ISSN: 0973-7510
E-ISSN: 2581-690X
The ability of plants to acclimatise and thrive in stressed environments can be attributed, in part, to the reserve of endophytic fungi that they harbour, that help enhance physiological and immunological defence and tolerance to various biotic and abiotic stressors. The present work has focussed on screening laccase producing endophytic fungi residing in different aquatic plants isolated from Hulimavu Lake, Bengaluru. This lake is well known for its water pollution contributed by anthropogenic factors. Survival of plants in this lake can hence be associated with their rich repertoire of endophytic fungi that enhance host plant defence towards stressors. Upon isolation and culturing of endophytic fungi, qualitative laccase detection using laccase specific growth media and quantitative laccase estimation using ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) substrate were performed. Differential production rates were observed for the laccase enzyme by different endophytic fungi; production rates also varied between fungi isolated from different parts like node, stem, root and leaf of the same plant species too. Phylogenetic analysis of fungal isolates with highest laccase production was performed and the species was found to be Cladosporium tenuissimum. Even the crude extract of this strain displayed laccase production of 42.16U/L, as revealed by ABTS assay. Hence this strain is a promising candidate for optimization studies for utilisation in the domain of bioremediation and industrial applications.
Endophytic Fungi, Abiotic Stressors, Laccase, ABTS, Bioremediation
Associated commonly with wood decay or decomposition, laccases are important enzymes commonly produced by many basidiomycetes1 among which, genus Trametes are well known producers of laccase.2 Laccases are glycosylated polyphenol oxidases with important biotechnological applications in industrial fields like bioremediation, food, pulp-paper industry, textiles and detoxification of pesticides and pollutants. They are known to catalyse different oxidation reactions for organic and inorganic substrates like polyphenols and aromatic amines.3 This group of enzymes are among the oldest and most studied groups with inherent properties of reactive radical production that have not yet been fully applied in various fields due to low commercial availability of laccase producing organisms.4 Known predominantly for lignin degradation, laccases also assist in detoxifying toxic polyphenols. Fungal laccases are hypothesised to partake in host protection against environmental stress through the production of dark pigmented polymers of dihydroxynaphthalene melanins.5 Extracellular laccase production in various organisms like plants, fungi, bacteria and insects has been reported to be only in small amounts, that can be enhanced using various substrates like phenols,6 aliphatic alcohols7 and aqueous plant extracts.8 While these reports of enhanced laccase production have been well documented, there remains more scope for research into effects of metal ions in laccase production. A study by Wang et al. reported a 10-fold increase in laccase production in the presence of Lanthanum (La3+) at a concentration of 1g/L in a bambusicolous fungus Shiraia bambusicola. At the molecular level, this was attributed to La3+ mediated upregulation of ROS (Reactive Oxygen Species) and NO (Nitric Oxygen) that led to enhanced laccase gene (lcc1) expression followed by increased cell membrane permeability that facilitated greater exudation of laccase.9 Highest laccase producers with industrial applications are white rot fungi belonging to the genera Pleurotus, Trametes and Phlebia.4 Despite the various advantages of laccases in different industrial applications, major drawbacks of commercial laccase production include lower yield and longer fermentation duration. Hence the need for novel laccase producing organisms is of paramount significance. Shiraia strains isolated from fruiting bodies were identified as novel fungal laccase producers that helped produce about 12000-16400 U/L laccase in shorter fermentation time when compared to white rot fungal species.10 Alongside lanthanum, similar research into effects of metal ions in promoting laccase production revealed upregulation in laccase activities in Trametes that was brought about by Cu2+ at concentrations of 1mmol/L.11 First of its kind, in a study to probe the effects of gamma irradiation on fungal endophytic laccase production, Navada and Kulal reported enhanced laccase production in 1.2kGy gamma irradiated endophytic fungus and laccase stability was also found to be unaffected even with metal concentrations upto 10mM.12
The symbiotic relationship between plants and endophytes is indispensable.13 While host plants are conferred with numerous advantages by endophytes, these microorganisms have life cycles that are partly or entirely inside plants without harming them or causing any diseases to their host plants.14 Of the various bioactive compounds produced by endophytic fungi, about 51% of the newly discovered compounds have pharmacological properties including promoting plants to better cope with different types of stressed conditions.15 They also significantly modulate plant gene expressions and secondary metabolites production. Apart from pharmaceutical applications, bioactive compounds secreted by fungal endophytes are also employed for biotransformation of pollutants and toxic compounds. Decomposition of a phenylurea herbicide diuron was reported to be performed by endophytic fungi Neurospora intermedia MF362953 isolated from Saccharum officinarum.16 Various studies have also reported endophytic fungi mediated in vitro degradation of different anti-inflammatory drugs like diclofenac, ibuprofen, piroxicam and so on.17 These bio-degradative properties are brought about through synergistic actions between enzymes from fungal endophytes and their host plants. Thus, endophytic fungi represent a repertoire of different enzymes like amylase, pectinase, xylanase, cellulase, laccase and so on that have numerous applications in different industrial processes. Few noteworthy examples of fungal endophytic enzymes isolated from different host plants include: xylanase and endoglucanase produced by Beauveria bassiana MN544934 isolated from host plant Allium cepa,18 amylase, cellulase and laccase produced by Fusarium equiseti isolated from host plant Cananga odorata,19 cellulase produced by from Fomitopsis cf. Meliae KYO isolated from host plant Bacopa monnieri.20
Among various enzymes produced by fungal endophytes, laccases display wide ranging applications in industries partly due to broad ranging substrates they have. Additionally, laccase mediated biocatalysis is efficient, sustainable and eco-friendly. However, factors like high cost and efficiency of isolating fungal endophytic laccases are the limiting factors in the industrial applications of these enzymes. With wide ranging applications in bioremediation of dyes, endophytic fungi are considered to be efficient, eco-friendly, cost-effective and viable alternatives because of their properties to degrade non-phenolic and phenolic dyes.21 In comparison to endophytic bacteria, endophytic fungi are preferred for dye biodegradation due to factors like wide mycelial network, ability to utilise xenobiotics as substrates and presence of catabolic enzymes with low substrate specificity.22 White-rot fungi are considered as most potent azo dye degrading fungal organisms in lieu of the production of lignin peroxidase, laccase and manganese peroxidase that have non-specific enzymatic activity.23 A study by Sun et al. reported decolourisation potential of fungal strain Myrothecium verrucaria towards dyes crystal violet, congo red, methyl orange and methyl red in the presence of ABTS.24 Another such similar study reported dye degrading capabilities of fungal endophytic organisms against textile dyes. These included fungal endophytes like Phlebia spp. isolated from P. hispidum Sw. that helped degrade textile dyes like Reactive Black 5 and Reactive Blue 19.25 Fungal laccases were first reported in exudates of plant Rhus vernicifera and have continued to be an extensive topic of research. Their roles have been implicated in different plant physiological processes like delignification, pigmentation and pathogenesis.26
Laccase production by fungal endophytes is a significantly promising area of research in lieu of various industrial applications of laccase in areas like bioremediation and detoxification of pollutants. In vivo conditions that favour fungal endophytic enzyme production cannot be fully replicated in vitro but various aspects can be introduced to enhance enzyme production. The present work was focussed on isolating fungal endophytes from different plants in Hulimavu Lake, Bengaluru in order to elucidate their laccase producing properties. As evident in this study, endophytic fungi cultured from different plant regions of Alternanthera exhibited differences in quantity of laccase production. Hence, optimising enzyme production would involve various factors that will be specific to host plants and fungal endophytic organisms being studied. Industrial applications of enzymes have significant research value due to the ability of enzymes to catalyse various reactions even when present in low quantities. However, the use of enzymes extracted from organisms that live in stressed habitats, as reflected in this study, have furthermore applications due to unique characteristics of these organisms to withstand biotic and abiotic stress without affecting enzyme production. In this regard, extraction of fungal enzymes from plants living in polluted or stressed conditions exemplifies the significance of these enzymes in industrial processes that involve extreme conditions of temperature, pH, etc. These enzymes thus help partake in catalysis of reactions involving extreme conditions without undergoing any negative effects on their activity or specificity. Laccase catalysed reactions are among the most commonly employed processes in industries.
Isolation of fungal endophytes from Hulimavu Lake flora
Three plants were selected for isolation of endophytes after initial screening of Hulimavu Lake and they were collected from the following geographical locations – Latitude 12°56’2’’N; Longitude 77°36’21’’E (for Alternanthera philoxeroides, Ricinus communis) and Latitude 12°53’12’’N; Longitude 77°35’22’’E (for Persicaria glabra). All plant samples were collected in clean plastic covers and brought to the laboratory where they were transferred to a large clean plastic tray and washed under running tap water for about 15-20 minutes (total). This wash was repeated thrice (in total) and water was changed during each wash. After thorough tap water wash the plant samples were transferred to a clean sheet of newspaper and dried at room temperature for ~15 minutes. A part of all plant samples was kept aside for preparation and deposition as herbarium specimens. These were later identified by a certified taxonomist as Alternanthera philoxeroides, Ricinus communis and Persicaria glabra. Remaining parts of all plant samples were taken to the LAF (Laminar Air Flow) for processing under sterile conditions. Plant samples were processed using ethanol and sodium hypochlorite as surface sterilising agents.27 Each plant sample was processed separately and under sterile conditions these plant parts were subjected to two surface sterilisation conditions, one set with 70% (v/v) ethanol and another set with 5% (v/v) sodium hypochlorite. Leaves were excised to make 0.5cm*0.5cm pieces, root and stem explants were prepared as 0.25cm, 0.5cm or 1cm segments and while inoculating the inside part of all explants was placed towards the growth media (and then slightly pressed using sterile forceps). Flower explants were not cut further since these were bud-like small flowers and hence were inoculated by placing the inside part towards the growth media and then slightly pressing the explants. After preparation of each explant for each plant sample and blot drying them on sterile filter paper pieces, 4 explants of each type were inoculated on each petri plate containing PDA (Potato Dextrose Agar) growth media (supplemented with streptomycin at a concentration of 20µg/mL). All these culture plates were kept for incubation at room temperature under dark conditions. These culture plates were observed every day for fungal growth and contamination (if any).
Qualitative screening for laccase production
After ~5 weeks of incubation, primary fungal endophytic cultures were subjected to subculturing onto PDA growth media (supplemented with streptomycin at a concentration of 20µg/mL) and kept for incubation at room temperature under dark conditions for ~15 days. After optimal mycelial growth in these subcultures, qualitative assay to detect laccase production was performed according to the protocol by Sunitha et al.28 This assay was based on growth of fungal subcultures in GYP (Glucose Yeast extract Peptone) agar media supplemented with 0.005% (g/v) of α-naphthol (growth media composition per L: glucose 1g, yeast extract 0.1g, peptone 0.5g, agar 16g, α-naphthol 0.05g, distilled water 1L) followed by incubation for ~4 days at room temperature (under dark conditions). Presence of laccase production was indicated by laccase catalysed oxidation of α-naphthol that induced media colour change from colourless to blue.
Quantitative screening for laccase production
Upon achieving sufficient mycelial growth in subcultured explants from PDA growth media, under aseptic conditions, 2-3 mycelial plugs were transferred from PDA growth media to PDB (Potato Dextrose Broth) growth media. These broth cultures were incubated for ~8 days at room temperature (under dark conditions) for subsequent quantitative laccase assay. Under aseptic conditions, broths of fungal cultures were filtered using filter paper to remove mycelial biomass. Filtrates thus obtained were stored at 4°C until further use for enzyme assays. Laccase production was quantified using the ABTS (2-azino-bis(3-ethylbenzthiazoline)-6-sulfonate)) assay method29 in which oxidation of ABTS was monitored as an increase in absorbance at A420. For this assay 0.3mL of 0.3mM ABTS and 0.9mL of 0.1M citrate buffer (pH=5.0) were mixed with 1.8mL of enzyme filtrate. Following this, the increase in absorbance was monitored every one minute at A420 (at room temperature) until the absorbance values decreased or remained constant.
Molecular identification of laccase producing fungal endophytes
Molecular phylogenetic analysis was done by DNA sequencing of ITS (Internal Transcribed Spacer) regions. After purification of these PCR amplicons, forward and reverse ITS-1 and 4 primers were used for DNA sequencing. BDT v3.1 Cycle sequencing kit was used on ABI 3730xl Genetic Analyzer. Results thus obtained were analysed using BLAST analysis and compared with the GenBank database using nucleotide homology. MEGA 10 tool was then used for constructing phylogenetic tree. Subsequently, consensus sequences were generated from forward and reverse primers using aligner software. Based on identity score matrix using BLAST (Basic Local Alignment Search Tool) analysis in GenBank database, amplified fragments of ITS regions were analysed for species identification through multiple alignment software program Clustal W. Distance matrix. Phylogenetic tree was then constructed using MEGA 10.).
Culturing and isolation of fungal endophytes
Plants selected from Hulimavu Lake were subjected to washing and surface sterilisation using 70% (v/v) ethanol and 5% (v/v) sodium hypochlorite. After inoculation of explants from different plant parts like leaf, stem, node and root (for Alternanthera philoxeroides), stem (for Ricinus communis) and flower (for Persicaria glabra) to PDA growth media, these cultures were incubated at room temperature (under dark conditions) to allow fungal growth. Regular observations were made in order to check for mycelial growth and contaminations if any. After four weeks of incubation optimal mycelial growth was obtained and these cultures were then further subcultured in PDA and incubated for about 15 days at room temperature (under dark conditions) for optimal mycelial growth. The culture was further inoculated in PDB (Potato Dextrose Broth) and incubated for about 7 days at room temperature (under dark conditions). These cultures were used for subsequent qualitative and quantitative assays for detection and estimation of laccase production. Different plants and plant regions harbour an extensive range of endophytes. Hence for the isolation of fungal endophytes these organisms need to be subcultured repeatedly to obtain pure cultures of desired organisms. Optimisation of subculturing conditions to obtain pure cultures of fungal endophytes further facilitates enhanced production and extraction of required enzymes and bioactive compounds. Growth rate of fungal endophytes was found to be dependent on temperature and culture conditions. Optimal growth was observed under dark conditions and at ~25°C.
External factors of growth media composition, pH, ratio of carbon-nitrogen sources and variations in culture conditions including incubation of fungal broth cultures under rotary shaking or static conditions significantly impact endophytic fungal growth, enzyme production and bioactive compounds production. Different components in fungal endophytic growth media promote growth at varied rates. PDA was selected as the growth media of choice in concurrence with literature reports that indicated this to be the best media that promotes growth of a broad range of fungal organisms.30 In a study by Wang et al. different pH, temperature (20-40°C) and growth media compositions were tested to optimise fungal endophytic growth of Monotospora sp. Strain W823 and its laccase production. It was reported that maximum laccase production was obtained at pH of 8.5, temperature of 30°C and with growth media consisting of maltose at 2g/L and ammonium tartrate at 10g/L.31 Thus, growth media composition along with extraneous culture conditions like temperature and pH affect the growth rate, type and quanta of endophytic fungal bioactive compounds. In a study by More et al., kinetics of laccase production was studied across different temperature and pH ranges and optimal production was achieved at 65°C and at pH 4.5. Activity of enzyme significantly increased from 60-65°C after which there was a decline after 70°C. Influence of different metal ions and inhibitors on laccase production was also analysed of which zinc was noted to completely inactivate laccase at a concentration of 2mM and other metal ions like manganese, mercury and iron inhibited nearly 60% laccase activity at 2mM concentration. Among the inhibitors tested, sodium azide, EDTA and SDS exhibited significant inactivation of laccase enzyme.32
Qualitative detection of laccase production
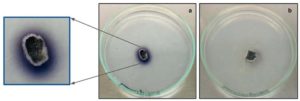
Detection of laccase production was performed qualitatively by inoculating the fungal endophytes in GYP agar media supplemented with 0.005% (w/v) of α-naphthol. Incubation was performed at room temperature (under dark conditions) for ~4 days and observed regularly. Laccase production was indicated by oxidation of α-naphthol that resulted in growth media colour change from colourless to light blue. Fungal endophytes isolated from stem, root and node regions of Alternanthera philoxeroides, stem region of Ricinus communis and flower regions of Persicaria glabra showed presence of laccase production indicated by growth media colour change to blue from colourless. In few of these cases, intensity of growth media colour change to blue for stem explants of Alternanthera was more enhanced than explants of Ricinus and Persicaria. This could be attributed to variations in the amount of laccase enzyme production by different fungal endophytes isolated from different regions of their host plants. Figure 1 depicts the bluish purple colour spreading in the media around the fungal mycelia of the explant from A. philoxeroides indicating that it is laccase positive.
Figure 1. Qualitative laccase assay for fungal endophytes isolated from Alternanthera philoxeroides stem explants showing bluish purple coloration in the GYP medium: a) Laccase positive fungal isolate b) Laccase negative fungal isolate
Various parameters like growth media composition (carbon source, nitrogen source, ionic balance, etc.), pH, temperature, growth culture volume, fermentation conditions (static and shaking), nature of host plant, type of plant part and fungal organisms being analysed affect the ultimate production and yield of various enzymes and bioactive compounds. Certain biotic and abiotic stressors in the environment of host plants also affect the physiological secretions by resident fungal endophytes that in turn favourably help their host plants to become tolerant to stress. In a study by Chanyal S. et al. different substrates of laccase were used in the qualitative analysis of fungal laccase production. Tannic acid, ABTS, syringaldehyde and guaiacol were each added to each GYP agar media to test the presence of laccase production by fungal endophytes. Though all substrates facilitated detection of laccase enzyme based on their specific modes of action, ABTS was reported as the best substrate for laccase enzyme. Tannic acid was considered to be less efficient than the other substrates due to its non-specificity as a substrate for laccases.33
Quantitative estimation of laccase production
Fungal endophytic subcultures incubated in PDB at room temperature for ~8 days (under dark conditions) were used for the quantitative estimation of laccase production. After incubating these subcultures in PDB the broth cultures were filtered using filter paper and filtrates were stored at 4°C for subsequent use in enzyme assays. Laccase estimation was performed by ABTS assay in which the oxidation of ABTS per minute by laccase enzyme (at A420) was calculated as per the formula: Enzyme units per L (U/L) = (δE×Vt )/(ε × d ×Vs).
where δE is the change in excitation of light per minute at A420, Vt is the total volume measured, Vs is the volume of enzyme stock solution added to ABTS stock solution, d is the layer (in cm) of thickness of cuvette and ε is the molar absorption coefficient of ABTS at A420 (3.6 × 104 M/cm-1). Thus enzyme activity of 1 unit under standard conditions is considered as 1µmol of ABTS being oxidised in each minute.29 As summarised in Table, different plant regions like stem, root and node of Alternanthera displayed significant differences in laccase production. While crude enzyme extracts of fungal endophytic cultures from HEFAPhS1, i.e. Hulimavu Lake Endophytic Fungus Alternanthera philoxeroides Stem (isolate 1) produced the highest amount of laccase (42.16U/L), those from its root and nodal regions produced lower amounts of 9.305U/L and 9.28U/L of laccase, respectively. From among the organisms tested for laccase production, crude enzyme extracts of fungal endophytic cultures from Persicaria flower reported lowest laccase production with enzyme units of 4.416U/L. Aslam, M.S. et al. reported partial purification of extracellular laccase with an output of 0.19U/L by Cladosporium cladosporioides that was further subjected to kinetics assays to determine the kinetic constants and physicochemical properties of laccase.34 Fungal laccases which are monomeric proteins have molecular weights ranging from 50-90kDa.35,36,37 General research on fungal laccases dwells around organisms like Phanerochaete chrysosporium, Pycnoporus sanguineus and Trametes versicolor.34 Partial purification of laccase from Schizophyllum commune IBL-06 gave an yield of 0.367U/L. Here, while 1mM of CuSO4 enhanced its production, AgNO3, TEMED and mercaptoethanol decreased laccase production by 25%.38
Table :
Quantitative ABTS assay based estimation of laccase production by endophytic fungi isolated from different plant parts of Hulimavu lake flora.
| Fungal strains | Absorbance values (A420) | Avg. δt (min.) |
Laccase activity (U/L) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | Avg. δE | |||
| HEFAPhR1 | 0.018 | 0.055 | 0.025 | NA | NA | NA | NA | NA | NA | NA | NA | 0.0335 | 0.3 | 9.305 |
| HEFAPhS1 | 0.003 | 0.008 | 0.012 | 0.018 | 0.248 | 0.24 | NA | NA | NA | NA | NA | 0.0506 | 0.1 | 42.16 |
| HEFRCoS1 | 0.028 | 0.027 | NA | NA | NA | NA | NA | NA | NA | NA | NA | 0.0275 | 0.5 | 4.583 |
| HEFAPhN1 | 0.346 | 0.355 | 0.361 | 0.37 | 0.393 | 0.405 | 0.421 | 0.424 | 0.423 | 0.416 | 0.416 | 0.01114 | 0.1 | 9.28 |
| HEFPGlF1 | 3.614 | 3.642 | 3.646 | 3.562 | 3.514 | NA | NA | NA | NA | NA | NA | 0.0106 | 0.2 | 4.416 |
Identification of the Fungal Endophyte by rDNA Sequence Analysis
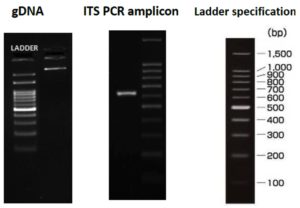
Endophytic fungi isolated from the stem region of Alternanthera philoxeroides exhibited maximum laccase production. This fungal isolate was identified by 28S rRNA sequencing by PCR amplification of ITS-1 and 4 regions. Based on identity score matrix using BLAST (Basic Local Alignment Search Tool) analysis in GenBank database, amplified fragments of ITS regions were analysed for species identification through multiple alignment software program Clustal W. Distance matrix. As depicted in Figure 2, after nucleotide homology, phylogenetic analysis using Maximum Likelihood Method and comparison with GenBank database, the fungal endophytic species was identified as Cladosporium tenuissimum. The sequence obtained was deposited in the Genbank database (Accession number ON505945). The genus Cladosporium species is considered to be heterogeneous and 993 organisms belonging to this genera come under the category of common endophytes.39 This fungus has been researched globally for its production of secondary metabolites. Examples include C. tenuissimum from Swietenia mahagoni in Indonesia,40 from Pinus wallichiana in Kashmir41 and from Cronartium flaccidum in Italy.42 Being an extensively researched organism, C. tenuissimum finds immense potential for industrial and therapeutic applications of its bioactive compounds. Different bioactive compounds have been known to be isolated from this genera of fungal endophytes and few examples include o-hydroxyphenyl,41 plumbagin (5-hydroxyl-2-methyl-naptalene-1,4dione)30 and hydroxyemodin.43 Ethyl acetate extracts of antioxidants from Cladosporium tenuissimum have revealed high antioxidant activity along with IC50 value of 85.35µg/mL.40 There are many products, like cladosporin identified in 1971,44 that have been reported to be produced by fungal organisms belonging to the genus Cladosporium. These include secondary metabolites like azaphilones, coumarins and isocoumarins, lactones, sterols and so on.13 Biological properties of these compounds have since been extensively studied. Laccase production by Cladosporium tenuissimum remains an area of research that needs further exploration. From among the few reports published in this area, a study by Dhakar K. and Pandey A. reported laccase production from a newly isolated psychrotolerant Cladosporium tenuissimum strain NFCCI 2608. This strain recorded laccase activity of 15.10±0.78U/L at 14°C when compared to its activity of 8.05±0.94U/L at 24°C, with efficiency of laccase production being maximum at lower temperature.41 Comparatively, the present study demonstrated high laccase production by Cladosporium tenuissimum isolated from Alternanthera philoxeroides growing in highly polluted Hulimavu Lake, Bangalore, without the addition of any laccase inducers and at standard temperature of ~25-28°C.
Figure 2. Species identification of fungal cultures isolated from the stem region of Alternanthera philoxeroides: Genomic DNA isolation (left), ITS region PCR amplification (middle) and DNA ladder specifications.
A study by Navada et al. aimed at enhancing laccase production upto 1.6 fold in Phomopsis sp. through 0.2kGy gamma-irradiation. Laccase enzyme from irradiated fungal cultures retained its activity in broad ranges of pH from 4-8 and temperature from 25-45°C conditions, while also displaying tolerance to metals like Zinc, calcium, copper and chromium at concentrations upto 10mM.45 Another study also reported increased laccase production upto 225.05mg/L six days after incubation of Shiraia bambusicola fungal cultures. Here, laccase activity was enhanced using lanthanum (La3+) at a concentration of 1-2g/L. While lanthanum inhibited mycelial biomass, the laccase production was enhanced almost 10 fold compared to control fungal cultures.9 In a study by Revankar and Lele, laccase production by fungal strain belonging to the genus Gandoderma was maximised by optimising growth media micronutrients. This strain naturally produced high amounts of laccase (124U/mL) compared to other strains that produced between 4-100U/mL of laccase. Using orthogonal matrix method of experimental design, enhanced laccase production was achieved by calculating optimal levels of micronutrients in the fermentation media. Starch based growth media supplemented with copper sulphate and 2,5-xylidine (laccase inducer) enhanced the laccase production upto 692U/mL by this fungal strain.46
Laccase production by fungal endophytes is a significantly promising area of research in lieu of the various industrial applications of laccase in areas like bioremediation and detoxification of pollutants. In vivo conditions that favour fungal endophytic enzyme production cannot be fully replicated in vitro but various aspects can be introduced to optimise and enhance enzyme production. As evident in this study, endophytic fungi cultured from different plant regions of Alternanthera exhibited differences in quantity of laccase production. Hence, optimising enzyme production would involve various factors that will be specific to host plants and fungal endophytic organisms being studied. Industrial applications of enzymes have significant research value due to the ability of enzymes to catalyse various reactions even when present in low quantities. However, the use of enzymes extracted from organisms that live in stressed habitats have furthermore applications due to unique characteristics of these organisms to withstand biotic and abiotic stress without affecting enzyme production. In this regard, extraction of fungal enzymes from plants living in polluted or stressed conditions exemplifies the significance of these enzymes in industrial processes that involve extreme conditions of temperature, pH, etc.47,48 These enzymes thus help partake in catalysis of reactions involving extreme conditions without undergoing any negative effects on their activity or specificity. Laccase catalysed reactions are among the most commonly employed processes in industries. The present work aimed at probing laccase producing fungal endophytes through qualitative detection and quantitative estimation of laccase production. From among the plants studied in this work, fungal endophytes from stem regions of Alternanthera philoxeroides reported highest laccase production and those from flower regions of Persicaria glabra reported the least laccase production. Laccase production varied among the different plant regions like node, root and stem of Alternanthera, thus demonstrating the effect of variations in plant regions and type of fungi on laccase production.The current study helps demonstrate the variations in fungal endophytic composition within a single plant, on the type and quanta of fungal endophytic enzymes produced. Hulimavu lake was chosen for this study due to the high levels of biotic and abiotic stress present in this lake, brought about by factors like pollution by nearby commercial habitation and dumping of waste into this lake. Owing to this, fungal endophytes present in plants of this lake were hypothesised to harbour useful wide ranging enzymes and bioactive compounds that conferred their host plants with the ability to withstand extreme conditions. Since laccase is an industrially important enzyme, research into optimising fungal endophytic laccase production would pave the way for standardising large scale fermentation reactions by using high laccase producing fungal endophytes, especially ones which can tolerate extreme conditions. The present study revealed the high laccase production by C. tenuissimum endophytic strain which showed 42.16 U/L laccase output even from crude filtrate of the fungal culture. Hence the study points to an excellent laccase producer which can be used for further characterization and optimization of laccase production by altering cultural conditions, using elicitors etc.
ACKNOWLEDGMENTS
The authors would like to thank Dr. NM Ganesh Babu, Associate Professor, Heading Centre for Herbal Gardens, FRLHT, Bengaluru, for providing his expertise in authenticating the plant samples for this study. The authors would also like to acknowledge the Department of Life Sciences, CHRIST (Deemed to be University), Bengaluru for providing the infrastructural support to carry out this study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
SS and SJ conceptualised the study. SJ and SS wrote the manuscript. SS, MU, IP and SB reviewed and edited the manuscript. SS supervised the study. All authors read and approved the final manuscript for publication.
FUNDING
The authors extend their gratitude to Karnataka Science and Technology Promotion Society (KSTePS)- Department of Science and Technology (DST) Ph.D. fellowship (award letter number LIF-05:2021-22/1017).
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Gianfreda L, Xu F, Bollag JM. Laccases: a useful group of oxidoreductive enzymes. Bioremediat J. 1999;3(1):1-26.
Crossref - Jang MY, Ryu WR, Cho MH. Laccase production from repeated batch cultures using free mycelia of Trametes sp. Enzyme Microb Technol. 2002;30:741-746.
Crossref - Eggert C, Temp U, Dean JFD, Eriksson K-EL. Laccase mediated formation of the phenoxazinone derivative, cinnabarinic acid. FEBS Lett. 1995;376(3):202-206.
Crossref - Upadhyay P, Rahul S, Pavan Kumar A. Bioprospecting and biotechnological applications of fungal laccase. 3 Biotech. 2016;6(1):1-12.
Crossref - Bell AA, Wheeler MH. Biosynthesis and functions of fungal melanins. Ann Rev Phytopathol. 1986;24(1):411-451.
Crossref - Bollag JM, Leonowicz A. Comparative studies of extracellular fungal laccases. Appl Environ Microbiol. 1984;48(4):849-854.
Crossref - Lee IY, Jung KH, Lee CH, Park YH. Enhanced production of laccase in Trametes versicolor by the addition of ethanol. Biotechnol Lett. 1999;21:965-968.
Crossref - Ardon O, Kerem Z, Hadar Y. Enhancement of laccase activity in liquid cultures of the ligninolytic fungus Pleurotus ostreatus by cotton stalk extract. J Biotechnol. 1996;51(3):201-207.
Crossref - Wang Y, Zhang X, Lu C, Li X, Zhou J, Wang J. Lanthanum: a novel inducer for enhancement of fungal laccase production by Shiraia bambusicola. Journal of Rare Earths. 2020.
- Du W, Sun C, Liang J, Han Y, Yu J, Liang Z. Improvement of laccase production and its characterization by mutagenesis. J Food Biochem. 2015;39(1):101-108.
Crossref - Galhaup C, Haltrich D. Enhanced formation of laccase activity by the white-rot fungus Trametes pubescens in the presence of copper. Appl Microbiol Biot. 2001;56(1-2):225-232.
Crossref - Navada KK, Kulal A. Enhanced production of laccase from gamma irradiated endophytic fungus: A study on biotransformation kinetics of aniline blue and textile effluent decolourisation. J Environ Chem Eng. 2019;8(2):103550.
Crossref - Salvatore MM, Andolfi A, Nicoletti R. The Genus Cladosporium: A Rich Source of Diverse and Bioactive Natural Compounds. Molecules. 2021;26(13):3959.
Crossref - Strobel G. The emergence of endophytic microbes and their biological promise. J Fungi. 2018;2:57.
Crossref - Liu Z, Zhao JY, Sun SF, Li Y, Liu YB. Fungi: outstanding source of novel chemical scaffolds. J Asian Nat Prod Res. 2020;22(2):99-120.
Crossref - Morais PV, Wang Y, Li H, Feng G, Du L, Zeng D. Biodegradation of diuron by an endophytic fungus Neurospora intermedia DP8-1 isolated from sugarcane and its potential for remediating diuron-contaminated soils. Plos One. 2017;12:e0182556.
Crossref - Gonda S, Kiss-Szikszai A, Szucs Z, Balla B, Vasas G. Efficient biotransformation of non-steroid anti-inflammatory drugs by endophytic and epiphytic fungi from dried leaves of a medicinal plant, Plantago lanceolate L. Int Biodeter Biodegr. 2016;108:115-121.
Crossref - Amobonye A, Bhagwat P, Singh S, Pillai S. Enhanced xylanase and endoglucanase production from Beauveria bassiana SAN01, an entomopathogenic fungal endophyte. Fungal Biol. 2021;125:39-48.
Crossref - Toghueo RMK, Zabalgogeazcoa I, de Aldana BV, Boyom FF. Enzymatic activity of endophytic fungi from the medicinal plants Terminalia catappa, Terminalia mantaly and Cananga odorata. South African Journal of Botany. 2017;109:146-153.
Crossref - Katoch M, Salgotra A, Singh G. Endophytic fungi found in association with Bacopa monnieri as potential producers of industrial enzymes and antimicrobial bioactive compounds. Braz Arch Biol Technol. 2014;57:714-722.
Crossref - Libra JA, Borchert M, Banit S. Competition strategies for the decolorization of a textile-reactive dye with the white-rot fungi Trametes versicolor under non-sterile conditions. Biotechnol Bioeng. 2003;82(6):736-744.
Crossref - Deng Z, Cao L. Fungal endophytes and their interactions with plants in phytoremediation: a review. Chemosphere. 2017;168:1100-1106.
Crossref - Bergsten-Torralba LR, Nishikawa MM, Baptista DF, Magalhães DdP, Silva Md. Decolorization of diferent textile dyes by Penicillium simplicissimum and toxicity evaluation after fungal treatment. Braz J Microbiol. 2009;40(4):808-817.
Crossref - Sun J, Guo N, Niu LL, et al. Production of laccase by a new Myrothecium verrucaria MD-R-16 isolated from Pigeon Pea [Cajanus cajan (L.) Millsp.] and its application on dye decolorization. Molecules. 2017;22:673.
Crossref - Bulla LMC, Polonio JC, Portela-Castro ALB, Kava V, Azevedo JL, Pamphile JA. Activity of the endophytic fungi Phlebia sp. and Paecilomyces formosus in decolourisation and the reduction o reactive dyes’ cytotoxicity in fish erythrocytes. Environ Monit Assess. 2017;189(88):1-11.
Crossref - Arora DS, Sharma RK. Ligninolytic fungal laccases and their biotechnological applications. Appl Biochem Biotechnol. 2010;160:1760-1788.
Crossref - Hazalin NAMN, Ramasamy K, Lim SSM, Wahab IA, Cole ALJ, Majeed ABA. Cytotoxic and antibacterial activities of endophytic fungi isolated from plants at the National Park, Pahang, Malaysia. BMC Complement Altern Med. 2009;9:46.
Crossref - Sunitha VH, Devi DN, Srinivas C. Extracellular enzymatic activity of endophytic fungal strains isolated from medicinal plants. World J Agric Sci. 2013;9(1):01-09.
- Soumya PS, Lakshmi MSK, Nambisan P. Application of response surface methodology for the optimization of laccase production from Pleurotus ostreatus by solid state fermentation on pineapple leaf substrate. J Sci Ind Res. 2016;75:306-314.
- Venkateswarulu N, Shameer S, Bramhachari PV, Basha SKT, Nagaraju C, Vijaya T. Isolation and characterization of plumbagin (5- hydroxyl- 2- methylnaptalene-1,4-dione) producing endophytic fungi Cladosporium delicatulum from endemic medicinal plants. Biotechnol Rep. 2018;20:e00282.
Crossref - Wang JW, Wu JH, Huang WY, Tan RX. Laccase production by Monotospora sp., an endophytic fungus in Cynodon dactylon. Bioresource Technology. 2006;97(5):786-789.
Crossref - More SS, PS R, Malini S, SM V. Isolation, purification, and characterization of fungal laccase from Pleurotus sp. Enzyme Research. 2011;2011:248735.
Crossref - Chanyal S, Agrawal PK. Preliminary Screening for Laccase Producing Endophytic Fungi from Cupressus Torulosa D.Don, Int J Sci Eng Manag. 2016;1(2).
- Aslam MS, Aishy A, Samra ZQ, Gull I, Athar MA. Identification, purification and characterization of a novel extracellular laccase from Cladosporium cladosporioides. Biotechnol Biotechnol Equip. 2012;26(6):3345-3350.
Crossref - Garcia T, Santiago M, Ulhoa C. Properties of Laccases Produced by Pycnoporus sanguineus Induced by 2,5-xylidine. Biotechnol Lett. 2006; 28:633-636.
Crossref - Ko EM, Leem YE, Choi HT. Purification and characterization of laccase isozymes from the white-rot basidiomycete Ganoderma lucidum. Appl Microbiol Biotechnol. 2001;57(1-2):98-102.
Crossref - Zouari MH, Mechichi T, Dhouib A, Sayadi S, Martinez AT, Martinez MJ. Laccase purification and characterization from Trametes trogii isolated in Tunisia: decolorization of textile dyes by the purified enzyme. Enzym Microb Technol. 2006;39(1):141-148.
Crossref - Irshad M, Asgher M, Sheikh MA, Nawaz H. Purification and characterization of laccase produced by Schyzophylum commune IBL-06 in solid state culture of banana stalks. BioResources. 2011; 6(3):2861-2873.
- Bensch K, Braun U, Groenewald JZ, Crous PW. The genus Cladosporium. Stud Mycol. 2012;72(1):1-401.
Crossref - Fadhillah F, Yohandini H, Widjajanti H. Chemical compound isolated from antioxidant active extract of endophytic fungus Cladosporium tenuissimum in Swietenia mahagoni leaf stalks. Biodiversitas Journal of Biological Diversity. 2019;20(9).
Crossref - Naseer S, Bhat KA, Qadri M, Riyaz-Ul-Hassan S, Malik FA, Khuroo MA. Bioactivity-guided isolation, antimicrobial and cytotoxic evaluation of secondary metabolites from Cladosporium tenuissimum associated with Pinus wallichiana. ChemistrySelect. 2017;2:1311-1314.
Crossref - Nasini G, Arnone A, Assante G, Bava A, Moricca S, Ragazzi A. Secondary mould metabolites of Cladosporium tenuissimum, a hyperparasite of rust fungi. Phytochemistry. 2004;65:2107-2111.
Crossref - Akpotu, MO, Eze PM, Abba CC, et al. Antimicrobial activities of secondary metabolites of endophytic fungi isolated from Catharanthus roseus. J Health Sci. 2017;7(1):15-22.
Crossref - Scott PM, Van Walbeek W, MacLean WM. Cladosporin, a new antifungal metabolite from Cladosporium cladosporioides. J Antibiot. 1971;24(11):747-755.
Crossref - Navada KK, Sanjeev G, Kulal A. Enhanced biodegradation and kinetics of anthraquinone dye by laccase from an electron beam irradiated endophytic fungus. Int Biodeterior Biodegradation. 2018;132:241-250.
Crossref - Revankar MS, Lele SS. Enhanced production of laccase using a new isolate of white rot fungus WR-1. Process Biochemistry. 2006;41(3):581-588.
Crossref - Jayaram S, Sarojini S. Bioprospecting of Fungal Endophytes in Hulimavu Lake for Their Repertoire of Bioactive Compounds. Electrochem Soc. 2022;107(1):10471-10481.
Crossref - Sivakumar B, Rao NR, Poornamath BP, Jayaram S, Sarojini S. Multifarious pigment producing fungi of Western Ghats and their potential. Plant Science Today. 2022; 9(3):733-747.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.