Masjedi Mansoor1, Zand Farid2, Sabetian Golnar3,
Maghsoudi Behzad4 and Savaie Mohsen5*
1Assistant Professor of Anesthesiology and Critical Care Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
2Professor of Anesthesiology and Critical Care Medicine, Anesthesiology and Critical Care
Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
3Assistant Professor of Anesthesiology and Critical Care Medicine, Trauma Research Center,
Shiraz University of Medical Sciences, Shiraz, Iran.
4Associate Professor of Anesthesiology and Critical Care Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
5Anesthesiologist, Subspecialty Resident of Critical Care Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
Abstract
Ventilator associated pneumonia may occur as a complication, in intubated patients under mechanical ventilation. In this study, we investigated the impact of early replacement of conventional endotracheal tube with an endotracheal tube with subglottic suction port on the incidence of ventilator associated events. We designed a randomized clinical trial, and enrolled 60 critical care trauma patients (31 in control group and 29 in intervention group). Conventional endotracheal tube was replaced with an endotracheal tube with subglottic suction port during first 12 hours of arrival in ICU in the intervention group. The incidence of ventilator associated conditions includinge ventilator associated pneumonia was measured, and compared between two groups. The incidence of ventilator associated conditions, infection-related ventilator associated complications, ventilator associated pneumonia according to center of disease control and prevention (CDC) criteria, and ventilator associated pneumonia according to clinical pulmonary infection score (CPIS) in control group versus intervention group were: 12.9% vs. 20.7% (P= 0.419), 3.23% vs. 13.8% (P= 0.419139), 54.8% vs. 44.8% (P= 0.438), and 34.5% vs. 32.3% (P= 0.855), respectively. Ventilator free days, intensive care unit length of stay and hospital costs in control group versus intervention group were: 10.26±10.26 days vs. 15.14±10.34 days (P= 0.062), 19.10±14.89 days vs. 16.70±12.37 days (P= 0.604), and 1057.64±1303.54$ vs. 1189.14±1072.72$ (P= 0.186), respectively. According to our study results, the replacement of conventional endotracheal tube with an endotracheal tube with subglottic
suction port, cannot be recommended as routine, because of undetermined its capability
to reduce ventilator associated events and hospital costs, and also concerns about some
risks such as airway loss and pulmonary aspiration. Further investigations are
recommended.
Keywords: Intensive care unit, Trauma, Ventilator associated pneumonia, Endotracheal intubation.
Introduction
Mechanical ventilation is a basic treatment in critically ill patients. Some ventilator-associated events (VAE) may occurr during mechanical ventilation. Center for Disease Control and Prevention (CDC) has provided some definitions in this context, in order to increase objectivity, measurability, reliability, effectiveness and comparability of VAE surveillances (1).
When the patient’s respiratory status becomes worse after a two-day period of stability or partial recovery, ventilator-associated conditions (VAC) have occurred; If the VAC is along with the evidence of infection including fever or leukocytosis and prescription of a new antibiotic drug, infection-related ventilator-associated complication (IVAC) have occurred; Ultimately, ventilator-associated pneumonia (VAP) is one of the types of pneumonia developed in intubated patients, which underwent mechanical ventilation for at least 48 hours, before presentation of pneumonia (2).
The most common organisms causing VAP, include gram-negative bacilli, particularly multi-drug resistant (MDR) types (Pseudomonas aeruginosa, Acinetobacter baumannii, Serratia, Enterobacter and Klebsiella), gram-positives such as Staphylococcus aureus and methicillin-resistant staphylococcus aureus (MRSA), Candida and Aspergillusis (3, 4).
The importance of these complications during mechanical ventilation are: an increase the duration of mechanical ventilation, length of ICU stay and hospital costs.
Considering the importance of the issue, recommendations for the prevention of VAP proposed, named VAP bundle; these include: the use noninvasive ventilation in selected populations, manage patients without sedation whenever possible, interrupt sedation daily, daily assess about readiness for extubation, perform spontaneous breathing trials with sedatives turned off, facilitate early mobility, utilize endotracheal tubes with subglottic secretion drainage ports for patients expected to require greater than 48 or 72 hours of mechanical ventilation, change the ventilator circuit only if visibly soiled or malfunctioning, elevate the head of the bed to 30–45 degrees, selective oral or digestive decontamination, regular oral care with chlorhexidine and prophylactic probiotics, ultrathin polyurethane endotracheal tube cuffs, automated control of endotracheal tube cuff pressure, saline instillation before tracheal suctioning and mechanical tooth brushing (5).
Accumulation and micro-aspiration of oropharyngeal secretions at the top of the endotracheal tube cuff, in the subglottic region, is known as a key factor in the development of VAP (6). In order to prevention of micro-aspiration, some endotracheal tubes with a special port for drainage of subglottic secretions using medical suctions or a simple syringe, are commercially available (7, 8).
In this study, the clinical outcome of replacing a conventional endotracheal tube with endotracheal tube with subglottic suction port were scrutinized.
Materials and Methods
This study was performed in a tertiary referral teaching hospital with six intensive care units, include 54 intensive care beds, designed for trauma patients.
After the proposal approved by deputy of research and ethics committee, IRCT registration done. Any adult trauma patients, referred from emergency department, operation room or floor, to the one of these six intensive care units, initially evaluated by our research team; if the patient has including criteria, then enrolled in our study, after obtaining informed consent from the patient or his/her legal guardian: has an endotracheal tube, anticipating the need for mechanical ventilation for more than 48 hours, hasn’t any history or evidences of pneumonia, heart disease, airway problem, cervical spine injuries, immune deficiency state, obtaining immune-suppressant medications (cytotoxic chemotherapy or taking corticosteroids at a dose greater than 160 mg, hydrocortisone, prednisone 40 mg, 6 mg of dexamethasone or its equivalent). Patients excluded from study if the patient or his/her family refused during study, early discontinuation of mechanical ventilation or extubation (in first 48 hours) or patient died during less than 48 hours of initiation.
Any enrolled patient, was randomly assigned in control or intervention group, using a predefined random numbers sheet.
If the patient has enrolled in control group, we have continued the mechanical ventilation with their conventional endotracheal tube with an inner diameter of 7 or 7.5 mm for women and 8 or 8.5 mm for men; but If the patient has enrolled in intervention group, previous conventional endotracheal tube was replaced with an endotracheal tube with subglottic suction port (Mallinckrodt™ TaperGuard Evac Oral Tracheal Tube; Covidien, Mexico) with an inner diameter of 7 or 7.5 mm for women and 8 or 8.5 mm for men, through direct laryngoscopy, according to a written guide based on Doyle et al. study with few variations (8):
- Ensure about the availability of all the equipment and medications needed for reintubation.
- Suction of the nasogastric tube
- Suction of oral cavity and endotracheal tube with separate suction catheters using infection control rules.
- Pre-oxygenation with 100% oxygen for atleast 5 minutes
- Use of propofol, midazolam, fentanyl or cis-atracurium as needed, for patient comfort and facilitation of the intubation.
- Preparation of a proper size of endotracheal tube with subglottic suction port and insertion an intubation stylet in its lumen.
- Use of direct laryngoscopy and suction catheter to ensure the complete drainage of secretions from hypo-pharynx.
- Deflation of the previous tube’s cuff, after proper visualization of laryngeal inlet and remove it and insertion of the new endotracheal tube and inflation of cuff.
- Ventilation of lungs and ensure the establishment of the tube in a convenient location.
- Fixation of the tube and connection of the patient to ventilator.
In two cases, tube replacement was done using a tube exchanger. Then we have continued mechanical ventilation as fashion. Intermittent subglottic suctioning was taken in the intervention group with a regular intervals (every 6 hours), through subglottic port, using a 10 ml simple syringe.
Endotracheal tube cuff pressure measured and regulated in both groups, using a manometer, set on 20 mm Hg (every 6 hours).
During the study, if tracheostomy planed for patient, surgical tracheostomy was done; in the control group we used conventional tracheostomy tube, but in intervention group, a tracheostomy tube with subglottic suction port (Mallinckrodt TM TaperGuard Evac Tracheostomy Tube; Covidien, Mexico) was used.
In order to ensure the equal intensive care for both groups during the study, data including: head of bed elevation compliance, stress ulcer prophylaxis prescription, hand hygiene compliance, oral hygiene with chlorhexidine mouthwash, proper use of heat-moist exchanger filters and obtaining scheduled surveillance cultures, investigated and recorded without any intervention.
Staff awareness of ventilator-associated pneumonia, preventing the unplanned departure of the patient’s endotracheal tube and active monitoring of microbial colonization of the ICU patients were evaluated
Hand hygiene compliance, were audited based on the CDC checklist. The staff awareness of ventilator-associated pneumonia and its prevention were evaluated based on a questionnaire which previously its validity and reliability, have been proved in our country by Yeganeh et al. (9).
All patients information, including: demographic data, APACHE IV score, hemodynamic measures (heart rate, blood pressure), ventilatory settings (Fio2, respiratory rate, tidal volumes, PEEP), respiratory measures (respiratory rate, tidal volumes, lung imaging, breathing sounds, arterial blood O2 and CO2, change in previous pattern of respiratory secretions and suction need, cough, dyspnea), change in mental status, infection criteria (body temperature, white blood cell count, the number of prescribed antibiotics, purulent sputum), were checked and recorded during hospitalization, since ICU arrival, till discharge from hospital or death.
Data were analyzed using IBM SPSS software 23; Kolmogorov-Smirnov test was used to check the normality; as well as, Q-square, Student T test, Mann-Whitney U and logistic regression was used to compare variables between the two groups.
Results
Demographic, clinical, laboratory and radiologic data were obtained from 60 patients in both groups. There was not any significant differences in age and apache IV score between two groups (Table1).
Table 1. Patients data
Control group |
Intervention group |
P |
|
Number of cases |
31 |
29 |
|
Age (Years) |
43.35±24.71 |
38.24±24.71 |
0.801 |
Sex (Male/ Female)
|
19/12 |
27/2 |
|
APACHE IV Score |
82.81±32.88 |
70.65±19.82 |
0.087 |
The incidence of ventilator associated conditions, infection-related ventilator associated complications, ventilator associated pneumonia according to CDC criteria (PNU1), and ventilator associated pneumonia according to clinical pulmonary infection score (CPIS) were 12.9% vs. 20.7% (P= 0.419), 3.23% vs. 13.8% (P= 0.319), 54.8% vs. 44.8% (P= 0.438), 34.5% vs. 32.3% (P= 0.855), in control and intervention group respectively (Figure1). The mean ventilator free days, hospital length of stay and intensive care unit length of stay were 10.26±10.26 days vs. 15.14±10.34 days (P= 0.062), 25.93±19.97 days vs. 26.03±17.29 days (P= 0.842) and 19.10±14.89 days vs. 16.70±12.37 days (P= 0.604) in control and intervention group respectively. Tracheostomy rate was 51.67% in control group versus 41.4% in intervention group (P= 0.427). The mean costs of endotracheal tube, tracheostomy tube and antibiotics were 1057.64±1303.54$ in control group versus 1189.14±1072.72$ in intervention group (P= 0.186) (Table2).
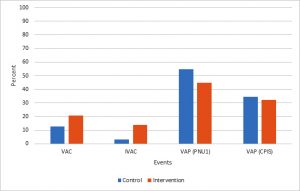 Fig. 1. Comparison of ventilator-associated events between two groups
Fig. 1. Comparison of ventilator-associated events between two groups
VAC: Ventilator-associated condition, CDC: IVAC: Infection-related ventilator-associated complication, VAP: Ventilator-associated pneumonia, CDC: Center for Disease Control and Prevention, CPIS: Clinical pulmonary infection score.
Table 2. Comparison of length of stay and costs between two groups
Control group |
Intervention group |
P |
|
Ventilator free days days) |
10.26±10.26 |
15.14±10.34 |
0.062 |
Hospital length of stay (days) |
25.93±19.97 |
26.03±17.29 |
0.842 |
ICU length of stay (days) |
19.10±14.89 |
16.70±12.37 |
0.604 |
Costs ($) |
1057.64±1303.54 |
1189.14±1072.72 |
0.186 |
ICU: Intensive Care Unit |
The analysis of hand hygiene surveillance revealed that, the overall hand hygiene compliance among intensive caregivers was 48.41±5.02.
The intensive care unit caregivers got the mean score of 6.1±1.76 of 10 in evaluation about VAP prophylaxis knowledge. Doctors, nurses, nurse assistances, radiology technicians, physiotherapists and service personnel gained a mean score of 7.5±2.12, 6.32±1.57, 5.37±1.85, 6.5±2.12, 6.5±2.12 and 4.9±2.28 of 10, respectively. During this study, some complications occurred: 4 cases had difficult intubation with laryngoscopy grade 3, according to Cormack-Lehane system. Fortunately all were successfully intubated using simple intubation stylet, after 3-4 tries. Re-intubation failed in one patient because of difficulty in passing the tube. She was intubated using simple endotracheal tube and excluded from study.
Discussion
According to the results of this study, replacing the conventional endotracheal tube with endotracheal tube with subglottic suction port, didn’t decrease significantly the occurrence of ventilator-associated pneumonia based on CDC (PNU1) and CPIS criteria (10, 11); and also, didn’t decrease hospital costs, intensive care unit length of stay or hospital length of stay, and didn’t increase ventilator free days, significantly. On the other hand, our study revealed a trend to increase VAC and IVAC, after this intervention.
Two separate studies in 2014, conducted by Al-Sayaghi et al. (12) and Safdari et al. (13), concluded that intensive care nurses’ information about prevention of VAP is low, but the average personnel information was acceptable (> 60 %) in our study. Blot et al. reported that, only 70 percent of nurses in intensive care units are familiar with the suction of the subglottic secretions in order to prevent VAP (14), this result is similar to our centers (67.06%).
Training the nurses is in adhere to the highly effective preventive principles as mentioned in Tolentino-de los Reyes et al. study (15), therefore the need to continuing education in this field will be fully felt. Our study also showed, despite the acceptable awareness of personnel, hand hygiene compliance is less than expected; so these educations must be combined with frequent reminders and ongoing surveillance, as reported by Hamishehkar et al. (16)
Despite of clinical evidences about the usefulness of endotracheal tube with the ability of subglottic suction for prevention of VAP (17, 18, 19, 20, 21, 22), its use, does not take place widespread (23); this maybe, because of higher prices of these endotracheal tubes, compared to the conventional endotracheal tubes (5-10 times). Most patients who admitted in our intensive care units and need prolong mechanical ventilation, have a conventional endotracheal tube, in this context we thought about a new solution: “elective replacement of conventional endotracheal tube using endotracheal tube with subglottic suction port, in intensive care unit”, early after decision to mechanical ventilation for at least 48 hours, an idea that had beneficial effects in the study of Doyle et al. (8); In that study, endotracheal tube replacement fallowed by using PneuX system (Venner Medical, Singapore), a certain tube with cuff pressure monitoring, warrant sealing, to reduce the aspiration of the secretions, together with intermittent suction of subglottic secretions; their study showed significant reduction in VAP incidence. In our study we also used intermittent suction of subglottic secretions but didn’t use automated cuff pressure monitoring, because of unavailability and limited financial resources; Different results may be due to importance of sealing, using continuous cuff pressure monitoring and adjustment in order to prevent micro-aspirations.
Replacement of endotracheal tube always is associated with some concerns about the risk of cardiac or respiratory complications, may be occurred during or after procedure: loss of airway due to difficult intubation and aspiration pneumonia are the historical concerns about reintubation (24, 25). These complications mostly occurred with reintubation after accidental extubation or extubation failure, and are associated with increase morbidity, mortality, hospital length of stay and intensive care unit length of stay (24, 25). Our study was based on elective endotracheal tube replacement with predesigned preparations and percautions, including the mouth secretions suction and extubation under direct laryngoscopy, so may create different conditions and we expect these complications, rarely; it seems, we are thought right.
We have chosen intermittent versus continuous subglottic suction, due to concerns about complications of continuous suction (possible tracheal mucosal prolapse into the suction port and injury of tracheal mucosa), reported by Switzerland et al. (26), Dragoumanis et al. (27) and Wang et al. (19).
Because of our limitations in the field of quantitative bacterial cultures, two non-culture based measures of pneumonia (PNU1 and CPIS) were used for diagnosis of VAP.
Our study showed that VAC and IVAC are more detected after endotracheal tube replacement with endotracheal tube with subglottic suction port, and the VAP was not significantly reduced. VAC may occurred because of atelectasis, pulmonary edema, acute respiratory distress syndrome (ARDS) or VAP (1) and increased VAC in this study may be due to other reasons, specially, atelectasis and segmental collapse fallowing discontinuation of positive pressure ventilation during reintubation; Also, IVAC may be due to a VAC plus a fever do not related to the pneumonia (other infections, drug induced, etc.); However, it may be caused by pneumonia (VAP).
VAP relatively, more occurred in trauma patients, than nontrauma patients (17.8% vs. 3.4% in Cook et al. study), it may be due to patient’s condition and environmental insults include invasive procedures and gram negative organisms (28, 29). In our study, the incidence of VAP was 49.95% in trauma patients. One of the probable reason for our inability to reduce VAP rate, may be, the conducting of our study in a trauma center with the patients, who may have rib fractures and lung contusions (29).
One of our challenges during this study was the concerns of physicians and nurses about the risk of unwanted life-threatening events during reintubation as discused before; this problem was partly solved by their justification according to scientific evidences, proper case inclusion and safe reintubation based on a pre-written protocol.
This pilot study, provided a realistic incidence of ventilatory associated events (VAE) and possible problems related to the replacement of conventional endotracheal tube with endotracheal tube with subglottic suction port. Our study has some limitations, including small sample size that could not provide conclusive results, but it can help us to design future studies. To achieve conclusive results in this field, multi center studies with a sample size of at least ten times is recommended
According to the results of this study and previous studies, it seems that, the logic strategies are: 1. the use of endotracheal tube with subglottic suction port for patients who are scheduled for elective or emergent intubation and mechanical ventilation. 2. Patients transferred to intensive care unit with a conventional endotracheal tube, should be continued with same tube, until risk-benefit and cost-benefit of tube replacement to be determined through future studies. 3. Obviously, if we need to replace endotracheal tube for other reasons such as malfunction, the use of endotracheal tube with subglottic suction port should be considered.
Conclusion
According to our study results, the early replacement of conventional endotracheal tube with an endotracheal tube with subglottic suction port in intensive care unit, cannot be recommended as routine, because of its undetermined capability to reduce ventilator associated events and hospital costs, aside from concerns about some life-threatening events such as airway loss and pulmonary aspiration.
Acknowledgement
Thanks to the research deputy of Shiraz Medical University and Shiraz medical faculty, who was responsible for financial support of the project. Thanks to all the staff, especially nurses in intensive care units, who helped us in conducting this research. Thanks to Mrs. Feizi (infection control supervisor of hospital), Mr.Darabizadeh and Mr. Izadpanah for their important role in this research.
References
- Klompas M, Branson R, Eichenwald EC, Greene LR, Howell MD, Lee G, Magill SS, Maragakis LL, Priebe GP, Speck K, Yokoe DS. Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infection Control & Hospital Epidemiology. 2014 Sep 1;35(S2):S133-54.
- Centers for Disease Control and Prevention. Pneumonia (ventilatorassociated [VAP] and non-ventilator-associated pneumonia [PNEU]) event. Device-associated Module PNEU/VAP. 2015.
- Demirdal T, Sari US, Nemli SA. Is inhaled colistin beneficial in ventilator associated pneumonia or nosocomial pneumonia caused by Acinetobacter baumannii?. Annals of clinical microbiology and antimicrobials. 2016 Feb 24;15(1):1.
- Montravers P, Harpan A, Guivarch E. Current and Future Considerations for the Treatment of Hospital-Acquired Pneumonia. Advances in therapy. 2016 Feb 1;33(2):151-66.
- Klompas M, Branson R, Eichenwald EC, et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infection Control and Hospital Epidemiology 2014; 35(8): 915-36.
- Nseir S1, Zerimech F, Fournier C, Lubret R, Ramon P, Durocher A, Balduyck M. Continuous control of tracheal cuff pressure and microaspiration of gastric contents in critically ill patients. Am J Respir Crit Care Med 2011; 184(9): 1041-47.
- Steven Deem, Miriam M Treggiari. New Endotracheal Tubes Designed to Prevent Ventilator-Associated Pneumonia: Do They Make a Difference? Respiratory Care 2010;55(8):1046 -55.
- Doyle A, Fletcher A, Carter J, Blunt M, Young P. The incidence of ventilator-associated pneumonia using the PneuX System with or without elective endotracheal tube exchange: A pilot study. BMC research notes. 2011 Mar 30;4(1):1.
- Yeganeh M, Yekta H, Farmanbar R, Khalili M. Knowledge of evidence‐based guidelines in Ventilator‐Associated Pneumonia prevention. Journal of Evidence‐Based Medicine. 2016 Jan 1.
- Kalanuria AA, Zai W, Mirski M. Ventilator-associated pneumonia in the ICU. Critical Care. 2014 Mar 18;18(2):1.
- Zilberberg MD, Shorr AF. Ventilator-associated pneumonia: the clinical pulmonary infection score as a surrogate for diagnostics and outcome. Clinical Infectious Diseases. 2010 Aug 1;51(Supplement 1):S131-5.
- Al-Sayaghi KM. Prevention of ventilator-associated pneumonia A knowledge survey among intensive care nurses in Yemen. Saudi medical journal. 2014 Mar 10;35(3):269-76.
- Safdari R, Yazdannik A, Abbasi S. Effect of intermittent subglottic secretion drainage on ventilator-associated pneumonia: A clinical trial. Iranian journal of nursing and midwifery research. 2014 Jul;19(4):376.
- Blot SI, Labeau S, Vandijck D, Van Aken P, Claes B, Executive Board of the Flemish Society for Critical Care Nurses. Evidence-based guidelines for the prevention of ventilator-associated pneumonia: results of a knowledge test among intensive care nurses. Intensive care medicine. 2007 Aug 1;33(8):1463-7.
- Tolentino-de los Reyes AF, Ruppert SD, Shiao SY. Ventilator associated pneumonia evidence-based practice: use of the ventilator bundle to prevent. Am J Crit Care. 2007;16:20-7.
- Hamishehkar H, Vahidinezhad M, Mashayekhi SO, Asgharian P, Hassankhani H, Mahmoodpoor A. Education alone is not enough in ventilator associated pneumonia care bundle compliance. Journal of research in pharmacy practice. 2014 Apr 1;3(2):51.
- Hudson JK, McDonald BJ, MacDonald JC, Ruel MA, Hudson CC. Impact of subglottic suctioning on the incidence of pneumonia after cardiac surgery: a retrospective observational study. Journal of cardiothoracic and vascular anesthesia. 2015 Feb 28;29(1):59-63.
- Damas P, Frippiat F, Ancion A, Canivet JL, Lambermont B, Layios N, Massion P, Morimont P, Nys M, Piret S, Lancellotti P. Prevention of ventilator-associated pneumonia and ventilator-associated conditions: a randomized controlled trial with subglottic secretion suctioning. Critical care medicine. 2015 Jan 1;43(1):22-30.
- Wang F, Bo L, Tang L, Lou J, Wu Y, Chen F, Li J, Deng X. Subglottic secretion drainage for preventing ventilator-associated pneumonia: an updated meta-analysis of randomized controlled trials. Journal of Trauma and Acute Care Surgery. 2012 May 1;72(5):1276-85.
- Frost SA, Azeem A, Alexandrou E, Tam V, Murphy JK, Hunt L, O’Regan W, Hillman KM. Subglottic secretion drainage for preventing ventilator associated pneumonia: a meta-analysis. Australian critical care. 2013 Nov 30;26(4):180-8.
- Dezfulian C, Shojania K, Collard HR, Kim HM, Matthay MA, Saint S. Subglottic secretion drainage for preventing ventilator-associated pneumonia: a meta-analysis. The American journal of medicine. 2005 Jan 31;118(1):11-8.
- Juneja D, Javeri Y, Singh O, Nasa P, Pandey R, Uniyal B. Comparing influence of intermittent subglottic secretions drainage with/without closed suction systems on the incidence of ventilator associated pneumonia. Indian Journal of Critical Care Medicine. 2011 Jul 1;15(3):168.
- Williams TA. Is the evidence for the use of subglottic drainage to prevent ventilated-associated pneumonia sufficient to change practice?. Australian Critical Care. 2012 Aug 31;25(3):200-4.
- Mort TC, Braffett BH. Conventional versus video laryngoscopy for tracheal tube exchange: Glottic visualization, success rates, complications, and rescue alternatives in the high-risk difficult airway patient. Anesthesia & Analgesia. 2015 Aug 1;121(2):440-8.
- Bittner EA, Schmidt UH. Tracheal Reintubation: Caused by “Too Much of a Good Thing”?. Respiratory care. 2012 Oct 1;57(10):1687-91.
- Suys E, Nieboer K, Stiers W, De Regt J, Huyghens L, Spapen H. Intermittent subglottic secretion drainage may cause tracheal damage in patients with few oropharyngeal secretions. Intensive and Critical Care Nursing. 2013 Dec 31;29(6):317-20.
- Dragoumanis CK, Vretzakis GI, Papaioannou VE, Didilis VN, Vogiatzaki TD, Pneumatikos IA. Investigating the failure to aspirate subglottic secretions with the Evac endotracheal tube. Anesthesia & Analgesia. 2007 Oct 1;105(4):1083-5.
- Cook A, Norwood S, Berne J. Ventilator-associated pneumonia is more common and of less consequence in trauma patients compared with other critically ill patients. Journal of Trauma and Acute Care Surgery. 2010 Nov 1;69(5):1083-91.
- Mangram AJ, Sohn J, Zhou N, Hollingworth AK, Ali-Osman FR, Sucher JF, Moyer M, Dzandu JK. Trauma-associated pneumonia: time to redefine ventilator-associated pneumonia in trauma patients. The American Journal of Surgery. 2015 Dec 31;210(6):1056-62.

