ISSN: 0973-7510
E-ISSN: 2581-690X
Mango anthracnose, caused by Colletotrichum gloeosporioides, is a significant disease affecting mango production. This study aimed to develop and evaluate a bio-formulation of Serratia nematodiphila GCSR38 for controlling mango anthracnose. The antagonistic activity of S. nematodiphila GCSR38 was tested using the dual culture method, achieving a 40.21% inhibition rate against C. gloeosporioides. Three powder formulations were developed using a low-heat drying process, differing in their composition of corn starch, talcum, calcium carbonate, and carboxymethyl cellulose (CMC). The formulations exhibited varying physical characteristics, water solubility, and moisture stability, with pH values ranging from 7.90 to 8.00. The survival of S. nematodiphila GCSR38 in the powder formulations remained substantial over 120 days, with final bacterial counts between 2.50 × 107 and 5.10 × 107 CFU/g. Efficacy testing on mango leaves showed that Bio-Formulation 3 provided the highest inhibition of C. gloeosporioides, achieving 51.00% inhibition in preventive application and 48.30% in curative application. These results were comparable to the chemical fungicide Carbendazim (56.11% and 49.31%, respectively), with no significant differences observed. Preventive application proved more effective than curative treatment. Overall, S. nematodiphila GCSR38 shows promise as a biocontrol agent for mango anthracnose, offering an eco-friendly alternative to chemical fungicides.
Serratia nematodiphila GCSR38, Mango Anthracnose, Powder Bio-product, Preventive Application, Curative Application
Anthracnose, caused by fungi of the Colletotrichum genus, is a major plant disease affecting a wide range of economically important crops, including mango (Mangifera indica), chili (Capsicum annuum), papaya (Carica papaya) and pitaya (Hylocereus spp.).1,2 The disease is characterized by dark, sunken lesions on fruits, leaves, and stems, leading to severe yield losses and reduced market value.3,4 Conventional management strategies primarily rely on synthetic fungicides; however, their excessive use poses several challenges, including environmental contamination, chemical residues in food, and the emergence of fungicide-resistant Colletotrichum strains.5,6 These concerns have led to an increasing demand for environmentally friendly and sustainable disease management alternatives, such as biocontrol agents.
Biological control using antagonistic bacteria has gained attention as a promising alternative to synthetic fungicides. Various bacterial species, including Bacillus spp., Pseudomonas spp., and Serratia spp., exhibit strong antifungal activity against Colletotrichum spp. through multiple mechanisms, such as competition, antibiosis, and induced systemic resistance.7,8 Among these, Serratia nematodiphila has been identified as a potential biocontrol agent due to its ability to inhibit fungal pathogens through the production of secondary metabolites and hydrolytic enzymes.9 Furthermore, Sutthisa10 evaluated the efficacy of antagonistic microorganisms and secondary metabolites of the entomopathogenic bacterium S. nematodiphila GCSR38 against Xanthomonas oryzae pv. oryzae (Xoo), the causal agent of rice bacterial leaf blight. The agar well diffusion assay demonstrated that S. nematodiphila GCSR38 effectively inhibited Xoo, with a 19.15 mm inhibition zone when using a cell suspension and a 17.60 mm inhibition zone when using 2,000 µg/ml of secondary metabolite crude extracts. However, for successful application in agricultural fields, the formulation and stability of biocontrol agents must be optimized to ensure their long-term viability and efficacy.11
This study aims to develop a stable bio-product formulation of S. nematodiphila GCSR38 and evaluate its effectiveness in controlling anthracnose disease. The efficacy of the bacterial strain was assessed through dual culture assays against C. gloeosporioides, followed by the development of three bio-product formulations using different ratio of carrier materials. The study further examined the survival of S. nematodiphila GCSR38 in the formulations over four months of storage. The results of this research contribute to the development of an eco-friendly and sustainable alternative for anthracnose management, reducing reliance on chemical fungicides and promoting environmental safety.
Testing the efficacy of Serratia nematodiphila GCSR38 in inhibiting Colletotrichum gloeosporioides using the dual-culture technique
Serratia nematodiphila GCSR38 and Colletotrichum gloeosporioides were obtained from the Microbiology Laboratory, Department of Biology, Faculty of Science, Mahasarakham University. The antagonistic activity of S. nematodiphila GCSR38 against C. gloeosporioides was assessed using the dual-culture method. A 0.8 cm agar plug from a 7-day-old C. gloeosporioides PDA culture was placed at the center of a fresh PDA plate. S. nematodiphila GCSR38, grown in nutrient broth (NB) for 24 h, was streaked in four directions 3 cm away from the fungal plug using a sterile loop. Plates were incubated at 28 ± 2 °C for 5 days in triplicate. Fungal radial growth was measured on both test (with antagonist) and control (without antagonist) plates. The percentage inhibition of fungal growth was calculated as follows12:
% inhibition = [(C – T)/C] × 100
Where,
C = Diameter of the fungal colony in the control plate
T = Diameter of the fungal colony in the test plate
Development of Serratia nematodiphila GCSR38 powder bio-products
S. nematodiphila GCSR38 was cultured on nutrient agar (NA) at 28 ± 2 °C for 24 h. A single colony was inoculated into 100 mL NB and incubated at 150 rpm, 28 ± 2 °C for 24 h. A 10% inoculum was then transferred to 500 mL fresh NB and incubated under the same conditions. Bacterial cells were harvested by centrifugation at 7,000 rpm for 10 min, washed twice with 0.85% NaCl, and adjusted to 108 CFU/mL (OD600 = 0.2). Powdered formulations were prepared by mixing 900 g sterilized carrier with 100 mL bacterial suspension (Table 1). The mixture was dried at 45 °C for 4 days and stored in aluminum foil bags at room temperature.
Table (1):
Composition and ratio of powdered bio-products formulations
Formula |
Composition |
Ratio |
|---|---|---|
1 |
Corn starch:Talcum:Calcium carbonate:Carboxyl methyl cellulose (CMC) |
10:87.5:1.5:1 |
2 |
Corn starch:Talcum:Calcium carbonate:Carboxyl methyl cellulose (CMC) |
20:77.5:1.5:1 |
3 |
Talcum:Calcium carbonate:Carboxyl methyl cellulose (CMC) |
97.5:1.5:1 |
Evaluation of the efficiency of Serratia nematodiphila GCSR38 powder bio-products
Water solubility test
Water solubility was determined by dissolving 1 g of each formulation in 99 mL distilled water under magnetic stirring (200 rpm). Dissolution time was recorded and classified into five levels13: (1) 1-5 min, (2) 6-10 min, (3) 11-30 min, (4) 31-60 min, and (5) not dissolved. All tests were conducted in triplicate.
Bacterial viability
Survival of S. nematodiphila GCSR38 in powder formulations was determined using the dilution plate count method.12 Samples were serially diluted (10-5 to 10-7), and 0.1 mL of each dilution was plated onto NA in triplicate. Plates were incubated at 28 ± 2 °C for 24-48 h. Colony counts were recorded on day 0, day 30, and after 4 months of storage.
Moisture content
Moisture content was measured by drying samples at 105 °C for 5 h. Each sample was weighed in aluminum cups before and after drying. The moisture percentage was calculated as14:
Moisture content (%) = [(W2 – W1) – (W3 – W1) /(W2 – W1)] × 100
Where,
W1 = Weight of the aluminum foil cup (g)
W2 = Weight of the biological product before drying (g)
W3 = Weight of the aluminum foil cup + dried biological product (g)
pH measurement
To measure pH, 1 g of powdered formulation was suspended in 100 mL distilled water (1% w/v). pH was measured using a pH meter (AB33PH-F, OHAUS, USA) and recorded as an average of three replicates.14
Evaluation of the efficacy of Serratia nematodiphila GCSR38 powder bio-products in inhibiting Colletotrichum gloeosporioides on test plants
Plant and pathogen preparation
Mango leaves were cleaned with 70% ethanol. Stalks were wrapped in sterile cotton, dipped in sterile water, and sealed with foil. C. gloeosporioides was cultured on PDA for 7 days. A 0.8 cm plug from the actively growing edge was used for inoculation.
Treatment methods
Method 1 (Preventive): Bio-products and carbendazim were applied to wounded leaves. After 24 h in a humidified box, C. gloeosporioides was inoculated.
Method 2 (Curative): C. gloeosporioides was inoculated first; after 24 h, bio-products or carbendazim were applied. All treatments used 80 g/20 L water, applying 50 µL per leaf. Each treatment included three replications:
- Formula 1 (corn starch, talcum, calcium carbonate, CMC, 10:87.5:1.5:1)
- Formula 2 (corn starch, talcum, calcium carbonate, CMC, 20:77.5:1.5:1)
- Formula 3 (talcum, calcium carbonate, CMC, 97.5:1.5:1)
- Carbendazim (chemical fungicide, 30 g/20 L water)
- Control (dH2O)
Leaves were incubated for 14 days under high humidity. Disease severity was evaluated daily, and disease reduction was calculated using12:
Disease reduction (%) = ((A – B) / A) × 100
Where,
A = Disease severity in the control
B = Disease severity in the treatment
Efficacy testing of S. nematodiphila GCSR38 against anthracnose fungi using the dual culture method
The efficacy of the antagonistic bacterium S. nematodiphila GCSR38 in inhibiting the growth of anthracnose fungi in mango was evaluated using the dual culture method. After a 7-day incubation period, S. nematodiphila GCSR38 demonstrated an inhibitory effect on the hyphal growth of the anthracnose fungi, achieving a 40.21% inhibition rate (Figure 1).
Figure 1. Efficacy testing of antagonistic bacterium Serratia nematodiphila GCSR38 in controlling anthracnose fungi: (a) test plate with S. nematodiphila GCSR38; (b) control plate without S. nematodiphila GCSR38
Development of S. nematodiphila GCSR38 powder bio-products
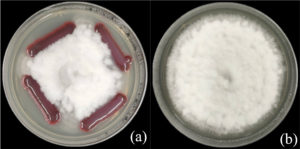
Three formulations of S. nematodiphila GCSR38-based bio-products were developed using a low-heat drying process: Formula 1 (corn starch: talcum: calcium carbonate: CMC at a ratio of 10:87.5:1.5:1), Formula 2 (corn starch: talcum: calcium carbonate: CMC at a ratio of 20:77.5:1.5:1), and Formula 3 (talcum: calcium carbonate: CMC at a ratio of 97.5:1.5:1). The resulting powders exhibited distinct physical characteristics: Formula 1 was a fine white powder, Formula 2 formed a white powder with some clumping, and Formula 3 exhibited significant clumping. The formulations were then packaged in aluminum foil bags and stored at room temperature for further evaluation (Figure 2).
Figure 2. Biological characteristics of S. nematodiphila GCSR38 in powder form: (a) Formula 1 (corn starch:talcum: calcium carbonate:CMC in the ratio of 10:87.5:1.5:1); (b) Formula 2 (corn starch:talcum:calcium carbonate:CMC in the ratio of 20:77.5:1.5:1); (c) Formula 3 (talcum:calcium carbonate:CMC in the ratio of 97.5:1.5:1)
Efficiency of S. nematodiphila GCSR38 powder bio-products
The powdered formulations were evaluated for physical and microbiological parameters to assess their efficiency. All three formulations demonstrated rapid water solubility, classified as Level 1 (1-5 min). Formula 2 exhibited the least precipitation upon standing, followed by Formulas 1 and 3. The pH values were similar across formulations, with Formulas 1 and 2 measuring 7.90, and Formula 3 at 8.00 (Table 2). Moisture content across all formulations remained relatively stable over a four-month storage period, with no statistically significant changes observed (Table 3). Initially, Formula 1 had the highest bacterial count at 1.50 × 108 CFU/g, but all formulations experienced a decline in viability over 120 days. After four months, viable counts ranged between 2.50 × 107 and 5.10 × 107 CFU/g, and the differences among them were not statistically significant.
Table (2):
Water solubility and pH of S. nematodiphila GCSR38 powder bio-products
Bio-product Formula |
Dissolution level |
pH |
|---|---|---|
1 |
1 |
7.90 |
2 |
1 |
7.90 |
3 |
1 |
8.00 |
Table (3):
Moisture content of S. nematodiphila GCSR38 powder bio-products after storage
| Bio-product Formula | Moisture content (%) | S. nematodiphila GCSR38 (CFU/g) | ||
|---|---|---|---|---|
| 1 Day | 120 Days | 1 Day | 120 Days | |
| 1 | 13.02 ± 0.45ns | 12.35 ± 0.07ns | 1.50 × 108ns | 2.50 × 107ns |
| 2 | 12.70 ± 3.39 | 14.01 ± 0.31 | 1.00 × 108 | 5.10 × 107 |
| 3 | 12.31 ± 0.59 | 11.29 ± 0.25 | 1.10 × 108 | 5.10 × 107 |
ns = no statistically significant difference
Testing the efficacy of S. nematodiphila GCSR38 powder bio-products in inhibiting C. gloeosporioides on test plants
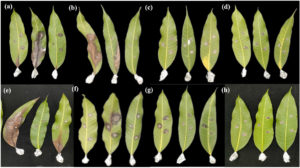
The bio-products were tested on mango leaves using two application methods: preventive and curative. Bio-formulation 3 showed the highest inhibitory effect among all formulations, with 51.00% inhibition in the preventive method and 48.30% in the curative method. This was comparable to the chemical fungicide Carbendazim, which showed 56.11% and 49.31% inhibition in the preventive and curative methods, respectively. No statistically significant difference was observed between bio-formulation 3 and Carbendazim. In contrast, bio-formulations 1 and 2 exhibited significantly lower inhibition rates, around 30-32% in both methods. Preventive treatment consistently resulted in slightly higher inhibition percentages than curative application, suggesting that early application of the bio-products enhances effectiveness against C. gloeosporioides infection (Figure 3, Table 4).
Table (4):
Efficacy of powder bio-products of S. nematodiphila GCSR38 in inhibiting anthracnose pathogen
| Bio-product Formula | Inhibition (%) | |
|---|---|---|
| Method 1 Preventive Application | Method 2 Curative Application | |
| 1 | 31.27 ± 0.84b | 29.42 ± 0.63b |
| 2 | 31.93 ± 2.60b | 30.08 ± 1.01b |
| 3 | 51.00 ± 2.44a | 48.30 ± 2.32a |
| Carbendazim (30 g/20 L) | 56.11 ± 5.90a | 49.31 ± 5.82a |
Means followed by different letters (a, b) indicate significant differences based on the Least Significant Difference (LSD) method
Figure 3. Efficacy of powder bio-products of S. nematodiphila GCSR38 in inhibiting anthracnose pathogen on mango leaves (a-d) Method 1: Preventive application; (a) Formula 1; (b) Formula 2; (c) Formula 3; (d) Carbendazim; (e-h) Method 2: Curative application; (e) Formula 1; (f) Formula 2; (g) Formula 3; (h) Carbendazim
The development of S. nematodiphila GCSR38-based bioproducts in this study aimed to provide an environmentally friendly alternative to chemical control for anthracnose disease in mango caused by C. gloeosporioides. The dual culture method demonstrated that S. nematodiphila GCSR38 achieved a 40.21% inhibition rate against the growth of C. gloeosporioides, highlighting its potential as an effective biocontrol agent. This finding is consistent with previous studies showing that S. nematodiphila and other Serratia species exhibit antagonistic properties against various plant pathogens, including fungi such as Colletotrichum.15
The development of three different formulations of S. nematodiphila GCSR38 powder was successful, with distinct physical characteristics. The formulations varied in their composition, which influenced their appearance, with Formula 1 being a fine white powder and Formula 3 exhibiting significant clumping. This variation in formulation could potentially influence the stability and release rate of the biological agent. Similar formulations using corn starch, talcum powder, calcium carbonate, and CMC have been used for the formulation of biocontrol agents, where the stability and ease of handling are essential for field application.16
The solubility tests indicated that all three formulations dissolved quickly in water, which is essential for field application. The rapid dissolution ensures that the bioagent can be efficiently dispersed during application. These results are consistent with the findings of Elnahal et al.,17 who reported that rapid solubility is crucial for the practical application of biocontrol agents, enabling faster establishment of the biocontrol agent on the plant surface.
In terms of pH, all three formulations exhibited near-neutral pH values (around 7.9-8.0), which is advantageous since neutral pH conditions are typically optimal for the growth of many plant-associated microorganisms, including Serratia species.18 Furthermore, the moisture content analysis revealed that the formulations maintained stable moisture levels over four months of storage, indicating that the bioproducts were stable under standard storage conditions. This result is significant for the commercialization of biocontrol agents, as stability during storage is a key factor for product shelf life.19
The survival and viability of S. nematodiphila GCSR38 in powdered bio-products were evaluated over a storage period of 120 days. The results indicated that all three bio-formulations (Formula 1, Formula 2, and Formula 3) maintained a substantial bacterial population throughout the storage period, though a decline in bacterial counts was observed. This is consistent with previous studies that have reported a decrease in the survival of microbial populations in bio-formulations during storage, attributed to various factors such as environmental stress, nutrient depletion, and unfavorable conditions like temperature or humidity.20,21
At the beginning of the study, Formula 1 exhibited the highest bacterial count (1.50 × 108 CFU/g), followed by Formula 3 (1.10 × 108 CFU/g) and Formula 2 (1.00 × 108 CFU/g). This initial difference in bacterial populations could be due to variations in the formulation’s composition, including the choice of carrier materials, stabilizers, and the method of product preparation.22 Formula 1 may have contained components that provided better protection or more suitable conditions for bacterial survival compared to the other two formulations. After four months of storage, a general decline in bacterial counts was observed in all formulations, with Formula 1 showing a final count of 2.50 × 107 CFU/g, and Formulas 2 and 3 showing 5.10 × 107 CFU/g. While the bacterial population significantly decreased over time, the levels remained relatively high in comparison to other studies on microbial bio-formulations. For example, studies on the survival of beneficial bacteria in powdered formulations have reported similar survival trends, where bacterial populations often decrease but remain viable enough to exhibit biological activity.23 Notably, the differences in survival rates among the three formulations were not statistically significant. This suggests that while there were variations in initial bacterial counts, the formulations provided similar environments for the survival of S. nematodiphila GCSR38 over time. The lack of significant differences might also indicate that other factors, such as the microbial strain’s inherent robustness, could have played a more significant role in survival than the formulation itself.16 However, the overall trend indicates that the formulation’s composition did impact the initial survival, even if the differences became less pronounced after storage.
The ability of S. nematodiphila GCSR38 to maintain viable populations in powdered form for four months is a promising feature for its potential use as a bio-control agent. Several studies have emphasized the importance of formulation stability for long-term storage and effectiveness of microbial inoculants. Research has shown that microbial viability is a crucial factor for ensuring the efficacy of bio-products, as low survival rates can directly reduce their biological control potential.24 For instance, formulations incorporating appropriate carriers such as starch or clay minerals have been shown to offer better protection for microbial populations.22 The findings from this study suggest that S. nematodiphila GCSR38 retains sufficient viability even after prolonged storage, making it a potential candidate for development into a stable, long-lasting bio-product.
The efficacy of S. nematodiphila GCSR38 powder bio-product in inhibiting C. gloeosporioides was evaluated using both preventive and curative application methods. The results indicate that bio-formulation 3 demonstrated the highest inhibitory effect among the three formulations, with 51.00% inhibition in the preventive method and 48.30% inhibition in the curative method. Although slightly lower than Carbendazim, a conventional fungicide, bio-formulation 3 showed no statistically significant difference in efficacy, suggesting its potential as an eco-friendly alternative to chemical treatments. The higher efficacy of bio-formulation 3 in the preventive method aligns with previous studies indicating that early intervention with biocontrol agents enhances their ability to suppress fungal pathogens before infection establishes.25 Preventive application allows for better colonization of beneficial microorganisms on the plant surface, creating a protective barrier that inhibits pathogen invasion.26 This finding supports the concept that biocontrol products, when applied before infection, can effectively outcompete pathogens for nutrients and space, thereby reducing disease incidence.27
On the other hand, the slightly lower inhibition percentages observed in the curative method suggest that the bio-product may not be as effective once the pathogen has already established itself in plant tissues. This is consistent with findings from Palmieri et al.,28 who reported that biocontrol agents tend to perform better as preventive rather than curative treatments, as they act primarily by preventing pathogen colonization rather than eradicating established infections. Despite the promising results of bio-formulation 3, the significantly lower inhibition rates observed in bio-formulations 1 and 2 (approximately 30-32%) which highlight the importance of formulation composition in biocontrol efficacy. Variability in bio-formulation effectiveness could be attributed to differences in microbial viability, carrier materials, or the presence of additional bioactive compounds that enhance antagonistic activity.29 Furthermore, while Carbendazim achieved slightly higher inhibition rates (56.11% preventive, 49.31% curative), concerns over fungicide resistance and environmental impact necessitate the exploration of biocontrol alternatives. Previous studies have reported that excessive reliance on chemical fungicides can lead to the development of resistant fungal strains, reducing long-term disease control efficacy.30 Therefore, integrating biocontrol agents such as S. nematodiphila GCSR38 into disease management programs could serve as a sustainable approach to anthracnose control in mango leaves.
This study demonstrated the efficacy of S. nematodiphila GCSR38 as a biocontrol agent against C. gloeosporioides, the causal pathogen of mango anthracnose. The dual culture method confirmed its antagonistic potential, with a 40.21% inhibition rate. Among the three bio-formulations developed, bio-formulation 3 exhibited the highest efficacy, achieving over 50% inhibition in preventive applications. While its effectiveness was slightly lower than Carbendazim, the lack of statistical difference suggests that S. nematodiphila GCSR38 could serve as a viable eco-friendly alternative to chemical fungicides. The preventive application method was slightly more effective than the curative method, highlighting the importance of early intervention for optimal disease control. Additionally, all formulations maintained substantial bacterial viability over a four-month storage period, ensuring product stability. Future research should focus on improving formulation stability, evaluating field efficacy, and investigating potential synergistic effects with other biocontrol or chemical agents to enhance the management of mango anthracnose.
ACKNOWLEDGMENTS
The authors are grateful to Mahasarakham University for its support of research equipment and financial assistance. The authors are also grateful to Prof. Motoyuki Sumida for English language editing.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This research project was financially supported by Mahasarakham University.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
- Zakaria L. Diversity of Colletotrichum species associated with anthracnose disease in tropical fruit crops. Agriculture. 2021;11(4):297.
Crossref - dos Santos MH, de Oliveira Costa JF, da Silva Franca KR, et al. Characterization and pathogenicity of Colletotrichum species causing anthracnose on pitaya (Hylocereus spp.) in Brazil. Physiol Mol Plant Pathol. 2025;138:102657.
Crossref - de Silva DD, Crous PW, Ades PK, Hyde KD, Taylor PWJ. Lifestyles of Colletotrichum species and implications for plant biosecurity. Fungal Biol Rev. 2017;31(3):155-168.
Crossref - Jayawardena RS, Bhunjun CS, Hyde KD, Gentekaki E, Itthayakorn P. Colletotrichum: lifestyles, biology, morpho-species, species complexes and accepted species. Mycosphere. 2021;12(1):519-669.
Crossref - Corkley I, Fraaije B, Hawkins N. Fungicide resistance management: maximizing the effective life of plant protection products. Plant Pathol. 2022;71(1):150-169.
Crossref - Cortaga CQ, Cordez BWP, Dacones LS, Balendres MAO, Cueva FMD. Mutations associated with fungicide resistance in Colletotrichum species. Phytoparasitica. 2023;51(3):569-592.
Crossref - El-Saadony MT, Saad AM, Soliman SM, et al. Plant growth promoting microorganisms as biocontrol agents of plant diseases:mechanisms, challenges and future perspectives. Front Plant Sci. 2022;13:923880.
Crossref - Prasad B, Sharma D, Kumar P, Dubey RC. Biocontrol potential of Bacillus spp. for resilient and sustainable agricultural systems. Physiol Mol Plant Pathol. 2023;128:102173.
Crossref - Trejo-Lopez JA, Rangel-Vargas E, Gomez-Aldapa CA, et al. Isolation and molecular identification of Serratia strains producing chitinases, glucanases, cellulases, and prodigiosin and determination of their antifungal effect against Colletotrichum siamense and Alternaria alternata in vitro and on mango fruit. Int J Plant Biol. 2022;13(3):281-297.
Crossref - Sutthisa W. Comparison of the antagonistic potential of the entomopathogenic bacterium Serratia nematodiphila GCSR38 with other effective microorganisms for the control of rice bacterial leaf blight. J Pure Appl Microbiol. 2022;16(1):557-566.
Crossref - Fenta L, Mekonnen H. Microbial biofungicides as a substitute for chemical fungicides in the control of phytopathogens: Current rerspectives and research directions. Scientifica. 2024;2024(1):5322696.
Crossref - Sutthisa W, Popranom A, Taddeetrakool A, Khankhum S. Development of Trichoderma formulation and application to control durian anthracnose disease. Trends in Sci. 2024;21(1):7276.
Crossref - Barsagade P, Khetade R, Nirwan K, Agrawal T, Gotafode S, Lade U. Review article of dissolution test method development and validation of dosage form by using RP-HPLC. Int J Pharm Sci Rev Res. 2021;70(1):28-38.
Crossref - Sutthisa W, Srilawong J, Kliangros N, Khankhum S, Charirak P. Development and application of a Trichoderma-PGPR bioformulation to enhance rice growth. J Pure Appl Microbiol. 2025;19(1):296-306.
Crossref - Alijani Z, Amini J, Ashengroph M, Bahramnejad B. Antifungal activity of Serratia rubidaea Mar61-01 purified prodigiosin against Colletotrichum nymphaeae, the causal agent of strawberry anthracnose. J Plant Growth Regul. 2022;41(2):585-595.
Crossref - Teixido N, Usall J, Torres R. Insight into a successful development of biocontrol agents:production, formulation, packaging, and shelf life as key aspects. Horticulturae. 2022;8(4):305.
Crossref - Elnahal ASM, El-Saadony MT, Saad AM, et al. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: A review. Eur J Plant Pathol. 2022;162(1):759-792.
Crossref - Bonaterra A, Badosa E, Daranas N, Frances J, Rosello G, Montesinos E. Bacteria as biological control agents of plant diseases. Microorganisms. 2022;10(9):1759.
Crossref - Mawar R, Manjunatha BL, Kumar S. Commercialization, diffusion and adoption of bioformulations for sustainable disease management in Indian Arid agriculture: prospects and challenges. Circ Econ Sust. 2021;1(4):1367-1385.
Crossref - Eakjamnong W, Keawsalong N, Dethoup T. Novel ready-to-use dry powder formulation of Talaromyces tratensis KUFA0091 to control dirty panicle disease in rice. Biological Control. 2021;152:104454.
Crossref - Balla A, Silini A, Cherif-Silini H, Bouket AC, Alenezi FN, Belbahri L. Recent advances in encapsulation techniques of plant growth-promoting microorganisms and their prospects in the sustainable agriculture. Appl Sci. 2022;12(18):9020.
Crossref - Khan A, Singh AV, Gautam SS, et al. Microbial bioformulation: A microbial assisted biostimulating fertilization technique for sustainable agriculture. Front Plant Sci. 2023;14:1270039.
Crossref - Chaudhary R, Nawaz A, Khattak Z, et al. Microbial bio-control agents: A comprehensive analysis on sustainable pest management in agriculture. J Agri Food Res. 2024;18:101421.
Crossref - Yadav R, Singh S, Singh AN. Biopesticides:current status and future prospects. Proc Int Acad Ecol Environ Sci. 2022;12(3):211-233
- Negi R, Sharma B, Kaur S, et al. Microbial antagonists: diversity, formulation and applications for management of pest-pathogens. Egypt J Biol Pest Control. 2023;33(1):105.
Crossref - Li X, Zeng S, Winmierski M, et al. Current and future trends in the biocontrol of postharvest diseases. Crit Rev Food Sci Nut. 2022;64(17):5672-5684.
Crossref - Lahlali R, Ezrari S, Radouane N, et al. Biological control of plant pathogens:a global perspective. Microorganisms. 2022;10(3):596.
Crossref - Palmieri D, Ianiri G, Del-Grosso C, et al. Advances and perspectives in the use of biocontrol agents against fungal plant diseases. Horticulturae. 2022;8(7):577.
Crossref - Dutta P, Mahanta M, Singh SB, et al. Molecular interaction between plants and Trichoderma species against soil-borne plant pathogens. Front Plant Sci. 2023;14:1145715.
Crossref - Massi F, Torriani SFF, Borghi L, Toffolatti SL. Fungicide resistance evolution and detection in plant pathogens: Plasmopara viticola as a case study. Microorganisms. 2021;9(1):119.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.