.37a | 4.47a | 4.34a | 21.14a | 18.92a | 11.37ab | 8.82ab | 240.36a | 166.28a | 10.21a | 7.22ab |
| T5 (RI-3+ RIII-4) | 4.33ab | 4.23ab | 4.17ab | 4.11ab | 18.11b | 17.42ab | 11.97a | 8.77ab | 216.77a | 152.80ab | 9.75a | 7.28ab |
| T6(RII-4+ RIII-4) | 3.83b | 4.00bc | 3.84bc | 3.97ab | 14.67cd | 15.90bc | 10.42bc | 9.03ab | 152.70bc | 143.45bc | 7.70b | 7.57a |
| T7(RI-3+RII-4
+RIII-4) |
4.10b | 4.00bc | 4.01b | 3.99ab | 16.43bc | 15.96bc | 9.18cd | 9.12ab | 150.88bc | 145.14abc | 7.33b | 6.80abc |
| T8(Control) | 4.07b | 3.87c | 3.44c | 3.85ab | 13.76d | 14.88c | 8.27d | 8.24b | 114.08d | 122.44c | 5.62c | 6.19c |
| SEm± | 0.192479 | 0.098246 | 0.141529 | 0.152505 | 0.620776 | 0.644512 | 0.477586 | 0.43907 | 10.71265 | 6.955806 | 0.367753 | 0.238649 |
Expected reason for reduced performance in non-sterilized soil may be due to strong competition from native microflora (Biro et al. 2000). There may be inhibitory effects of one member of consortia on another member. As in study by Couillerot et al. (2011) Pseudomonas fluorescens producing 2, 4-DAPG has adverse effects on Azospirillum sp. which in turn reduce the effects of other.
Looking at the ecological compatibility, microbes with apparently no deleterious effects in plate assays may have negative effects on field. Azospirillum sp. has apparently no effects on Arbuscular Mycorrhiza Fungi (AMF) but mixed inoculation of Azospirillum + Pseudomonas fluorescen s+ Glomus sp. reduced the root colonization by AM fungi as compared to dual inoculation with P. fluorescens+ Glomus sp. (Couillerot et al. 2013).
On the other hand, two apparently antagonists may work in synergy after inoculation. 2, 4-DAPG producing P. fluorescens F113 strain has antifungal activity but when inoculated with AM fungi, there was no deleterious effects observed on colonization of AM fungi (Couillerot ET AL. 2013).
In case of microbial consortia, even though they are compatible, their combinations may not always result in higher yield. There are many other ecological interactions going on in the rhizosphere upon application of consortium. These interactions may affect the performance of bioinoculant, be it positively or negatively. Inoculants and its consortium do have its effect on plant growth and yield but a lot many combinations have to be tried to get higher ecological success. This study may help in future for selecting ecologically more suitable combination of consortium which can perform better in vivo and thus help in enhancing the yield of pulses.
ACKNOWLEDGMENTS
Present work has been carried out at ICAR-NBAIM, Mau in collaboration with Chandra Shekhar Azad University of Agriculture and Technology, Kanpur, Uttar Pradesh under the project “Bioprospecting of microbes from different agro-ecological niche for promotion of low input green technology for sustainable and organic farming in eco-agriculture”. Authors are grateful to UP Council of Agricultural Research, Lucknow for funding the project.
- Beck, D. P., Roughley, R. J.: Biological nitrogen fixation as a limitation to food legume production in Asia. In: Food legume Improvement for Asian Farming System (G Blair et al. ed.). ACIAR, Australia. 1987; pp 121-127.
- Wani, S. P., Lee, K. K. Role of biofertilizers in upland crop production. In: Fertilizers of organic manures, Recycle waste and biofertilizers, (Tandon, H. L.S. ed.). ‘Fertilizer development and Consultaion Organization, New Delhi, 1991; pp. 91–112.
- Gaind, S., Gaur, A. C. Thermotolerant phosphate solubilising microorganisms and their interactions in mungbean. Plant and Soil, 1991;133: 141–149.
- Mayak, S., Tirosh, T., Glick, B. R. Stimulation of the growth of tomato, Pepper and Mung bean plants by the plant growth promoting bacterium Enterobacter cloacae CAL3. Biological Agriculture and Horticulture, 2001;19(3):261-274.
- Ahmad, M., Zahir, A. Z., Asgher, H. N. and Ashgar, M. Inducing salt tolerance in mung bean through co-inoculation with rhizobia and plant growth promoting rhizobacteria containing 1-aminocyclopropane-1- carboxylate deaminase. Can J Microbiol, 2011; 57: 578-589.
- Alagawadi, A. R., Gaur, A. C. Inoculation of Azospirillum brasilense and phosphate solubilizing bacteria on yield of sorghum [Sorghum bicolor (L.) Moench] in dry land’. Tropical Agriculture. 1992; 69: 347–350.
- Rather, S. A., Hussain, M. A., Sharma, N. L. Effect of biofertilizers on growth, yield and economics of field pea (Pisum sativum L.). Int. J. Agric. Sci., 2010; 6: 65-66.
- Schwyn, B. Neilands, J. B. Universal chemical assay for the detection and determination of siderophores. Analytical Biochemistry, 1987; 160: 47–56.
- Milagres, A. M.F., Napolea, ˜o D., Machuca, A. Detection of siderophore production from several fungi and bacteria by a modiûcation of chrome azurol S (CAS) agar plate assay. Journal of Microbiological Methods, 1999; 37: 1–6.
- Winter Version 2014.web.clark.edu/rrausch/biolabs/260/NitrateReduction.pdf
- Cappuccino, J. C., Sherman, N.(ed) In: Microbiology: A Laboratory Manual, third ed. Benjamin/Cummings Pub. Co. New York, 1992; pp 125–179.
- Nautiyal, C. S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiology Letters, 1999; 170: 265–270.
- Nautiyal, C. S., Mehta, S. An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr. Microbiol., 2001; 43:51–56.
- Jackson, M . L. In: Soil chemical analysis New Delhi: Prentice Hall of India Pvt Ltd., 1973; pp. 25–214.
- Loper, J. E. and Schroth, M. N. Influence of bacterial sources of indole-3-acetic acid on root elongation of sugar beet.Plant Pathol., 1986; 76:386–389.
- Gordon, S. A., Weber, R. P. Colorimetric estimation of indole acetic acid. Plant Physiol., 1951; 26:192–195.
- Nikam, P. S., Jagtap, G. P., Sontakke, P. L. Management of chickpea wilt caused by Fusarium oxysporum f. sp. ciceri.African J. Agric. Res., 2007; 2(12): 692-697.
- Amer, G. A., Utkhede, R. S. Development of formulations of biological agents for management of root rot of lettuce and cucumber. Can. J. Microbiol., 2000; 46: 809-816
- Little, T. M., Hills, J. F., Agricultural experimentation. (John Wiley and sons, New York, USA), 1978.
- Couillerot, C., Ramírez-Trujillo, A., Walker, V., Felten, A., Jansa, J., Maurhofer, M., Défago, G., Prigent-Combaret, C., Comte, G., Caballero-Mellado, J., Moënne-Loccoz, Y. Comparison of prominent Azospirillum strains in Azospirillum–Pseudomonas–Glomus consortia for promotion of maize growth. Appl. Microbiol. Biotechnol., 2013; 97(10): 4639-4649.
- Fan, D. D., Ren, Y. X., Zxu, X. L., Ma, P., Liang, L. H. Optimization of culture conditions for phosphate solubilization by Acinetobacter calcoaceticus YC-5a using response surface methodology. Afri. J. Microbiol. Res., 2011; 5(20): 3327-3333.
- Kang, S. M., Joo, G. J., Muhammad, H., Na, C. I., Shin, D. H., Kim, H. Y., Hong, J.K., Lee, I. J. Gibberellin production and phosphate solubilization by newly isolated strain of Acinetobacter calcoaceticus and its effect on plant growth. Biotechnol. Lett., 2009; 31: 277-281.
- Shehata, S. M., Abdel-Azem, H. S., El-Yazied, A. A. Interactive effect of mineral nitrogen and biofertilization on the growth, chemical composition and yield of celeriac plant. Eur J Sci Res, 2010; 47:248-255.
- Ahmad, F., Ahmad, I., Khan, M. S. Indole Acetic Acid Production by the Indigenous Isolates of Azotobacter and Fluorescent Pseudomonas in the Presence and Absence of Tryptophan. Turk. J. Biol., 2005;29: 29-34.
- Balemi, T., Pal, N., Saxena, A. K. Response of onion (Allium cepa L.) to combined application of biological and chemical nitrogenous fertilizers. Acta Agric Slovenica, 2007; 89: 107-114.
- Sachin, D. N. , Misra, P. Effect of Azotobacter chroococcum(PGPR) on the growth of bamboo (Bambusa bamboo) and maize (Zea mays) Plants. Biofrontiers, 2009; 1: 24-31.
- Brahmaprakash, G. P., Sahu, P. K. Biofertilizers for sustainability. Journal of Indian Institute of Sciences, 2012; 92(1): 37–62.
- Lavanya, G., Sahu, P. K., Manikanta, D. S., Brahmaprakash, G. P. Effect of fluid bed dried formulation in comparison with lignite formulation of microbial consortium on finger millet (Eleucine coracana Gaertn.). J. Pure Appl. Microbiol., 2015; 9(2): 193-199.
- Biro, B., Köves-Péchy, K., Vörös, I., Takács, T., Eggenberger, P., Strasser, R. J. Interrelations between Azospirillum and Rhizobium nitrogen-fixers and arbuscular mycorrhizal fungi in the rhizosphere of alfalfa in sterile, AMF-free or normal soil conditions. Appl. Soil Ecol., 2000 15:159–168.
- Couillerot, O., Combes-Meynet, E., Pothier, J.F., Bellvert, F., Challita, E., Poirier, M.A., Rohr, R., Comte, G., Moënne-Loccoz, Y., Prigent- Combaret, Y. The role of the antimicrobial compound 2,4- diacetylphloroglucinol in the impact of biocontrol Pseudomonas fluorescens F113 on Azospirillum brasilense phytostimulators. Microbiology, 2011; 157:1694–1705.
© The Author(s) 2016. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


