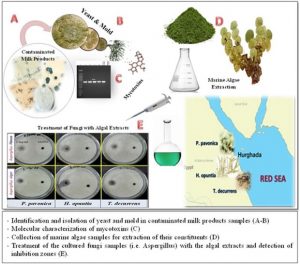
ISSN: 0973-7510
E-ISSN: 2581-690X
Fungal and mycotoxin contamination of milk products constitute a potential hazard to human health and food safety. Isolation and identifications of mold and yeast out of 140 milk products samples collected from dairy shops in Qena, Egypt were done through conventional microbiological methods. Aflatoxin-M1, aflatoxin-B1 and ochratoxin-A were characterized by thin-layer chromatography; aflR regulatory gene identified by using PCR. Marine algal extracts of Halimeda opuntia, Padina pavonica and Turbinaria decurrens species were studied for their antimicrobial activity. Overall of 80 and 64% dairy products samples were positive for mold and yeast contamination. A total of 38 mold and 15 yeast species were isolated. Aspergillus and Candida spp. were the most abundant isolated species. Furthermore 25, 40 and 27% of cheese and 71, 78 and 73.3 of dairy desserts samples were contaminated with AFM1, AFB1 and OTA, respectively; with average estimated dietary intake level much more than the acceptable daily intake for infant and adult. PCR identified aflR gene among four selected aflatoxigenic A. flavus. The major constituents of H. opuntia extract were 2,4-Decadienal, (E,E)- (21.56%) and 9,12-Octadecadienoic acid (Z,Z)- (36.16%). Ethyl acetate extract of Halimeda opuntia (3mg/ml) exhibited the strongest fungicidal activity with inhibition zones of 16.5 and 22.3 mm against A. flavus and A. niger. It exhibited potent candidacidal activity against C. tropicalis; 11 log10 orders of killing at 750 µg/ml. The discovered antimicrobial activity of H. opuntia is a promising candidate for designing novel antifungal agents which can be used in food preservatives and medicine industry.
Milk products, Aspergillus, Candida, Mycotoxins, Algae, Antimicrobial activity.
Mold and yeast can invade the dairy products during unhygienic processing and handling conditions which constitute a public health hazard1. Mold and yeast in milk and dairy products might act as allergen and an irritant to human health and may be the reason for the gastrointestinal disease2,3. Species of Aspergillus, Fusarium, penicillium, Rhizopus and Trichderma are common contaminants of dairy products and known as spore formers4. Mold species are also responsible for many serious diseases through production of toxic metabolites called mycotoxins5. Thus, mold and yeast counts are considered the standard test of milk hygiene6. Aspergillus and Penicillium species are common spore forming contaminants in the dairy derivatives7.
Mycotoxins; aflatoxin-B1 (AFB1), aflatoxin-M1 (AFM1) and ochratoxin-A (OTA) are fungal toxic metabolites characterized by heat stable toxicity, mutagenicity, teratogenicity and carcinogenicity8. Aflatoxins and ochratoxins are the major mycotoxins affecting our health9. Aflatoxin is produced by several Aspergillus species including Aspergillus flavus and Aspergillus parasiticus10. Major aflatoxins are B1, B2, G1, G2 and plus two additional metabolites; M1 and M211. AFB1 and its metabolite AFM1 in cow’s milk are human carcinogenic toxins12. The AFB1 and AFM1 international permissible limits are 2.0 and 0.05µg/kg, respectively13. Ochratoxin-A is produced by Aspergillus and Penicillium on the surface of cheese during ripening14 and it is the most common and wide-scale carcinogenic toxin in the Ochratoxins’ family15. The international permissible limit of OTA is 5.0µg/ kg16.
Prevention of the toxigenic molds growth and mycotoxins synthesis in raw materials and end products to control its outbreak, is achieved traditionally by using of chemical preservatives17. Despite of its proven efficiency, their repeated applications has resulted in side effects on human health, acquisition of microbial resistance to the applied chemicals and accumulation of chemical residues in food18. The growing consumer demand for high quality food, safe, preservative free with extended shelf life has focused efforts in the discovery of new natural preservatives. On light of above, efforts have been directed to developing potentially effective, safer, healthy and natural food preservatives. Marine organisms including marine macroalgae (seaweeds) are source of various natural antimicrobial compounds with pharmacological and biological activities19. Macroalgae contain many different secondary metabolites which have become recognized as potential sources of bioactive compounds, such as antimicrobial active compounds. The existence of bioactive compounds with antifungal effect was recorded in crude extracts of different species of green, brown and red algae by many investigators20. Utilization of algal extracts as antimicrobial agents for food preservation could be a fascinating alternative to chemical and physical methods, and it has received much attention recently21. The algal extracts considered as natural antimicrobial agents, nutritionally safe and easily degradable22. The antimicrobial activity exhibited by algal extracts against mold and yeast has been demonstrated by several researchers23, 24. The present study aimed to investigate the mold and yeast species present in dairy products, and estimate the levels of mycotoxins; AFB1, AFM1 and OTA using thin-layer chromatography (TLC) considering their permissible limits and acceptable daily intakes (ADI) for human taking the age into consideration. Furthermore, Aspergillus flavus strains would be molecularly identified for the presence of aflR regulatory gene. Furthermore, the antimicrobial activity of some algal extracts, i.e. Padina pavonica, Halimeda opuntia and Turbinaria decurrens against Aspergillus and Candida species were evaluated.
All procedures of sampling, assays and analyses were strictly carried out according to the instructions and guidelines provided with the companies brochures for lab analysis.
Media and samples used for fungal isolation and identification
Sabouraud dextrose agar, Malt extract agar (MEA), Czapek yeast extract agar (CYA), 25% glycerol nitrate agar and TSB (pH 7.3) were used.
Samples
A total 140 random samples of cheese (Ras, Cheddar, Feta and Processed) and dairy desserts (Mahalabia, Custard and Rice Milk) were randomly collected from dairy markets. The samples were kept in polyethylene bags and preserved in ice box, then immediately transferred to the laboratory aseptically to be prepared for mycology.
Preparation of samples’ serial dilutions
The samples were released out aseptically from their packages and thoroughly mixed in a sterile mortar. From each prepared samples, 10 grams were transferred into sterile flask containing 90 ml sterile peptone water solution as a buffer. Ten-fold serial dilutions up to 10-8 from each sample were prepared.
Mold and yeast counting and identification
The developing yeast and mold colonies were counted and isolated for identification according to Chay et al. (2017)25 and Harrigan and MacCance (1966)26. The obtained results were compared with the maximum allowed mold or yeast counts of the Egyptian Organization for Standardization and Quality control (EQSOC). Mold isolates were inoculated in CYA, MEA and G25N (25%). Colony appearance, exudate production, reverse coloration and pigmentation were assessed. Colonies’ diameters were assessed after 7 days of growth at 25°C27. The characteristics of yeast colonies including; the pattern and rate of growth, colony consistency, size and its surface color were studied. Vegetative reproduction was examined on corn meal agar28. Yeast morphology was grossly and microscopically examined29. Yeast growth was studied after isolates culturing on slopes of Sabouraud dextrose media and incubating it at 37°C for 3-5 days. Urease and germ tube tests were performed according to Cruickshank et al. (1975)30 and Koneman et al. (1992)31.
Detection of Mycotoxins Residues in the Dairy Products
Extraction of aflatoxins out of dairy products samples was done in accordance with the method mentioned by Roberts and Patterson (1975)32. The final samples’ filtrates were combined and evaporated to dryness in a rotatory evaporator and saved for analysis. Ochratoxin-A (OTA) was detected by thin-layer chromatography (TLC) according to AOAC (1980)33. Mycotoxins residues were qualitatively estimated according to the method proposed by (Scott, 1965; Howell and Taylor, 1981)34, 35. The reference values, colors and intensities of unknown spots were compared to those standard reference values (Sigma, USA). Samples extracts which were found to contain mycotoxins by the qualitative technique were further calculated according to the standard formula:
µg/ kg =(S×Y×V) / (X×W)
S=µl of mycotoxin standard equal to unknown
Y= Concentration of mycotoxin inµg/ml
V=µl of final extract dilution.
X=µl of the extract emitting a spot intensity equal to S. W= Mass (weight) of the sample, represented by the final extract in gram.
The obtained results were compared with the maximum permissible limits (MPL) of the local Egyptian Organization for Standardization and Quality control (EQSOC) and International organizations latest guidelines standards.
Health risk assessment: estimated daily intake and estimated weekly intake
Calculations of the estimated daily intake (EDI) and estimated weekly intake (EWI) of mycotoxins in the examined milk products samples were performed according to previous accredited equations. The obtained results were compared to the acceptable daily intake (ADI) according to the international standards organization.
Detection of Aspergillus flavus ability to produce aflatoxins using PCR
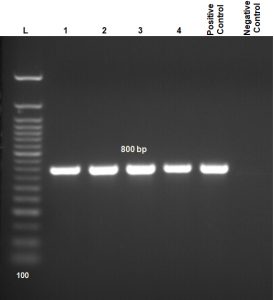
Genomic DNA was extracted from ground frozen Aspergillus flavus mycelium/spores using Spin Column DNeasy plant minikit (Geneaid, USA). The target fragment of regulatory aflatoxin gene fragments of aflatoxigenic DNA was amplified by PCR. The forward-reverse primers aflR1 regulatory gene was; 5¯ AACCGCATCCACAATCTCAT 3¯, and 5¯AGTGCAGTTCGCTCAGAACA 3¯, involved within 800-base pairs (bp) gene size36. The reaction mixtures consisted of extracted A. flavus target DNA, forward primer (F), reverse primer (R) and PCR Master Mix: DreamTaq Green PCR Master Mix (2X) (Thermo Scientific, USA, Cat., No. K1081). Amplification of DNA was performed via optimized PCR reactions; denaturation, primer annealing and extension. Subsequently, 35 amplification cycles were done in a programmable thermocycler (Gradient Thermal cycler; 1000 S Thermal cycler BioRAD USA). The PCR-product was analysed by electrophoresis on agarose gel (1.5%) stained by ethidium bromide. The gel image was visualized by trans-illuminator. A 100 bp-size ladder is the marker for amplicons size.
Algal collection and extraction preparation
Three marine algae; Halimeda opuntia (Chlorophyta), Padina pavonica, and Turbinaria decurrens (Phaeophyta) were collected during October, 2018 from Hurghada coast, Red Sea Coast, Egypt. Seaweeds were collected in sterilized polyethylene bags, kept in ice box till reach the laboratory for identification, preparation and analysis37. Algal extraction was performed by using different solvents; ethyl acetate for Padina pavonica, and petroleum ether for Halimeda opuntia and Turbinaria decurrens. The extracts were suspended in dimethyl sulfoxide (DMSO) to a final concentration of 50 mg/ml, and then stored in airtight bottles at 4 ºC till used38.
Gas chromatography-mass spectrometry (GC-MS) analysis of algal extract
Algal extracts were identified by using GC-MS (Thermo Scientific Technologies, Trace-1310, capillary column TG-5; 30m×250μm×0.25μm). Split mode-mass detector and helium gas with flow rate carrier 1.5 ml/minutes (min) were used. Injector was operated at 230°C, and initial oven temperature 60°C for 2 min and ramp 10/min to 300°C for 8 min. Mass spectra were taken at 70 eV and the total GC running time was 35 min.
Microorganisms
Strains of Aspergillus flavus, Aspergillus niger and Candida tropicalis as identified by Mycology Department, Animal Health Research Institute, Cairo, Egypt were used to study the antifungal activities of algal extract. All isolates were cultured onto Sabouraud dextrose agar, 24 hrs prior to assay.
Antifungal assay
Inoculums of Aspergillus flavus and Aspergillus niger cultures were suspended in a sterile saline solution (0.85 %). Suspension turbidity was modified by the spectrophotometry at 530 nm till obtain a final concentration of 0.5 McFarland standards (0.5-2.5×103). The antifungal activity was evaluated by using well diffusion method on the agar plates of Sabouraud dextrose media39, where the inhibition zones were detected after 48 hours of incubation at 27°C. The assay was done in triplicates (n = 3).
Candidacidal assay
Candida tropicalis blastoconidia grown for 24 hrs in 1% TSB (pH 7.3) were rinsed, resuspended to 106 – 107 CFU/ml in Sabouraud dextrose broth, and then mixed with DMSO, in equal volume, containing series of the algal extracts; 3 mg/ml, at 2-fold serial dilutions. The mixture was plated on Sabouraud dextrose media and incubated at 28°C for 24 hrs followed by further incubation at 30°C for another 24 hrs. The algal colonies were finally counted in log CFU/ml in the assay media40.
Statistical analysis
The inhibition zones in response to treatment of the fungi with algae were analysed by using two-way analysis of variance (ANOVA) and Bonferroni was the post-hoc test. All data were set as mean ± standard error (SEM) for three replicate-assays. Graph-Pad Prism software, San-Diego, USA, v. 5 was used. The difference among groups was considered significant at P<0.05.
The examined dairy samples were variably contaminated with mold and yeast, ranged 50 – 100 % above the allowed limits; 10 and 400 CFU/g, respectively proposed by EOSQC (2005)41. The mean values of mold detected ranged from 1.84×103 up to 7.25×104 CFU/g (Table 1), and that of yeast ranged from 2.14×103 to 1.73×105 CFU/g (Table 1). Aspergillus spp. were mostly the dominant species isolated from cheese and milk desserts with 42 and 25 %, respectively, i.e. A. flavus, A. niger, A. ochraceus, A. fumigatus and A. parasiticus (Table 2). Also, the most predominant yeast species were those of Candida, i.e. C. tropicalis, C. krusei and C. parapsilosis (Table 3).
Table (1):
Mold and yeast counts, presented as colony forming unit (CFU)/g, in different dairy products’ samples (n = 20), showing the safety from mold and yeast according to EOSQC [41].
| A | Milk products | +ve Mold (CFU/g) | |||||
|---|---|---|---|---|---|---|---|
| No. | % | Min. | Max. | Mean±S.E | > 10 (%) | ||
| Cheese | Ras cheese | 17 | 85 | 1.00×101 | 1.60×104 | 3.54×103±1.14×103 | 80 |
| Cheddar | 10 | 50 | 3.00×101 | 1.10×104 | 1.84×103±5.43×102 | 50 | |
| Feta | 17 | 85 | 1.00×101 | 3.00×104 | 4.85×103±1.58×103 | 85 | |
| Processed | 12 | 60 | 7.00×102 | 2.50×105 | 3.33×104±1.29×102 | 60 | |
| Dairy desserts | Mahalabia | 18 | 90 | 3.30×102 | 5.50×104 | 1.31×104±3.94×103 | 90 |
| Custard | 20 | 100 | 3.00×102 | 1.34×104 | 6.84×103±9.40×102 | 100 | |
| Rice Milk | 19 | 95 | 1.30×102 | 3.30×104 | 7.25×104±2.43×103 | 95 | |
| B | Milk products | +ve Yeast (CFU/g) | |||||
| No. | % | Min. | Max. | Mean±S.E | >400 (%) | ||
| Cheese | Ras cheese | 15 | 75 | 1.00×101 | 8.70×104 | 1.39×104±5.96×103 | 60 |
| Cheddar | 11 | 55 | 2.40×102 | 6.00×105 | 1.73×105±3.72×104 | 50 | |
| Feta | 10 | 50 | 1.00×102 | 4.00×104 | 6.66×103±1.98×103 | 40 | |
| Processed | 12 | 60 | 1.20×102 | 1.00×105 | 1.78×104±5.74×103 | 55 | |
| Dairy desserts | Mahalabia | 12 | 60 | 2.00×102 | 5.00×104 | 9.58×103±3.19×103 | 35 |
| Custard | 15 | 75 | 3.00×102 | 4.40×104 | 5.75×103±2.32×103 | 60 | |
| Rice Milk | 15 | 75 | 2.00×102 | 1.20×104 | 2.14×103±6.82×102 | 45 | |
Table (2):
Percent of contamination of mold isolates in different dairy products samples (n = 20).
| Isolates of molds | Percent of contamination (%) | ||||||
|---|---|---|---|---|---|---|---|
| Cheese samples | Dairy desserts | ||||||
| Ras | Cheddar | Feta | Processed | Mahalabia | Custard | Rice Milk | |
| Acremonium strictum | 0.0 | 0.0 | 0.0 | 0.0 | 3.7 | 8.3 | 0.0 |
| Arthriniumphaeo spermum | 0.0 | 0.0 | 0.0 | 0.0 | 3.7 | 0.0 | 0.0 |
| Aspergillus flavus Link | 17.1 | 9.1 | 16.2 | 6.3 | 3.7 | 12.5 | 6.5 |
| Aspergillus fumigatus | 5.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Aspergillus nigerTiegh. nom. cons. | 11.4 | 9.1 | 18.9 | 43.7 | 14.8 | 14.6 | 12.9 |
| Aspergillus ochraceus | 5.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Aspergillus parasiticus | 2.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Aspergillus sydowii | 5.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Aspergillus terreus Thom | 0.0 | 0.0 | 5.4 | 0.0 | 0.0 | 4.2 | 0.0 |
| Aureobasidium pullulans | 0.0 | 0.0 | 0.0 | 0.0 | 3.7 | 8.3 | 3.2 |
| Botrytis cinerea | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 6.5 |
| Cladosporium cladosporioides G.A. de Vries | 17.1 | 9.1 | 24.4 | 18.7 | 22.2 | 8.3 | 32.3 |
| Cladosporium herbarum Link | 0.0 | 0.0 | 5.4 | 12.5 | 0.0 | 0.0 | 0.0 |
| Emericella nidulans | 0.0 | 0.0 | 0.0 | 0.0 | 3.7 | 0.0 | 0.0 |
| Eurotium chevalieri | 0.0 | 0.0 | 0.0 | 0.0 | 7.4 | 4.2 | 3.2 |
| Eupenicillium spp. | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 18.8 | 0.0 |
| Fusarium chlamydosporum | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 4.2 | 0.0 |
| Fusarium poae Wollenw | 0.0 | 18.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Mucor plumbeus | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 6.5 |
| Paecilomyces variotii | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 6.5 |
| Penicillium aurantiogriseum Dierckx | 0.0 | 18.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Penicillium caseifulvum Lund, Filt. &Frisvad | 8.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Penicillium chrysogenum | 14.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Penicillium citreonigrum Dierckx | 0.0 | 0.0 | 2.7 | 0.0 | 3.7 | 0.0 | 0.0 |
| Penicillium citrinum Thom | 2.9 | 0.0 | 5.4 | 0.0 | 7.4 | 8.3 | 9.7 |
| Penicillium corylophilum Dierckx | 5.7 | 18.2 | 8.1 | 6.3 | 0.0 | 0.0 | 0.0 |
| Penicillium digitatum Sacc. | 2.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Penicillium implicatum Biourge | 0.0 | 9.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Penicillium paneum Frisvad | 0.0 | 0.0 | 0.0 | 12.5 | 0.0 | 0.0 | 0.0 |
| Penicillium paxilli Bainier | 0.0 | 9.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Penicillium purpurogenum Stoll | 0.0 | 0.0 | 2.7 | 0.0 | 0.0 | 0.0 | 0.0 |
| Penicillium raistrickii G. Sm | 0.0 | 0.0 | 2.7 | 0.0 | 0.0 | 0.0 | 0.0 |
| Penicillium restrictum | 0.0 | 0.0 | 0.0 | 0.0 | 18.5 | 6.3 | 12.9 |
| Penicillium simplicissimum Thom | 0.0 | 0.0 | 5.4 | 0.0 | 0.0 | 0.0 | 0.0 |
| Penicillium variabile Sopp | 0.0 | 0.0 | 2.7 | 0.0 | 0.0 | 0.0 | 0.0 |
| Penicillium variable | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.1 | 0.0 |
| Rhizopus microsporus | 0.0 | 0.0 | 0.0 | 0.0 | 3.7 | 0.0 | 0.0 |
| Scopulariopsis brevicaulis | 0.0 | 0.0 | 0.0 | 0.0 | 3.7 | 0.0 | 0.0 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Table (3):
Percent of contamination of yeast isolates in different dairy products samples (n = 20).
| Isolates of Yeast | Percent of contamination (%) | ||||||
|---|---|---|---|---|---|---|---|
| Cheese samples | Dairy desserts | ||||||
| Ras | Cheddar | Feta | Processed | Mahalabia | Custard | Rice Milk | |
| Candida famata | 0.0 | 0.0 | 0.0 | 0.0 | 11.8 | 0.0 | 5.0 |
| Candida guilliermondii | 0.0 | 0.0 | 7.7 | 6.7 | 5.9 | 0.0 | 5.0 |
| Candida holmii | 6.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Candida krusei | 6.7 | 0.0 | 15.4 | 0.0 | 5.9 | 4.3 | 0.0 |
| Candida parapsilosis | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 4.3 | 0.0 |
| Candida tropicalis | 13.3 | 10.0 | 23.1 | 13.3 | 17.6 | 17.4 | 20.0 |
| Cryptococcus albidus | 20.0 | 0.0 | 0.0 | 6.7 | 11.8 | 34.8 | 20.0 |
| Debaryomyces hansenii | 13.3 | 30.0 | 15.4 | 20.0 | 5.9 | 8.7 | 10.0 |
| Geotrichum candidum Link | 20.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 5.0 |
| Pichia anomala | 6.7 | 0.0 | 7.7 | 0.0 | 0.0 | 0.0 | 0.0 |
| Pichia membranaefaciens | 0.0 | 10.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Rhodotorula glutinis | 0.0 | 20.0 | 0.0 | 0.0 | 29.4 | 8.7 | 20.0 |
| Rhodotorula spp. | 6.7 | 20.0 | 15.4 | 6.7 | 0.0 | 0.0 | 5.0 |
| Saccharomyces cerevisiae | 6.7 | 10.0 | 15.4 | 40.0 | 5.9 | 17.4 | 10.0 |
| Trichosporon spp. | 0.0 | 0.0 | 0.0 | 6.7 | 5.9 | 4.3 | 0.0 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Dairy products showed heterogeneous mycotoxins that were chromatographically detected as shown in Table 4. The concentrations of aflatoxin-M1 (AFM1), aflatoxin-B1 (AFB1) and ochratoxin-A (OTA) in the examined samples ranged; 0.0-13.9, 11.1-13.8 and 4.5-13.9µg/kg, in 40-85, 15-85 and 15-80 %, respectively. Mostly all values of AFM1 and AFB1 estimated were exceeding their permissible limits according to Egyptian regulation permissible limits and EU regulation13,42,43. However, 100, 100, 67, 88, 92, 6 and 94% of the examined ras, cheddar, feta, processed cheese, mahalabia, custard and rice milk had Ochratoxin A above the allowed limit (> 5µg/g) according to Creppy (2002)16 (Table 4 A).
Table (4):
Table 4: (A) Maximum permissible limits (MPL; µg/kg) of mycotoxins in dairy products (n = 20); aflatoxin-M1 (AFM1), aflatoxin-B1 (AFB1) and ochratoxin-A (OTA) by the thin-layer chromatography (TLC). Letters (a, b, c, d) denote the international standards for MPLs. E.R.: Egyptian Regulation; I.S.: International Standards – a: EOSQC [43], b, c: European commission regulation [13], d: Creppy [16]. (B) Acceptable and estimated intakes (µg/kg b.w) of mycotoxins in the dairy products consumed by children and adult (n = 20). Letters (a, b, c) denote the international standards for the acceptable daily intake (ADI). STD = Standard references – a: Kuiper-Goodman [44], b: Brera et al. [46], c: JECFA [45] – ADI, acceptable daily intake; EDI, estimated daily intake; EWI, estimated weekly intake; PTWI, provisional tolerable weekly intake.
| Dairy Products | (A) Maximum Permissible Limits | (B) Acceptable vs. Estimated Intakes | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| +ve Cases | Mean ± SE(µg/kg) | MPL | STD | Children | Adults | |||||||||||||||
| E.R. | >MPL | I.S. | >MPL | |||||||||||||||||
| No. | % | No. | % | No. | % | ADI | PTWI | DI | WI | DI | WI | |||||||||
| EDI | >ADI % | EWI | >PTWI % | EDI | >ADI % | EWI | >PTWI % | |||||||||||||
| AFM1 | Ras Ch. | 8 | 40 | 8.9±1.1 | 0.0a | 8 | 100 | 0.05b | 8 | 100 | 0.002a | 0.014 | 15.9 | 40 | 111.8 | 40 | 6.1 | 40 | 43.0 | 40 |
| Ched. Ch. | 0 | 0 | — | – | – | – | – | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | |||||
| Feta Ch. | 3 | 15 | 9.4±1.0 | 3 | 100 | 3 | 100 | 16.9 | 15 | 118.4 | 15 | 6.5 | 15 | 45.6 | 15 | |||||
| Proc. Ch. | 9 | 45 | 13.9±1.8 | 9 | 100 | 9 | 100 | 25.0 | 45 | 175.1 | 45 | 9.6 | 45 | 67.4 | 45 | |||||
| Mahalabia | 12 | 60 | 10.3±1.5 | 12 | 100 | 9 | 75 | 43.5 | 60 | 304.3 | 60 | 16.7 | 60 | 117.0 | 60 | |||||
| Custard | 14 | 70 | 8.6±1.1 | 14 | 100 | 10 | 71 | 34.7 | 70 | 242.6 | 70 | 13.3 | 70 | 93.3 | 70 | |||||
| Rice Milk | 17 | 85 | 10.6±1.2 | 17 | 100 | 16 | 94 | 45.1 | 85 | 315.8 | 85 | 17.4 | 85 | 121.5 | 85 | |||||
| AFB1 | Ras Ch. | 11 | 55 | 11.5±1.8 | 0.0a | 11 | 100 | 2.0c | 11 | 100 | 0.0b | 0.0 | 20.6 | 55 | 144.3 | 55 | 7.9 | 55 | 55.5 | 55 |
| Ched. Ch. | 3 | 15 | 13.2±1.1 | 3 | 100 | 3 | 100 | 23.7 | 15 | 166.1 | 15 | 9.1 | 15 | 63.9 | 15 | |||||
| Feta Ch. | 12 | 60 | 13.4±1.8 | 12 | 100 | 11 | 92 | 24.2 | 60 | 169.5 | 60 | 9.3 | 60 | 65.2 | 60 | |||||
| Proc. Ch. | 6 | 30 | 13.7±1.6 | 6 | 100 | 6 | 100 | 24.6 | 30 | 172.3 | 30 | 9.5 | 30 | 66.3 | 30 | |||||
| Mahalabia | 13 | 65 | 12.7±1.7 | 13 | 100 | 11 | 85 | 53.9 | 65 | 377.2 | 65 | 20.7 | 65 | 145.1 | 65 | |||||
| Custard | 17 | 85 | 9.9±1.2 | 17 | 100 | 16 | 94 | 42.1 | 85 | 295.1 | 85 | 16.2 | 85 | 113.4 | 85 | |||||
| Rice Milk | 17 | 85 | 11.1±1.2 | 17 | 100 | 16 | 94 | 46.9 | 85 | 328.2 | 85 | 18.0 | 85 | 126.2 | 85 | |||||
| OTA | Ras Ch. | 7 | 35 | 13.9±1.5 | 0.0a | 7 | 100 | 5.0d | 7 | 100 | 0.014c | 0.098 | 25.0 | 35 | 175.2 | 35 | 9.6 | 35 | 67.4 | 35 |
| Ched. Ch. | 4 | 20 | 9.9±0.9 | 4 | 100 | 4 | 100 | 17.8 | 20 | 124.6 | 20 | 6.9 | 20 | 47.9 | 20 | |||||
| Feta Ch. | 3 | 15 | 6.2±0.6 | 3 | 100 | 2 | 67 | 11.2 | 15 | 78.4 | 15 | 4.3 | 15 | 30.1 | 15 | |||||
| Proc. Ch. | 8 | 40 | 8.0±1.0 | 8 | 100 | 7 | 88 | 14.4 | 40 | 100.8 | 40 | 5.5 | 40 | 38.8 | 40 | |||||
| Mahalabia | 12 | 60 | 7.5±1.0 | 12 | 100 | 11 | 92 | 31.9 | 60 | 223.2 | 60 | 12.3 | 60 | 85.8 | 60 | |||||
| Custard | 16 | 80 | 4.5±0.42 | 16 | 100 | 1 | 6 | 18.9 | 80 | 132.5 | 80 | 7.3 | 80 | 51.0 | 80 | |||||
| Rice Milk | 16 | 80 | 8.1±0.9 | 16 | 100 | 15 | 94 | 34.3 | 80 | 240.4 | 80 | 13.2 | 80 | 92.5 | 80 | |||||
The acceptable intakes (AI) compared to those estimated mycotoxins (EI) for children and adult human, either per day or week, were presented in Table 4 (B). The estimated daily intake (EDI) of mycotoxins from milk products was evaluated by using the consumed amount of the milk products and the average concentrations of mycotoxin estimated in each product type, considering the body weight average of the different groups. The current study indicated that all EDI levels of AFM1, AFB1 and OTA for infant and adult much exceeded over their acceptable daily intakes (ADI); 0.002, 0.0 and 0.014µg/kg b.w., respectively, according to the international regulation limits proposed by Kuiper-Goodman (1990); JECFA (2007); Brera et al. (2008)44-46.
Electrophoretic banding of aflR1 set was detected from four morphologically identified Aspergillus flavus as demonstration in connection with aflatoxin production which makes its detection is easily in compared to conventional plating techniques. The sizes of DNA fragments (800 bp) were estimated in accordance to commercial DNA ladder 100 bp (Fig. 2).
Fig. 2. Agarose gel electrophoresis (1.5%) of PCR amplification for DNA showing 800bp of aflatoxin regulatory gene-1 (aflR1). Lane 1-4 = samples; compared with control positive and control negative; Lane (L) DNA ladder 100 bp.
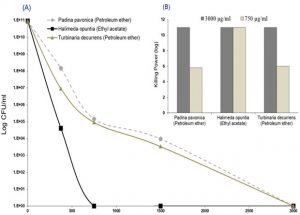
Fig. 3. Antifungal screening of algal extracts (3 mg/ml); Padina pavonica, Halimeda opuntia and Turbinaria decurrens, and dimethyl sulfoxide (DMSO), as negative control, against Aspergillus flavus and Aspergillus niger. The activity was determined by zone of inhibition using Sabouraud agar well diffusion method. Only +ve values of the examined algal extracts were recorded (Fig. 3A). DMSO showed no inhibition. Effect of each algal extract on Aspergillus species was shown in Fig. 3B. All data were set as mean ± SEM of three replicates (n = 3). The difference between groups were considered significant at *P<0.05 and ***P<0.001.
Fig. 4. Anticandidal activity of three algae extracts (log CFU/ml); P. pavonica, H. opuntia and T. decurrens, against Candida tropicalis were shown in Fig. 4A. The Killing powers of two concentrations (3000 and 750 µg/ml) per each extract against the C. tropicalis were shown in Fig. 4B. The assays were performed in triplicate (n = 3).
The antifungal activity of the algal extracts was reported in Fig. 3 and 4. The results revealed that Padina pavonica (petroleum ether extract), Halimeda opuntia (ethyl acetate extract) and Turbinaria decurrens (petroleum ether extract) were effective in suppressing the growth of A. flavus, A. niger and C. tropicalis with variable potency. Ethyl acetate extract of Halimeda opuntia at the concentration of 3 mg/ml was the most effective retarding the growth of all tested pathogenic fungi with inhibition zones of 16.5 ± 0.6 and 22.3 ± 0.73 mm against A. flavus and A. niger, respectively. It is followed by petroleum ether extracts of Padina pavonica and Turbinaria decurrens with inhibition zones of 9.2 ± 0.6 and 6.8 ± 0.44 mm, against A. flavus and 10.0 ± 0.58 and 9.7 ± 0.33 mm against A. niger, respectively (P<0.5), and the later showed higher sensitivity response to all algal extracts rather than the former, A. flavus (Fig. 3).
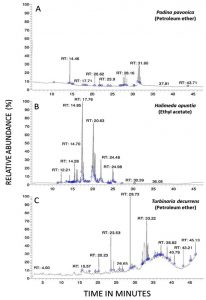
The candidacidal activity of algal extracts by using the dilution method was reported in Fig. 4 (A and B). The algal extracts incubated with C. tropicalis for 24 hr exhibited strong candidacidal activity in dose-dependent manner (Fig. 4 A). Ethyl acetate extract of Halimeda opuntia was very effective against C. tropicalis resulting in severe reduction in CFU of yeast (11 log10 order of killing). Also, the extracts of P. pavonica and T. decurrens showed effective candidacidal activity (5-6 log10 order of killing power) (Fig. 4 B). The constituents of algal extracts detected by GC-MS were shown in Table 5 and Fig. 4. It showed presence of nine compounds in the petroleum ether extract of P. pavonica (Fig. 5 A), eleven compounds in the ethyl acetate extract of H. opuntia (Fig. 5 B), and eleven compounds in petroleum ether extract of T. decurrens. The major constituents of H. opuntia extract were 2, 4-Decadienal, (E, E; 21.56 %), hexadecanoic acid (11.46 %) and 9, 12-octadecadienoic acid (Z, Z; 36.16 %) (Fig. 5 C).
Table (5):
Major bioactive chemical constituents identified in the algal extracts according to the gas chromatography-mass spectrometry (GC-MS) chromatogram analysis.
| Algae | Extract | Sample No. | RT (min.) | Compound Name | Molecular Formula | Molecular Weight | % |
|---|---|---|---|---|---|---|---|
| Padina pavonica | Petroleum ether | 1 | 7.30 | 4H-Pyran-4-0ne,2,3-dihydro-3,5-dihydroxy-6-methyl- | C6H8O4 | 144 | 1.66 |
| 2 | 12.21 | cis-a-Farnesene | C15H24 | 204 | 1.24 | ||
| 3 | 15.62 | Santalol | C15H24O | 220 | 1.01 | ||
| 4 | 16.00 | Bisabolone oxide | C15H24O2 | 236 | 1.77 | ||
| 5 | 17.50 | Bisabolol oxide A | C15H26O2 | 238 | 48.62 | ||
| 6 | 20.23 | En-in-dicycloether | C13H12O2 | 200 | 27.63 | ||
| 7 | 22.04 | Hexadecanoic acid, ethyl ester | C18H36O2 | 284 | 5.22 | ||
| 8 | 24.98 | Ethyl linoleate | C20H36O2 | 308 | 2.21 | ||
| 9 | 25.08 | 8, 11, 14-Eicosatrienoic acid | C20H34O2 | 306 | 1.53 | ||
| Halimeda opuntia | Ethyl acetate | 1 | 14.46 | 2,4-Decadienal, (E,E)- | C10H16O | 152 | 21.56 |
| 2 | 15.07 | Eugenol | C10H12O2 | 164 | 1.91 | ||
| 3 | 20.62 | cis-Asarone | C12H16O3 | 208 | 3.32 | ||
| 4 | 21.73 | 9,12,15-Octadecatrienal | C18H30O | 262 | 1.44 | ||
| 5 | 26.91 | Alantolactone | C15H20O2 | 232 | 2.97 | ||
| 6 | 27.71 | Eudesma-5,11(13)-dien-8,12-olide | C15H20O2 | 232 | 3.39 | ||
| 7 | 28.16 | Hexadecanoic acid | C16H32O2 | 256 | 11.46 | ||
| 8 | 28.65 | Eremanthin | C15H18O2 | 230 | 2.76 | ||
| 9 | 31.60 | 9,12-Octadecadienoic acid(Z,Z)- | C18H32O2 | 280 | 36.16 | ||
| 10 | 31.71 | Oleic Acid | C18H34O2 | 282 | 6.39 | ||
| 11 | 31.95 | Octadecanoic acid | C18H36O2 | 284 | 3.23 | ||
| Turbinaria decurrens | Petroleum ether | 1 | 15.57 | 5-Hydroxymethylfurfural | C6H6O3 | 126 | 1.51 |
| 2 | 20.23 | 2-Allyl-5-t-butylhydroquinone | C13H18O2 | 206 | 2.61 | ||
| 3 | 23.53 | Ar-tumerone | C15H20O | 216 | 8.26 | ||
| 4 | 24.34 | Curlone | C15H22O | 218 | 1.68 | ||
| 5 | 28.73 | Hexadecanoic acid, methylester | C17H34O2 | 270 | 13.47 | ||
| 6 | 29.93 | Hexadecanoic acid | C16H32O2 | 256 | 13.42 | ||
| 7 | 32.54 | Octadecanoic acid, methylester | C19H38O2 | 298 | 5.31 | ||
| 8 | 33.22 | Oleic acid | C18H34O2 | 282 | 25.62 | ||
| 9 | 33.4 | Isochiapin B | C19H22O6 | 346 | 7.57 | ||
| 10 | 38.82 | Dotriacontane | C32H66 | 450 | 11.63 | ||
| 11 | 46.56 | Lucenin 2 | C27H30O16 | 610 | 3.67 |
Unhygienic handling of milk products may lead to contamination by different fungi and propagation of mycotoxins. However, our results declared that milk products were contaminated with mold and yeast more than the permissible limit according to EOSQC (2005)41. A total of 80.7 and 64.3% of all samples were contaminated with mold and yeast, respectively. These results agreed with former findings reported by researchers in Egypt. They stated that milk samples are contaminated with mold and yeast in majority of the examined regions and most of samples didn’t comply with the permissible limit predetermined by National and International Standards Organizations47, 48. Furthermore, Italian researches found mold in 54 of the 122 analysed cheese samples (44.3%), stated that the potentially toxigenic fungal species were mainly detected in cheese samples49.
Mold and yeast are widely distributed as environmental contaminants which can grow at variable temperature, so their presence in milk products could be attributed to unsanitary measures during manufacturing, processing and storage or using of bad quality raw ingredients. They induce undesirable changes such as off-flavor, color defects, rancidity and changes in texture50. Mold and yeast counts in dairy products are used as an index of the proper hygienic quality51. The mycotoxins-producing molds are potential hazard to food safety and human health51.
On the other hand, a total of 38 and 15 different species of mold and yeast were isolated and identified from examined milk products samples. Aspergillus and Candida spp. were the most dominant species of mold and yeast isolated from 32.2 and 29.2% of the milk products, respectively, which coincide with Khalifa et al. (2013)52 and ELbagory et al. (2014)53.
Candida species; C. tropicalis, C. krusei and C. parapsilosis are the most common human-specific fungi responsible for systemic and superficial infections originated from food or the environment54. Moreover, C. tropicalis is implicated in the higher mortality rates than other Candida spp. particularly in neutropenic and oncogenic patients. Several cases of C. tropicalis cross-resistance to antifungal agents have been reported in clinical isolates55. Aspergillus species including; A. flavus, A. niger, A. ochraceus, A. parasiticus, A. fumigatus have the potential to contaminate food and environment and linked to the life-threatening disorders, i.e. aspergillosis and mycotoxicosis56. Aspergillosis causes wide scaled clinical manifestations, i.e. allergy, pulmonary, ocular infections, otomycosis, endocarditis, osteomyelitis, and skin infections, and so the invasive aspergillosis could lead to high mortalities57. Moreover, Aspergillus is an important genus in foods, causing more spoilage and biodeterioration than other fungi58. Almost all food kinds including milk and its products are vulnerable to contamination by Aspergillus species especially in tropical and subtropical climates resulted in huge agro-economic losses in the world59.
Aspergillus is the most significant genera of mycotoxigenic fungi, over 40 species of Aspergillus have been known their ability to produce a wide range of mycotoxins having adverse effect on health of humans and animals consuming it. Aflatoxins are products of A. flavus and A. parasiticus and ochratoxin-A is produced by A. niger, A. ochraceus and A. carbonarius60. Furthermore, Drusch and Aumann (2005)61 declared that Mycotoxins can diffuse into the food without any sign of mycelium growth. Consequently, the absence of mold does not guarantee mycotoxins free food.
The results showed heterogeneous mycotoxins mixtures; 25, 40 and 27.5% of cheese and 71.7, 78.3 and 73.3 % of dairy desserts were contaminated with mycotoxins; AFM1, AFB1 and OTA, respectively (Table 4). The concentrations of mycotoxins detected were exceeding more than the permissible limits declared by National/International Standards Organizations13,16,42,43. These values are in accordance with the results reported by other Egyptian researchers who stated that, mycotoxins residues were contaminated milk products samples in various levels more than the permissible limit set by Egyptian and European regulation limits48,62. They stated that the highest incidence of mycotoxins recorded in milk products may refer to unhygienic conditions in processing, package or storage which provide favourable conditions for mold growth and subsequently toxin production. Moreover, Turkish and Iranian researchers studied mycotoxins concentration in milk products samples, stated that mycotoxins concentrations were more than the maximum residue limits recommended by National and International standard Organizations63,64. They concluded that the content of mycotoxins remains relatively stable during the different steps of dairy products production and storage and thermal processing used in dairy industry cannot inactivate it.
For what the data reported in this paper, the amounts of AFM1, AFB1 and OTA detected seem to be dangerous. In fact, the average consumption amount of cheese and dairy desserts for the adult and children assumed 45 and 106 g/day, respectively (data referred to Egypt – Cairo Nutrition Institute, 1996; 2007)65,66, and the EDI levels of mycotoxins for infant and adult were much more than the ADI proposed by international regulation standards44-46. The AFB1, the most dangerous mycotoxin, should be absent or the lowest recorded according to ALARA (as low as reasonably achievable) for food safety46. Our data is agreed well with El-Badry, (2016)67 and Milicevic et al. (2017)68 whom found that most of analysed milk and milk products samples collected from different localities in Egypt and Serbia were above ADI of mycotoxins declared by International Standards Organizations. In contrast to our results, former international researchers concluded that collected milk samples containing mycotoxins level that were accepted for human as compared with recommended limits69.
The carcinogenic properties of aflatoxins motivate us to develop rapid, sensitive and specific approach for the identification and detection of aflatoxin producing A. flavus from food samples. Aflatoxin regulatory aflR1 gene forms the accurate and specific marker for aflatoxigenic strains of A. flavus in foods70. In agreement with ELbagory et al. (2014)53, the aflR-specific primer designed for aflatoxigenic A. flavus was approved as an easily detecting method, so the detection of aflatoxigenic A. flavus from food using polymerase chain reaction (PCR) shows no false results. The PCR technique allows screening of many suspected samples in high sensitivity and accuracy, with the capacity to proceed a high number of samples in a short time.
Preventing food spoilage and protecting human health from the harmful effects of mold or yeast pathogens has become extremely challenging. The limited application of chemical preservatives, susceptibility, toxicity, microbial resistance and its adverse effects on human health increase the demand to search for natural, healthy, safer and potentially effective antifungal agents. Thus, antimicrobial activity of algal extracts can provides a key aspect of treatment of fungal infections and to be used as natural preservatives to ensure healthy and safe food. In the present study, the crude extracts of the three algae species including, Halimeda opuntia, Turbinaria decurrens and Padina pavonica, showed fungicidal activities against recovered three fungal pathogens, A. flavus, A. niger and C. tropicalis with various inhibitory actions depending on the seaweed species and the solvent used.
Various studies evaluated the antimicrobial activities of the marine algae viz., H. opuntia, P. pavonica and Turbinaria Species. Different solvents viz., petroleum ether, diethyl ether, ethyl acetate, ethanol, chloroform, hexane and water were used for algae extraction to investigate the antimicrobial activity against mold and yeast including A. flavus, A. niger, A. fumigatus, Fusarium moniliforme, Penicillium herquei, Candida tropicalis, Candida albicans, Candida kefir71,72. Some Egyptian researches reported the fungicidal activities of H. opuntia, P. pavonica and Turbinaria Species which showed the inhibitory effect were 12 to 32 mm of H. opuntia and Padina pavonica extracts73 and 10-25 mm of methanolic H. opuntia extract against Candida spp.74. Globally, Indira et al. (2013)75 stated that methanolic extracts of Halimeda species exhibit the highest antifungal activity against several fungal strains including Aspergillus flavus, Aspergillus niger, Candida albicans, Rhizopus Spp. and Pencillium Spp. in vitro using minimum fungicidal concentration and well diffusion method with the potential use of algal extracts as antimicrobial candidate. In addition, the ethanolic extract of Padina pavonica generated maximum inhibition zone against Candida spp.76. Moreover, it was reported that Turbinaria Species exhibited significant inhibition effect against Candida spp. (7.0±0.0 mm) and Aspergillus flavus (7.0±0.0 mm)77.
The current article identified the phytochemical algal constituents against the mycotic pathogens by using the GC-MS analysis, showing their active principles with retention times (RT), molecular formulae, molecular weight and relative concentration (%) in the different algal extracts. The GC-MS analysis reported the presence of 9 compounds in the extracted P. pavonica, eleven compounds in H. opuntia and eleven compounds in T. decurrens. The most predominant compounds in the P. pavonica extract were bisabolol oxide A (48.62%) and En-in-dicycloether (27.63%), whereas those in H. opuntia extract were 2, 4-decadienal, (E, E)-(21.56%), Hexadecanoic acid (11.46%) and 9, 12-Octadecadienoic acid (Z,Z) (36.16%). However, the T. decurrens mostly contained hexadecanoic acid, methylester (13.47%), hexadecanoic acid (13.42%), oleic acid (25.62%) and dotriacontane (11.63%).
The great efficacy of the seaweeds extracts against the pathogenic mold and yeast could be attributed to the active phytochemicals and metabolites compounds in addition to the fatty acids and their derivatives78,79. Those abundant compounds had been previously identified and characterized from various herbal and algal sources. The in vitro data presented intensively in previous literatures showed that Octadecadienoic acid and Bisabolol oxide A poses potent fungicidal activities against wide range of fungal species. There are several reports regarding that bisabolol has strong fungicidal and bactericidal proprieties80. Bisabolol produced nearly 98% loss in the viability of the germinating conidia of A. niger, A. flavus, A. fumigatus81. In addition, bisabolol showed antimicrobial activity against A. niger at concentrations above 125µg/ml with hyphal growth and conidial production inhibition82. Many of the studies in the scientific literature highlighted the antifungal activity Octadecadienoic acid (Z,Z)-. Furthermore, Ali et al. (2017)83 identified 9-octadecenoic acid (Z)-, methyl ester (10.27%) and 9-octadecenoic acid (Z)-, methyl ester (12.75%), that were found to be responsible for antifungal activity against various fungal genera. In addition to another study, their result indicated the abundances of methyl ester (Z,Z)-9,12-octadecadienoic acid, (E)- 9-octadecanoic acid, 9,12-octadecadienoic acid, which showed great antifungal agent against various fungus isolates84. Bisabolol can be used to prevent microbial growth in wide ranging applications such as food, cosmetics and topical antifungal owing to its excellent nontoxic properties81. Finally, it could be concluded that those identified molecules have potential antifungal activities and, notably, that the extracts of the algae where they are abundant also showed potent antifungal activity. The explored algal species could be more effective against the fungal infection rather than those traditional fungicidal agents. Therefore, it can be considered as natural preservatives providing healthy and safe food without the unpleasant effects of chemical one. The current study presented the ability of algae to promote as an antifungal agent via stable its biologically active compounds. Also, provides insights into designing of novel antifungal drugs for the clinical use or in food preservation.
Broad fungal diversity and heterogeneous mycotoxins mixture were detected in the locally produced dairy products which denote the unhygienic measures of either processing or preservation in dairy shops. Presence of mycotoxins in the dairy products despite of low levels represents public health hazards. Aspergillus and Candida spp. were mostly detected in unhygienic preserved milk products. Interestingly, the current study for the first time presented the antifungal activity of Egyptian marine algae; P. pavonica H. opuntia and T. Decurrens which exhibited effective fungicidal activity in vitro. The GC-MS revealed presence of 9 to 11 compounds in the algal extracts, including bisabolol oxide A, 2, 4-decadienal and oleic acid. Implementation of regulatory and monitoring measures for mitigation of mycotoxin production and inhibit of toxigenic molds growth in food and feed are very crucial to overcome the higher level AFB1 and AFM1 in dairy products. Future pharmacological studies are required for studying the comparative effects of the algal constituents on mold and yeast growth in vitro, and the possibility for using the marine macro-algal extracts as safe natural preservatives instead of the currently used chemical preservatives. Using the discovered novel antifungal agent of marine algae, as novel natural preservatives, could have positive impact on the safety and quality of dairy product and medicine industry as potent fungicidal agents. Moreover, toxicological studies are needed to be performed for the drug discovery; and comparative in vitro studies should be performed on the IC50 of those algal constituents on different species of mycotoxins-producing fungi.
ACKNOWLEDGMENTS
Not applicable.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through General Research Project (Number R.G.P.1/157/40). The authors would like to acknowledge South Valley University, Qena, Egypt for supports.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
All datasets generated or analysed during this study are included in the manuscript.
- Kure CF, Skaar I, Brendehaug J. Mould contamination in production of semi-hard cheese. International Journal of Food Microbiology, 2004; 93(1): 41-49.
Crossref - Karthikeyan N, Dhanalaksnmi B. Hygienic quality of Indian sweet milk products from different sources. Banglad J. Microbiol., 2010; 27: 32-37.
Crossref - Irshad MS, Emily J, Steven S, Khalil K. Molecular Identification of Isolated Fungi from Unopened Containers of Greek Yogurt by DNA Sequencing of Internal Transcribed Spacer Region. Pathogens, 2014; 3: 499-509.
Crossref - Gandomi H, Misaghi A, Basti AA, Bokaei S, Khosravi A, Abbasifar A, Javan AJ. Effect of Zataria multiflora Boiss. Essential oil on growth and aflatoxin formation by Aspergillus flavus in culture media and cheese. Food and chemical toxicology, 2009; 47: 2397-2400.
Crossref - Ghibaudo G, Peano A. Chronic monolateral otomycosis in a dog caused by Aspergillus ochraceus. Veterinary Dermatology, 2010; 21(5): 522-526.
Crossref - Khalifa E, Nossair M. Comparative mycological assay on prevalence of yeasts, molds and aflatoxin M1 (AFM1) in some fermented milk products in Alexandria, Egypt. Life Science Journal, 2016; 13(7): 20-29.
- Gandomi H, Misaghi A, Basti AA, Bokaei S, Khosravi A, Abbasifar A, Javan AJ. Effect of Zataria multiflora Boiss essential oil on growth and aflatoxin formation by Aspergillus flavus in culture media and cheese. Food and Chemical Toxicology, 2009; 47(10): 2397-2400.
Crossref - Martins ML, Martins HM, Bernardo F. Aflatoxins in spices marketed in Portugal. Food Additives and Contaminants, 2001; 18(4): 315-319.
Crossref - Bennett JW, Klich M. Mycotoxins. Clinical Microbiology Reviews, 2003; 16(3): 497-516.
Crossref - Frisvad JC, Skouboe P, Samson RA. Taxonomic comparison of three different groups of aflatoxin producers and a new efficient producer of aflatoxin B1, sterigmatocystin and 3-Omethyl sterigmatocystin, Aspergillus rambellii sp nov. Systematic and Applied Microbiology, 2005; 28(5): 442-453.
Crossref - Sengun I, Yaman D, Gonul S. Mycotoxins and mould contamination in cheese: a review. World Mycotoxin Journal, 2008; 1(3): 291-298.
Crossref - International Agency of Research in Cancer (IARC). Some traditional herbal medicines: Some mycotoxins, naphthalene and styrene. IARC Monograph on the evaluation of carcinogenic risks to humans. World Health Organization Lyon France, 2002; 82: 1-556.
Crosref - European Commission Regulation. Setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union, 2006; 364: 5-24.
Crossref - Varga J, Frisvad JC, Samson RA. Two new aflatoxin producing species and an overview of Aspergillus section flavi. Studies in Mycology, 2011; 69: 57-80.
Crossref - Awad EI, Abdelfattah ME, Abdelkaliek AA, El-diasty EM. Prevalence of ochratoxin in small and large scale produced roomy cheese in Sharkia Governorate. World Rural Observations, 2012; 4(3): 76-80.
- Creppy EE. Update of survey, regulation and toxic effects of mycotoxins in Europe. Toxicology Letters, 2002; 127(1-3): 19-28.
Crossref - Shan B, Cai Y, Brooks JD, Corke H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int. J. Food Microbiol., 2007; 117: 112-119.
Crossref - Bialonska D, Ramnani P, Kasimsetty SG, Muntha KR, Gibson GR, Ferreira D. The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int. J. Food Microbiol., 2010; 140: 175-182.
Crossref - Manilal A, Sujith S, Sabarathnam B, Kiran G, Selvin J, Shakir C, Lipton A. Bioactivity of the red alga Asparagopsis taxiformis collected from the south-western coast of India. Braz. J. Oceonogr. 2010, 58(2): 93-100.
Crossref - Galal HRM, Salem WM, Nasr El-Deen F. Biological control of some pathogenic fungi using marine algae extracts. Research Journal of Microbiology, 2011; 6(8): 645-657.
Crossref - Shannon E, Abu-Ghannam N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Marine drugs,2016; 14(4), 81.
Crossref - Charway GN, Yenumula P, Kim YM. Marine Algae and Their Potential Application as Antimicrobial Agents. Journal of Food Hygiene and Safety, 2018; 33(3): 151-156.
Crossref - Abedin RMA, Taha HM. Antibacterial and antifungal activity of cyanobacteria and green microalgae. Evaluation of medium components by Plackett-Burman design for antimicrobial activity of Spirulina platensis. Global Journal of Biotechnology and Biochemistry, 2008; 3: 22-31.
- Mayer A, Rodriguez AD, Taglialatela-Scafati O, Fusetani N. Marine pharmacology in 2009–2011: Marine compounds with antibacterial, antidiabetic, antifungal, anti inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs, 2013; 11: 2510–2573.
Crossref - Chay C, Dizon EI, Elegado FB, Norng C, Hurtada WA, Raymundo LC. Isolation and identification of mold and yeast in medombae, a rice wine starter culture from Kompong Cham Province, Cambodia. Food Research, 2017; 1(6): 213-220.
Crossref - Harrigan WF, MacCance Margaret E. Laboratory Methods in Microbiology. Academic Press, New York, USA, 1966.
Crossref - Pitt JI, Hoching AD. Fungi and Food spoilage, 3rd Ed. Published by Blackie Academic and professional Academic Press, Springer Science, New York, London, 2009.
- Conant NF, Smith DT, Baker RD, Callaway JL, Martin DS. Manual of clinical mycology, 3rd Ed. WB Saunders, Philadelphia, USA, 1971; 503: 527-540.
- Finegold SM, Martin WJ. Bailey and Scott’s diagnostic microbiology, 6th Ed. The CV. Mosby Company, St. Louis, 1982.
- Cruickshank R, Duguid JP, Marimion BP, Swain RH. Medical microbiology, the practice of medical microbiology, 12th Ed, Vol. 11. Churchill Livingstone Limited, Edinburgh, London and New York, 1975.
- Koneman EW, Allen SD, Janda WM, Scheckenberger PC, Winn WC. Color atlas and text book of diagnostic microbiology, 4th Ed. JB Lippincott Company, Philadelphia, 1992.
- Roberts BA, Patterson DSP. Detection of twelve mycotoxins in mixed animal feed stuff using a novel membrane clean up procedure. J. AOAC, 1975; 58: 1178.
Crossref - AOAC. “Association of Official Agricultural Chemists”. Official methods of analysis of the Association of Official Analytical Chemists, 13th Ed., 1980.
- Scott De B. Toxigenic fungi isolated from cereal and legume products. Mycopathologia et Mycologia Applicata, 1965; 25: 213-222.
Crossref - Howell MV, Taylor PW. Determination of aflatoxin, ochratoxin A and zeralenone in mixed feeds with detection by thin layer chromatography or HPLC. J. Assoc. Anal Chem., 1981; 64, 1356.
Crossref - Bintvihok A, Treebonmuang S, Srisakwattana K, Nuanchun W, Patthanachai K, Usawang S. A rapid and sensitive detection of aflatoxin-producing fungus using an optimized polymerase chain reaction (PCR). Toxicological Research, 2016; 32(1): 81-87.
Crossref - Patra JK, Rath SK, Jena KP, Rathod VK, Thatoi H. Evaluation of antioxidant and antimicrobial activity of seaweed (Sargassum sp.) extract: a study on inhibition of Glutathione-S transferase activity. Turkish Journal of Biology, 2008; 32(2): 119-125.
Crossref - Salem WM, Galal H, Nasr El-deen F. Screening for antibacterial activities in some marine algae from the red sea (Hurghada, Egypt). African Journal of Microbiology Research, 2011; 5(15): 2160-2167.
Crossref - Magaldi S, Mata-Essayag S, De Capriles CH, Perez C, Colella MT, Olaizola C, Ontiveros Y. Well diffusion for antifungal susceptibility testing. International Journal of Infectious Diseases, 2004; 8(1): 39-45.
Crossref - Hoq MI, Ibrahim HR. Potent antimicrobial action of triclosan–lysozyme complex against skin pathogens mediated through drug-targeted delivery mechanism. European Journal of Pharmaceutical Sciences, 2011; 42(1-2): 130-137.
Crossref - Egyptian Organization for Standardization and Quality Control (EOSQC) “1008/2005”, “1185-1/2005”, “1414/2005”. Egyptian Organization for Standardization and Quality Control. Egyptian Standards (E.S.) No.: 1008/2005, 1185-1/2005, 1414/2005, 2005.
- Van Egmond HB, Jonker MA. Current situation on regulation for mycotoxins, 2004; pp. 1–15. In Yoshizawa T, Kumagai S, Goto T, (eds.), New horizon of mycotoxicology for assuring food safety. Tokyo: Japanese Association of Mycotoxicology.
- Egyptian organization for standardization and quality control (EOSQC). Maximum levels of mycotoxin for foods and feeds. part-1: Aflatoxins No. 1-1875/2007, 2007.
- Kuiper-Goodman T. Uncertainties in the risk assessment of three mycotoxins: Aflatoxin, ochratoxin, and zearalenone. Canadian Journal of physiology and Pharmacology, 1990; 68(7): 1017-1024.
Crossref - Joint FAO/WHO expert committee on food additives (JECFA). Evaluation of certain food additives and contaminants: sixty-eighth report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Org 68th Meeting, 2007. www.who.int/iris/handle/10665/43870
- Brera C, De Santis B, Debegnach F, Miraglia M. Mycotoxins, pp. 363-427. In Yolanda P, (ed,), Comprehensive analytical chemistry Compre. Analytical Chemistry, 2008; 51.
Crossref - El-Leboudy AA, Amer AA, El-Gaml AM, Shahin HF. Sanitary Evaluation of Curd Dairy Products. Alexandria Journal of Veterinary Sciences,2015; 45(1): 51-56.
Crossref - Khalifa M, Shata RR. Mycobiota and Aflatoxins B1 and M1 Levels in Commercial and Homemade Dairy Desserts in Aswan City, Egypt. Journal of Advanced Veterinary Research, 2018; 8(3): 43-48.
- Montagna MT, Santacroce MP, Spilotros G, Napoli C, Minervini F, Papa A, Dragoni I. Investigation of fungal contamination in sheep and goat cheeses in southern Italy. Mycopathologia, 2004; 158(2): 245-249.
Crossref - Mislivec PB, Beuchat LR, Cousin MA. Yeasts and molds. Chapter 16 Compendium of methods for microbiological examination of food. 3rd Ed. American Public Health Association, Washington DC, 1992.
- Varga L. Microbiological quality of commercial dairy products, pp. 487-494. In Communicating current research and educational topics and trends in applied microbiology. Formatex microbiology Series, Formatex Badajoz, 2007. ISBN 978-84-611-9422-3.
Crossref - ELbagory AM, Eid AM, Hammad AM, Dawood SA. Prevalence of fungi in locally produced cheese and molecular characterization of isolated toxigenic molds. Benha Veterinary Medical Journal, 2014; 27(2): 9-20.
Crossref - Khalifa MI, Al-Ashmawy MA, Abdel-Khalik A, El-Sherbini M. Mycological evaluation of serving some dairy products with special reference to mycotoxins production in Azhar University student hostels.World Journal of Dairy & Food Sciences, 2013; 8(2): 165-170.
- Turner SA, Butler G. The Candida pathogenic species complex. Cold Spring Harbor Perspectives in Medicine, 2014; 4(9): a019778.
Crossref - Forastiero A, Mesa-Arango AC, Alastruey-Izquierdo A, Alcazar-Fuoli L, Bernal-Martinez L, Pelaez T, Lopez JF, Grimalt JO, Gomez-Lopez A, Cuesta I, Zaragoza O, Mellado E. Candida tropicalis antifungal cross-resistance is related to different azole target (Erg11p) modifications. Antimicrobial Agents and Chemotherapy, 2013; 57(10): 4769-4781.
Crossref - Gemeda N, Woldeamanuel Y, Asrat D, Debela A, Lemma H, Belete Y. Assessment of aflatoxigenic Aspergillus species in food commodities from local market of Addis Ababa. Research, 2014; 1: 1195.
Crossref - Khasawneh F, Mohamad T, Moughrabieh MK, Lai Z, Ager J, Soubani AO. Isolation of Aspergillus in critically ill patients: a potential marker of poor outcome. Journal of Critical Care, 2006; 21(4): 322-327.
Crossref - Dao T, Dantigny P. Control of food spoilage fungi by ethanol. Food Control, 2011; 22(3-4): 360-368.
Crossref - Pitt JI, Hoching AD. Fungi and Food spoilage. 3rd Ed. Published by Blackie Academic and Professional Academic Press, Springer Science, New York, London, 2009.
- Zain ME. Impact of mycotoxins on humans and animals. Journal of Saudi Chemical Society, 2011; 15(2): 129-144.
Crossref - Drusch S, Aumann J. Mycotoxins in fruits: Microbiology, occurrence and changes during fruit processing. Advances in Food and Nutrition Research, 2005; 50: 33-78.
Crossref - Asmaa SMM, El-hady WM, Abdelfatah EN. Screening of some dairy products in Egypt for aflatoxin-M1 contamination and some heavy metal residues. Int. J. Adv. Res., 2017; 5(10): 1536-1543.
Crossref - Bakirci I. A study on the occurrence of aflatoxin M1 in milk and milk products produced in Van province of Turkey. Food Control, 2001; 12: 47-51.
Crossref - Fallah AA, Rahnama M, Jafari T, Saei-Dehkordi SS. Seasonal variation of aflatoxin M1 contamination in industrial and traditional Iranian dairy products. Food Control, 2011;22(10): 1653-1656.
Crossref - Nutrition Institute, Cairo. Guide of healthy food for Egyptian family. 2nd Ed. Nutrition Institute, Cairo, A.R.E., 1996
- Nutrition Institute, Cairo. Daily consumption of adult person in Egypt. No. 43906, 2007. http://www.elharm.com/9134/INVES.HTM
- El-Badry S. Determination of Ochratoxin A Residues in Some Animal and Plant Milk Products. Zagazig Veterinary Journal, 2016; 44(2): 101-105.
Crossref - Milicevic D, Spiric D, Jankovic S, Velebit B, Radicevic T, Petrovic Z, Stefanovic S. Aflatoxin M1 in processed milk: Occurrence and seasonal variation with an emphasis on risk assessment of human exposure in Serbia. IOP Conference Series: Earth and Environmental Science, 2017; 85: 012040.
Crossref - dos Santos JS, Granella V, Pigatto GM, Reiniger LRS, Costabeber IH. Aflatoxin M1 in pasteurized and raw milk from organic and conventional systems. Journal fur Verbraucherschutz und Lebensmittelsicherheit, 2016;11(4): 299-304.
Crossref - Shapira R, Paster N, Eyal O, Minastherov M, Mett A, Salomon R. Detection of aflatoxigenic moulds in grains by PCR. Applied and Environmental Microbiology, 1996; 62(9): 3270-3273.
Crossref - Amaro HM, Guedes AC, Malcata FX. Antimicrobial activities of microalgae: an invited review. Science against Microbial Pathogens: Communicating Current Research and Technological Advances, 2011; 3: 1272-1284.
- Charway GN, Yenumula P, Kim YM. Marine Algae and Their Potential Application as Antimicrobial Agents., 2018; 33(3): 151-156.
Crossref - Hamza AH, Al-Bishri W, Omar HH, Danial EN. Potential antimicrobial, antioxidant and anityrosenase activities achieved by selected species of marine macroalgae. J. Pure Appl Microbiol, 2014; 8: 257-265.
- Selim SA. Antimicrobial, Antiplasmid and Cytotoxicity Potentials of Marine Algae Halimeda opuntia and Sarconema filiforme collected from Red Sea Coast. World Academy of Science, Engineering and Technology, 2012; 61.
- Indira K, Balakrishnan S, Srinivasan M, Bragadeeswaran S, Balasubramanian T. Evaluation of in vitro antimicrobial property of seaweed (Halimeda tuna) from Tuticorin coast, Tamil Nadu, Southeast coast of India. African Journal of Biotechnology, 2013; 12(3).
Crossref - Tuney I, Cadirci BH, Unal D, Sukatar A. Antimicrobial activities of the extracts of marine algae from the coast of Urla (Izmir, Turkey). Turkish Journal of Biology, 2006; 30(3): 171-175.
- Shibu A, Dhanam S. In vitro antifungal activity of Turbinaria conoides collected from Mandapam coast, Tamilnadu, India. Journal of Experimental Sciences, 2016; 27-30.
Crossref - Perez MJ, Falque E, Dominguez H. Antimicrobial action of compounds from marine seaweed. Marine Drugs, 2016; 14(3): 52.
Crossref - Mayer AMS, Rodriguez AD, Taglialatela-Scafati O, Fusetani N. Marine pharmacology in 2009-2011: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Marine Drugs, 2013; 11(7): 2510-2573.
Crossref - EL-Hefny M, Abo Elgat WA, Al-Huqail AA, Ali HM. Essential and Recovery Oils from Matricaria chamomilla Flowers as Environmentally Friendly Fungicides Against Four Fungi Isolated from Cultural Heritage Objects. Processes, 2019; 7(11): 809.
Crossref - De Lucca AJ, Pauli A, Schilcher H, Sien T, Bhatnagar D, Walsh TJ. Fungicidal and bactericidal properties of bisabolol and dragosantol. Journal of essential oil research, 2011; 23(3): 47-54.
Crossref - Tolouee M, Alinezhad S, Saberi R, Eslamifar A, Zad SJ, Jaimand K, Taeb J, Rezaee MB, Kawachi M, Shams-Ghahfarokhi M, Razzaghi-Abyaneh M. Effect of Matricaria chamomilla L. flower essential oil on the growth and ultrastructure of Aspergillus niger van Tieghem. International journal of food microbiology, 2010; 139(3): 127-133.
Crossref - Ali A, Javaid A, Shoaib A. GC-MS analysis and antifungal activity of methanolic root extract of Chenopodium album against Sclerotium rolfsii. Planta Daninha, 2017; 35.
Crossref - Ojinnaka CM, Nwachukwu KI, Ezediokpu MN. The Chemical Constituents and Bioactivity of the seed (Fruit) extracts of Buchholzia Coriacea Engler (Capparaceae). Journal of Applied Sciences and Environmental Management, 2015; 19(4): 795-801.
Crossref
© The Author(s) 2020. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.