ISSN: 0973-7510
E-ISSN: 2581-690X
Agriculture is the primary source of income for more than 50 % of the Indian population and the current challenge in the agricultural industry is the increased crop production with sustainable agricultural practices from the shrinking cropland area. Plant Growth Promoting Rhizobacteria (PGPR) has been used as a bio inoculants for increasing the crop yield and the effectiveness of PGPR as biofertilizers majorly depends on the selection of the best carrier material, proper formulation of microorganisms and mode of delivery of the formulation. So, the present study investigates the effect of PGPR bacterial strains isolated from the Siruvani forest region, Coimbatore, Tamil Nadu. We have tested the efficacy of these PGPR strains using both in vitro seed germination assay and in vivo pot culture studies in CR100G rice seeds. We have used the banana peel powder (Patent No: 202041010982) as a novel organic carrier material for the development of bioformulation, along with talc as an inorganic carrier material to perform the in vivo study. The results showed that the rice plants treated with banana peel powder based bioformulation gives the highest shoot length (15.78 cm) when compared to the control (10.48 cm) on the 14th day, 21st and 45th day of seed seeding. The grain yield also increased in the Non-Enriched Banana Single (NEBS) bacterium group (125%) when normalized with the control. Thus, our current study suggests that Banana peel powder could be the better approach to be used as an organic carrier material for the development of Biofertilizers in future.
Plant Growth Promoting Rhizobacteria (PGPR), Bio fertilizers, Carrier material, in vivo pot culture study
The biggest global challenges of new millennium are to increase production of food from shrinking crop land area with best practices toward sustainable agriculture. The demand for food to feed the increasing population made the producers to depend more on agrochemicals which resulted in better increase in yield and equally caused threat to soil health and environment1. So, the method of crop production in the present scenario emphasis on increased production with sustainable agricultural practices.
Plant Growth Promoting Rhizobacteria (PGPR) has always been an important alternative approach in sustainable agriculture. Many bacteria including Acinobacter, Azospirillum, Azotobacter, Bacillus, Pseudomonas have been effectively explored as bio inoculants for increasing crop yield2. Some important functions of PGPR include production of phytohormones like auxins, Soil aggregation, atmospheric Nitrogen fixation and Solubilization of minerals such as phosphorus. The PGPR which produces auxin substances are known to increase their biomass, thus in turn changes the root morphology, so that they could make use of more soil volumes and take up more soil nutrients3. Phosphorus is the second mineral nutrient next to N, which limits the growth of crop plants. The metabolic activities of phosphate solubilizers such as organic acid exudates, phosphatases have the potentiality to dissolve the rock soil phosphate4. Well aggregated soils are more stable and less susceptible to erosion, thus the PGPR strains which involved in soil aggregation are plays important role in soil health.
The continuous application of chemical fertilizers and pesticides exhibited the detrimental effect on agro-ecosystems whereas the development of Bioinoculants with PGPR properties, has been emerged as an alternative solution for this problem. Though the main objective of bioformulation is to use best bacteria available, the survivability and performance of bioinoculant in different soil and climatic conditions is major limiting factor in commercial sector5. The major portion of the bioformulation is the carrier material, which serves as the delivery vehicle for the live microorganisms from factory to field and it could be either organic, or inorganic, or synthesized from defined molecules . The good carrier material should possesses some of the following characteristics: Non- toxic, Autoclavable, pH buffering capacity, maximam shelf – life, make nutrients to the inoculants and prevent them from biotic and abiotic conditions, able to allow more than one PGPR strains, stable in diverse climatic conditions, biodegradable, water holding capacity non-polluting material inexpensive as well as easily available6,7. The carrier could be the inert material such as talc, kaolinite, vermiculite, perlite, calcium sulphate etc, or organic material such as Farm Yard Manure (FYM), compost, wheat bran, barley bran, rice husk, peat etc.8 Though, inorganic carrier materials like talc powder has been widely used for biofertilizer development, it is not giving nutritional value to the plants9. Organic carriers like FYM, cow and poultry manures enhance the plant growth by altering the physicochemical characteristics and biological characteristics of the soil10. Though, they are required in large quantities to fulfil the nutrient requirement of crops and they are expensive.. So, the organic carrier material with good availability and cost – effective as well as supports the plant growth promotion via providing the some essential nutrients is the emerging need in the field of sustainable agriculture. Apart from these two aforementioned factors – PGPR and carrier selection, the effectiveness of PGPR as Biofertilizer also relies on the mode of delivery of the formulation. The application methods of bioformulation could be either in the form of the seed treatment or soil application, in some cases, combinations of both 11. Taking all these points into consideration, we have collected the rhizosphere soil samples from Siruvani forest region, Coimbatore, Tamil Nadu and screen the bacterial isolates for PGPR traits followed by evaluating the efficacy of these PGPR strains using in vitro and in vivo conditions. We have attempted a novel approach to use banana peel powder as an organic carrier material (both in enriched and non-enriched form) and compares the growth promotion in rice variety of CR100G with Talc based formulations, and a commercially available biofertilizer. Since banana peel powder is an eco -friendly, bio degradable carrier material, and processes tryptophan amino acid which is the major precursor of IAA production, we have hypothesized that it could be the better alternative to the existing organic carrier materials in agricultural industries.
The Bacterial strains used in the present study were isolated from the rhizosphere soil samples of Siruvani forest region, Coimbatore, Tamil Nadu, India. The rice seeds of CR100G variety were obtained from Paddy Breeding Station, Tamil Nadu Agricultural University, Coimbatore,
Collection of soil and isolation of bacterial isolate
Rizosphere soil samples were collected from various location of Siruvani forest and brought to the laboratory using sterile polythene bags. The latitude and longitude readings of the site of sample collection were 10.938011°N 76.687177°E.
Isolation of bacteria from soil samples
Bacteria was isolated from the rhizospere soil samples by adding 10g of soil in 90ml sterile water in flasks and shaken on rotary shaker at 150 rpm for 30 min. Serial dilution of the soil suspension were plated on nutrient agar medium (SRL, Research Grade) and incubated at 28°C for 48 hrs. Morphologically distinct pure bacterial colony were picked and stored in nutrient agar medium at 4°C for further studies.
Screening for PGPR traits of bacterial isolates
IAA Production Screening
The bacterial cultures were inoculated in the nutrient broth with tryptophan (SRL, Research grade) (5µg/ml). After 72 hrs incubation, the cultures were centrifuged at 3500 rpm for 10 min and the supernatant was collected. 4ml of salkowski’s reagent (SRL, Research grade) was mixed thoroughly with one ml of the supernatant, incubate at room temperature for 20 min and the absorbance was measured at 535 nm in a UV- Visible spectrophotometer. Salkowski’s reagent mixed with uninoculated Trp-containing medium served as blank. The level of IAA produced was estimated by standard IAA graph and expressed as µg/mL12 .
Detection of phosphate solubilization
A loopful of fresh bacterial culture was streaked onto Pikovaskaya’s medium (SRL, Research grade) amended with inorganic phosphate and plates were incubated at 28 ± 2°C for 4 days. A clear halo around the bacterial colony indicated solubilization of mineral phosphate13 .
Production and extraction of Exopolysaccharides
The production of exopolysaccarides screening test was carried out in 250 ml flasks containing 50 ml of medium. The medium consisted of the following components (g/l): peptone 10 gm, meat extract 3 gm, sodium chloride 5gm and sucrose 2% (SRL, Research Grade) . The pH was adjusted to 6.5. The flasks were incubated on a rotary shaker at 37°C for 72 hrs. Cells were harvested by centrifugation for 20 min at 10,000 rpm. After centrifugation, two volumes of ice-cold isopropanol were added into it and stored overnight at 4°C. Precipitated material was collected by centrifugation (20 min at 10,000 rpm) and the pellets were dried at 100°C. After drying the pellet was weighed to know the isolate producing exopolysaccharides14. Among the all isolated bacterial colonies, the bacteria showing positive for these PGPR traits alone selected for further biochemical and molecular characterization.
Characterization of bacterial strains
Biochemical Characterization of bacterial strains
The following biochemical screening tests were performed to preliminarily characterize the selected bacterial isolates based on PGPR traits. 1. Gram Staining; 2. Indole Test; 3. Methyl Red Test; 4. Urease Test; 5. Citrate utilization test.
Molecular identification of the bacterial strains
The genomic DNA was extracted and purified from the bacterial isolates FS1 1.1 and FS18.1. 16S rRNA gene of the DNA was amplified using thermal-cycler (Eppendorf thermocycler) programmed for an initial denaturation cycle of 95°C for 5 min, followed by 35 cycles of denaturation at 95°C for 30s, annealing at 52.5°C for 30s, extension 72°C for 2 min, followed by a final extension at 72°C for 7 min. The Polymerase chain reaction (PCR) products were purified using the QIAquick PCR purification kit (Qiagen, Hilden, Germany). Then, they were subjected to sequencing and the sequences were obtained.
In vitro Seed germination bioassay
Seed germination tests for CR100G rice seeds were performed by roll towel method (ISTA 2003). The bacterial strains were grown in nutrient broth at 28°C in shaker incubator for different incubation periods of 24, 48 and 72 hrs. The bacterial inoculum of 107 cfu/ml used for the seed treatment. In brief, CR100G rice seeds were surface sterilized with 1% sodium hypo chloride for 2 mins followed by rinsing with sterile distilled water for four times and then air dried. The seeds were treated with the bacterial suspension of IAA producing Bacteria and along with the consortium for one hour and then placed on seed germination papers, incubated at 28°C. Three replicates in Completely Randomized Design (CRD) were prepared for each treatment and a control without any inoculation was also maintained. Percent of Germination, Seed Vigour Index (SVI) was calculated after 7 days, 14th and 21st day of seed sowing, respectively.15.
SVI = (mean root length + Mean Shoot Length) X germination%
Development of Bio formulation
Bacterial inoculants were prepared by growing the bacteria in Nutrient Broth medium for 48 hrs at 28°C for 120 rpm. 1×108 CFU /ml of inoculum was used to prepare the bio formulation16. Efficacy of plant growth promotion by selected bacterial strains were evaluated using two types of carrier material such as talc powder as inorganic and banana peel powder (Patent No.: 202041010982) as an organic carrier material. The carrier materials were also enriched with MS nutrient medium. Banana peels were processed in dryer at 50°C for 8 hours and then powdered. The carrier materials were sterilized at 121°C at 15 lbs for 15 mins. The bio formulation was prepared in the ratio of 1:50:4 of CMC, carrier material and bacterial suspension culture respectively. The chemical constituents of processed banana peel powder has been analyzed and reported.
In vivo Pot culture study
The pot culture study was designed with the following treatments and each treatment were taken in three replicates as Completely Randomized Design (CRD). Five seeds were sown in each pot. Bio formulation were either applied to the seeds or to the soil or combination of these two methods to evaluate the efficient method of application. The following treatment groups were used: Enriched (EB) and non – enriched banana peel powder based (NEB) formulation with single IAA producing bacteria (FS 1 1.1 – EBS or NEBS) and consortia ( FS 1 1.1, FS 18.1, AG 1.4 – EBC or NEBC); Enriched (ETC) and Non enriched Talc (NET) based formulation with Single IAA producing bacteria (ETS or NETS) and consortia (ETC or NETC); Commercially available talc based Pseudomonas fluorescens formulation obtained from TNAU, Coimbatore was used as positive control and seeds were coated only with the slurry of CMC without any bacterial inoculation serves as control. Additionally, the Bio formulations were applied to the soil either with or without Farmyard Manure (FYM).
Growth promotional studies were conducted by measuring Seed Vigor Index, shoot length and root length of rice seedlings from different treatments. The seeds were weighed at harvesting period.
Statistical Analysis
The data were statistically analyzed by Analysis of Variance (ANOVA), Least Significant Difference test at probability level of P 0.05 was used to check the means when ANOVA F test indicated significant effect of treatments using SPSS software.
Screening for PGPR Traits
A total of 128 bacterial isolates were isolated from the rhizosphere soil of various locations of Siruvani forest, Tamil Nadu. Among them, the isolate FS1 1.1 isolate was selected which exhibited better indole-3-acetic acid production and isolate AG 1.4 showing efficient phosphate solubilization were chosen for the present study. Briefly, the isolate FS 11.1 produced IAA 20µg/ml followed by AG6.11, AG6.12, FS1.3, FS2.6, FS2.10 and FS2.12. Isolate AG1.4 exhibited solubilization (0.7cm) of phosphate under in vitro conditions. Likewise, Among the 128 isolates 7 isolates FS18.1, AG6.11, FS8.1, FS9.1, FS12.1 FS17.1 and AG6.14 exhibited promising (Exo Poly Saccharide) EPS production. The bacterial isolate FS18.1 produced the highest EPS (5.8µg/ml) selected for further studies.
Characterization of bacterial strains
Biochemical Characterization
All the bacterial isolates were negative for catalase, and positive for urea hydrolysis and, negative for Indole, Methyl red, FS 18.1 was positive for citrate and methyl test and all other isolates were negative. FS 1 1.1 was gram positive, and the other two isolates (FS 18.1, Ag 1.4) were gram negative. (Table 1)
Table (1):
Bio-chemical Characterization of PGPR bacterial strains.
Test |
FS 18.1 |
FS 1 1.1 |
Ag 1.4 |
|---|---|---|---|
Indole test |
– |
– |
– |
Methyl test |
+ |
– |
– |
Citrate utilization test |
+ |
– |
– |
Urease test |
+ |
+ |
+ |
Catalase test |
– |
– |
– |
Gram staining |
+ |
+ |
– |
+ symbol indicates the presence of specific Biochemical characteristics in their respective bacterial strains and – symbol indicates the absence of those characteristics in the isolates.
Molecular characterization of bacteria using 16S rDNA sequencing
The 16S rDNA sequencing was performed for the two isolates FS1 1.1 and FS 18.1 and the partial sequence obtained was analyzed using BLAST and the isolate FS 1.1 was identified as Bacillus asahii (KU884468) and the isolate FS18.1 was identified to be Bacillus cereus (KU884469).
Seed germination and Seed Vigor Index (SVI)
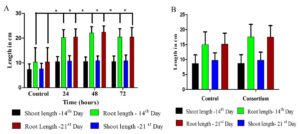
Seed inoculation of the bacterial strains significantly enhanced seed germination and seedling vigor of rice in in vitro conditions. CR100G rice seeds were treated with 24 hr, 48 hr and 72 hr old bacterial isolates of FS 1 1.1. The seed germination percentage (96%) and seeding vigor indexes (3162.24) were significantly higher in 48 hr old FS 1 1.1. treated groups compared to control (91%;1620.255). The similar trend was also observed in consortia (mixture of all the three selected PGPR strains). Consequently, increased root length has been observed both in the 14th and 21st day of FS 1 1.1 treated groups (Fig.1). So, we have choosed 48 hr old inoculants for our in vivo studies (Graph 1).
Graph 1. Efficacy of seed treatment with FS 1 1.1 (A) and consortium (B) on rice plant growth promotion under in vitro conditions.
In vivo pot culture study
The different treatment groups used for conducting in vivo pot culture study was summarized in Table 2, and we have also tested three different modes of application of bioformulation such as : (i) seed treatment, (ii) seed treatment + soil application and (iii) soil application with two different forms of soils (i) soil with FYM and (ii) Soil without FYM to check the efficacy of PGPR strains in growth promotion. Additionally, we have selected banana peel powder as an organic carrier material for our study (Patent No.: 202041010982) and their preparation steps involves (a) processing of banana peels in dryer at 50°C for 8 hours, (b) powdering, (c) sterilization at 121°C at 15 lbs for 15 mins. The presence of some essential nutrients for plant growth such as N(0.95 %; Analysis Method: IS 10158(1982) RA 2003), P (0.34 %; IS 10158 (1982) RA 2003) and K (7.29 %; EPA method – 3050 B 1996 (Rev.2)) in these processed banana peel powder could help us to reduce the chemical burden of NPK fertilizers. The other interesting phenomena to use this as an organic carrier material were the total organ carbon (43.72 %;Walkley black wet combustion method) and the presence of other important micronutrients as Fe (137.59 mg/Kg), Mn(30.19 mg/Kg), Zn (34.43 mg/Kg), Cu (84.50 mg/Kg), Ni (9.95 mg/Kg; CTL/SOP/ICH/002 ), B(12.7 mg/Kg; EPA 3050 – B -1996 (Rev. 2), Cl (2.79 % ;FAO method (pg. No 48) 2007 (Titrimetric method), Mg (288 mg/Kg ; EDTA titrimetric method) and Na (751 mg/Kg; EPA method-3050 B -1996 (Rev. 2)), which could able to support the health of soil, thus promoting the growth of rice plants.
Table (2):
Experimental setup and treatment designs for the in vivo pot culture study.
S. No. |
Treatment Groups |
Description of treatment groups |
|---|---|---|
1 |
NETS |
Talc + Single bacterium (FS 1 1.1) |
2 |
NETC |
Talc + consortium |
3 |
NEBS |
Banana peel powder + Single bacterium (FS 1 1.1) |
4 |
NEBC |
Banana peel powder + Consortium |
5 |
ETS |
Enriched talc + Single bacterium (FS 1 1.1) |
6 |
ETC |
Enriched talc + consortium |
7 |
EBS |
Enriched banana peel powder + Single bacterium (FS 1 1.1) |
8 |
EBC |
Enriched banana peel powder + consortium |
9 |
BIFC |
Biofertilizer control : Pesudomonas fluorescens |
10 |
C |
control |
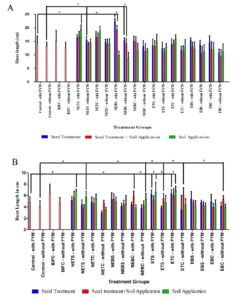
The highest shoot length was recorded in the NEBS – with FYM – Seed treatment group (15.78 cm) as compared to control (10.48 cm) on 14th day. Likewise, NEBS – with and without FYM – Seed treatment groups showed increased shoot length on 21st day of seed sowing when compared to control and other treatment groups (Graph 2A), (Table 3). In addition, the shoot length of NEB treated plants were found to be better than talc-based Pseudomonas fluorescens treated plants (15.14 ± 3.95 cm) used as positive control. However, NEBS – with FYM and without FYM – seed treatment; seed treatment + soil application groups and NETS – with FYM – soil application; NETS – without FYM – seed treatment; soil application groups also showed significant increase in the shoot length on 21st Day of seed sowing.
Graph 2. Effect of Bio formulation on growth of rice plant on 21st day of seed sowing in shoot length (A); and 30th day of seed sowing in root length (B) for the Soil with and without FYM groups.
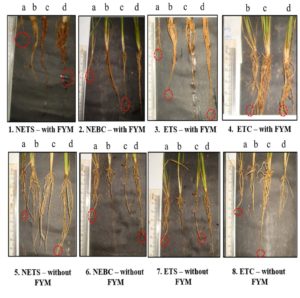
Highest root length was observed in plants treated with ETC – with FYM – Soil Application group (6.90 ±0.75) and ETC – without FYM – Seed + Soil Application (6.092± 1.30 cm) on 30th day of seed sowing when compared to control – with FYM (4.79 ± 1.00 cm) and control without FYM (4.09± 1.0 cm). The other treatment groups also showed significant increase in the root length are NETS – with and without FYM – soil application; NEBC – with FYM – seed and soil application; NEBC – without FYM – soil application; ETS – with FYM – seed treatment; soil application; ETS – without FYM – seed treatment and soil application; ETC – with FYM -seed treatment; ETC – without FYM – seed treatment and soil application; EBC – without FYM – seed treatment and soil application. (Table 3) (Fig. 1). Few rice plants from the groups which exhibited highest shoot length and root length were sacrificed on 45th day of seed sowing, and the different in measurements were represented in Fig. 2. The seeds were harvested and weighted for all the treatment group. Weight of thousand seeds of Commercially available Pseudomonas fluorescens is 77% (15.4 g), Non enriched banana Single bacterium is 125% (19.6 g) and Enriched Consortium based formulation is 119% (19.1 g) higher than the control group (8.71 g). The conclusion of the present study is that seed treatment of Non enriched banana peel powder-based Bio formulation is effective for Shoot development and enriched talc-based formulation with PGPR consortia is effective for root development in rice plant.
Table (3):
Effect of Bio formulation on growth of rice plant on 21st day of seed sowing in shoot length and 30th day of seed sowing in root length for the Soil with and without FYM groups.
| Measurement of shoot length on 21st day of seed sowing | ||||||
|---|---|---|---|---|---|---|
| Soil with FYM | Soil without FYM | |||||
| Combinations of Bio formulation | Seed treatment (cm) | Seed treatment + soil Application (cm) | Soil application (cm) | Seed treatment (cm) | Seed treatment + soil Application(cm) | Soil application(cm) |
| NETS | 16.36 ± 1.12 | 16.75 ± 1.93 | 19.32 ± 1.74* | 15.47 ± 1.31* | 14.26 ± 1.37 |
14.38 ± 1.48* |
| NETC | 16.44 ± 2.19 | 16.56 ± 1.52 | 18.84 ± 1.76 | 14.70 ± 1.04 | 13.97 ± 1.87 |
14.04 ± 1.91 |
| NEBS | 22.22 ± 1.28* | 19.26 ± 1.64* | 9.85 ± 1.56 |
15.34 ± 1.0* | 14.25 ± 1.78* |
8.71 ± 2.25 |
| NEBC | 15.15 ± 1.76 | 14.98 ± 1.73 | 11.52 ± 2.79 | 13.18 ± 2.03 | 11.42 ± 2.79 |
11.52 ± 0.86 |
| ETS | 13.46 ± 2.41 | 13.22 ± 2.00 | 14.57 ± 2.95 | 12.38 ± 2.24 | 10.98 ± 1.33 |
12.74 ± 1.47 |
| ETC | 13.85 ± 2.05 | 14.34 ± 1.09 | 15.79 ± 1.59 | 11.86 ± 1.33 | 11.07 ± 2.29 |
12.75 ± 2.90 |
| EBS | 15.47 ± 0.86 | 12.86 ± 1.77 | 12.17 ± 2.64 | 13.65 ± 1.76 | 10.54 ± 2.59 |
11.31 ± 2.48 |
| EBC | 14.48 ± 2.63 | 12.17 ± 2.73 | 11.93 ± 2.53 | 10.79 ± 1.36 | 9.61 ± 2.38 |
11.20 ± 2.84 |
| C | 14.22 ± 3.81 | 13.14 ± 2.55 | ||||
| BIFC | 15.14 ± 3.95 | 12.59 ± 2.08 | ||||
| Measurement on root length on 30th day of seed sowing | ||||||
|---|---|---|---|---|---|---|
| NETS | 5.14 ± 0.70 |
5.72 ± 1.02 | 6.26 ± 0.39* | 4.37 ± 0.87 | 3.83 ± 0.60 | 5.7± 1.01* |
| NETC | 4.87 ± 0.9 |
4.64 ± 0.92 | 5.33 ± 0.9 |
2.97 ± 0.34 | 4.61 ± 0.55 | 3.52 ± 0.42 |
| NEBS | 5.33 ± 1.16 |
5.36 ± 1.20 | 4.87 ± 0.89 | 4.16 ± 0.48 | 4.49 ± 0.69 | 4.39 ± 1.59 |
| NEBC | 4.39 ± 0.41 |
5.87± 0.91* | 3.80± 0.55 | 3.80± 0.55 | 4.35 ± 0.71 | 5.36 ± 1.04* |
| ETS | 6.202 ± 0.77* | 5.22 ± 0.73 | 6.50 ± 1.85 * | 3.81 ± 0.35 | 5.45 ± 0.90* | 4.76 ± 1.24 |
| ETC | 6.36 ± 0.68 * | 6.004 ± 1.23 | 6.90 ± 0.75 * | 3.49 ± 2.5 | 6.092 ± 1.30* | 4.33 ± 1.11 |
| EBS | 5.37 ± 1.35 | 4.86 ± 0.34 | 4.15 ± 0.93 | 4.83 ± 0.34 | 4.33 ± 0.58 | 4.22 ± 0.46 |
| EBC | 4.89 ± 1.30 | 3.95 ± 0.66 | 4.46 ± 0.78 | 4.19 ± 0.61 | 5.32 ± 0.7* | 3.91± 0.57 |
| C | 4.79 ± 1.00 | 4.09± 1.0 | ||||
| BIFC | 6.39 ± 2.63 | 4.72 ± 0.95 | ||||
Values are the mean ± standard deviation of the shoot length in cm. * P < 0.05
Fig. 1. Effect of different forms of Bioformulation on 30th day of seed sowing in root length. a – control; b – Seed Treatment; c – Seed treatment + Soil application; d – Soil application.
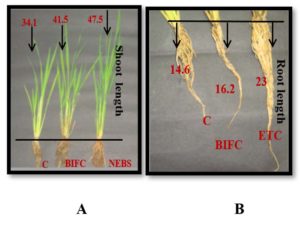
Fig. 2. Growth of rice seedlings after 45th day of seed sowing.
1 = Control (C) (34.1 cm); 2= Biofertilizer Control (BIFC) (41.5 cm); 3= Non – Enriched Banana peel Single bacterium group (NEBS) (47.5 cm).
1 = Control (C) (14.6 cm); 2 = Biofertilizer Control (BIFC) (16.2 cm); 3 = Enriched Talc Consortium group (ETC) (23 cm)
PGPR have been extensively studied and reported for their beneficial effects on plant growth by many direct – production of phytohormones and volatiles; Solubilization of phosphates; uptake of iron, and indirect mechanisms – production of antibiotics; Systemic Induce Resistance; inhibition of pathogen produced enzymes or toxins17-19. Microbial inoculant as Biofertilizer has been reported by numerous studies20-22. Isolation, Identification, and characterization of microorganisms, Screening for desirable characters, selection of efficient strains to the formulation and production of inoculum, field testing are the major steps for making use of this microbe-based technology. The common problem for the use of PGPR in agricultural field is the formation of suitable inoculant with the effective carrier material for reliable and consistent results under field conditions. Thus, the effectiveness of PGPR as Biofertilizer can rely on the following factors: (i) The selection of best carrier material, (ii) Proper formulation of microorganisms, (iii) Mode of delivery of the formulation (either to seeds or to the soil).
Talc has been proved as the best inorganic carrier material in various pot culture and field studies. For example, in addition to the reduction of sheath blight severity, plant height, grain yield also observed when talc-based formulation of Pseudomonas spp were applied to the rice23. Methyl Cellulose and Talc based P. fluorescens suppressed rice blast both in nursery and field conditions. Though, it could not be able to provide any additional nutrients either to PGPR or plant. So, organic carrier materials such as peat, vermi compost, animal manure and composted plant materials has been studied24. Peat has been used widely as organic carrier because of it’s high water holding, nutrients and buffering capacity,25 yet limited availability and increase cost price remains the problem26 . So, the search for alternative organic carrier material is greatest concern for the development of bio formulation. Banana peel powder contains various micro and macro nutrients, naturally. The presence of tryptophan amino acid which is the major precursor for the microbial production of IAA, in the banana peel powder is an encouraging phenomenon to be selected as organic carrier material for our study. Tryptophan amino acid has been considered as an important molecule that can alter the level of IAA bio synthesis27. The production of IAA has been strongly increased by exogenous application of tryptophan in various bacteria, such as Azospirillum, Pseudomonas putida, Rhizobium etc. 28,29,30. Similar studies reported in Brassica spp shows that L-tryptophan dependent auxin production by different PGPR strains increase the grain yield, number of branches and pods per plant31. IAA producing ability of PGPR depends on the availability of precursors and uptake of microbial IAA by plant32. So, Talc has been selected as inorganic and banana peel powder has been selected as organic carrier material for the development of Bio formulation. Plant growth hormones are important regulators of plant growth and mediate responses to both biotic and abiotic stresses. IAA is a Phyto hormone which has a cascading effect of plant development, due to its ability to influence plant growth, which in turn affects the nutrient uptake and ultimately the plant production. Phosphate solubilization by PGPR increase the availability of P to chickpea reported a possible mechanism for growth of chickpea33. Many studies are reported PGPR consortia as an effective method to increase the yield rather than using single bacterium34. To determine the ability of IAA producing bacteria and PGPR consortia in growth promotion of rice plants, we have developed the Bio formulation contains either group of bacteria. Application method of the bio formulation suggested for annual crops are soil surface or seed and combination of these two application methods. In addition, the inoculant survival has been improved by the addition of nutrients to seed pellets35 . Increased survival of PGPR bacteria as well as the better control over the leaf spot disease in groundnut plants has been observed when the formulation is supplemented with chitin36. Sachin Singh et al.37 studied enrichment of carrier material sawdust with CMC, sucrose, molasses, and gum Arabic. Enrichment with molasses brought maximum growth in chickpea and cell viability of the enriched bio formulation also shown to be increased, due to the presence of high carbon source. So, the objectives of this study is designed to select suitable carrier (talc or banana peel powder both in enriched and non-enriched form) for inoculating PGPR (either to single IAA producer or consortia) and to establish the most efficient mode of bio fertilizer application to the rice plants. And the results obtained in our study shows that seed treatment of Non enriched banana peel powder-based Bio formulation is effective for Shoot development and enriched talc-based formulation with PGPR consortia is effective for root development in rice plant. The possible reason for this increased growth promotion in NEBS – with FYM – Seed treatment group than other treatment groups could be the presence of Tryptophan amino acid and the other essential nutrients present in the banana peels38 .Since, banana peel powder based carrier material shows better growth promotion in rice seedlings and yield of grains than commercially available Pseudomonas fluorescens, this might be promising approach in future, to study and develop this novel carrier material based bio formulation with different PGPR strains for crops.
ACKNOWLEDGMENTS
We would like to thank the Karunya Institute of Technology and Sciences for providing the laboratory facilities and agriculture lands for performing the pot culture studies in the campus.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
ETHICS STATEMENT
Not applicable.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
- Gerhardson B. Biological substitutes for pesticides. Trends Biotechnol. 2002;20(8):338-343.
Crossref - Whipps JM. Microbial interactions and biocontrol in the rhizosphere. J Exp Bot. 2001;52(Issue Suppl 1):487-511.
Crossref - Malik KA, Bilal R, Mehnaz S, Rasul G, Mirza MS, Ali S. Association of nitrogen-fixing, plant-growth-promoting rhizobacteria (PGPR) with kallar grass and rice. Plant and Soil. 1997;194:37-44.
Crossref - Lal L. Phosphate Bio fertilizers. Agrotech Publishing Academy, Udaipur, India. 2002:224.
- Validov S, Kamilova F, Qi S, et al. Selection of bacteria able to control Fusarium oxysporum f. sp. radicis-lycopersici in stonewool substrate. J Appl Microbiol. 2007;102(2):461-471.
Crossref - Smith RS. Legume Inoculant Formulation and Application. Can J Microbiol. 1992;38:485-492.
Crossref - Malusa E, Sas-Paszt L, Ciesielska J. Technologies for beneficial microorganisms inocula used as biofertilizers. Sci World J. 2012;2012:49120.
Crossref - Bashan Y. Inoculants of plant growth promoting bacteria for use in agriculture. Biotechnology Advances. 1998;16(4):729-770.
Crossref - Sarma MV, Kumar V, Saharan K, et al. Application of inorganic carrier-based formulations of fluorescent pseudomonads and Piriformospora indica on tomato plants and evaluation of their efficacy. J Appl Microbiol. 2011;111(2):456-466.
Crossref - Ebaid RA, El-Refaee IS. Utilization of Rice Husk as an Organic Fertilizer to Improve Productivity and Water Use Efficiency in Rice Fields. 8th African Crop Science Society Conference, El-Minia, 2007, 1923-1928.
- Muresu R, Sulas S. Legume -Rhizobium Symbiosis: characteristics and prospects to inoculation. Rivoluzione Agronomica. 2003;37:33-45.
- Gordon SA, Paleg LG. Quantitative measurement of indole acetic acid. Physiol Plant Pathol. 1957;10:347-348.
- Verma SC, Ladha JK, Tripathi AK. Evaluation of plant growth promoting and colonization ability of endophytic diazotrophs from deep water rice. J Biotechnol. 2001;91(2-3):127-141.
Crossref - Pawar ST, Bhosale AA, Gawade TB, Nale TR. Isolation screening and optimization of exopolysaccharide producing bacterium from saline soil. J Microbiol Biotech Res. 2013;3(3):24-31.
- Abdul Baki AA, Anderson JD. Vigor determination in soybean seed by multiple criteria. Crop Science. 1973;13(6):630-633.
Crossref - Amer GA, Utkhede RS. Development of formulations of biological agents for management of root rot of lettuce and cucumber. Can J Microbiol. 2000;46(9):809-816.
Crossref - Podile AR, Kishore GK. Plant growth-promoting rhizobacteria. In: Gnanamanickam S.S. (eds) Plant-Associated Bacteria. Springer Dordrecht. 2007:195-230.
Crossref - Figueiredo MVB, Seldin L, de Araujo FF, Mariano RLR. Plant Growth Promoting Rhizobacteria: fundamentals and Applications. Plant Growth and Health Promoting Bacteria (Microbiology Monographs). 2010;18:21-43.
- Dardanelli MS, Carletti SM, Paulucci NS, et al. Benefits of Plant Growth Promoting Bacteria and Rhizobia in agriculture. Plant Growth and Health Promoting Bacteria (Microbiology Monographs). 2010;18:1-20.
Crossref - Antoun H, Beauchamp CJ, Goussard N, Chabot R, Lalande R. Potential of Rhizobium and Brady Rhizobium species as Plant Growth Promoting Rhizobacteria on non-legumes: effect on radishes (Raphanus sativus L.). Molecular Microbial Ecology of the Soil . 1998;204:57-67.
Crossref - Peix A, Mateos PF, Rodriguez-Barrueco C, Martinez-Molina E, Velazquez E. Growth promotion of common bean (Phaseolus vulgaris L.) by a strain of Burkholderia cepacia under growth chamber conditions. Soil Biol Biochem. 2001;33(14):1927-1935.
Crossref - Sarawgi SK, Tiwari PK, Tripathi RS. Uptake and balance sheet of nitrogen and phosphorus in gram (Cicerarietinum) as influenced by phosphorus, biofertilizers and micronutrients under rainfed condition. Indian J Agron. 1999;44:768-772.
- Nanda-Kumar R, Babu S, Viswanthan R, Sheela J, Raguchander T, Samivappan R. A new bio-formulation containing plant growth promoting rhizobacterial mixture for management of sheath blight and enhanced grain yield in rice. Bio control. 2001;46:493-510.
Crossref - Stephens JHG, Rask HM. Inoculant production and formulation. Field Crops Research. 2000;65(2-3):249-258.
Crossref - Burton JC. Modern concepts in legume production. Graham PH, Harris SC, (Eds.), Biological nitrogen fixation technology for tropical agriculture. 1982;105-114.
- Saha AK, Deshpande MV, Kapadnis BP. Studies on survival of Rhizobium the carriers at different temperatures using green fluorescent protein marker. Current Science. 2001;80(5):669-671.
- Spaepen S, Vanderleyden J, Remans R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev. 2007;31(4):425-448.
Crossref - Brandl MT, Lindow SE. Environmental signals modulate the expression of an indole 3 acetic acid biosynthetic gene in Erwania herbicola. Molecular plant -Microbe Interactions. 1997;10:499 -505.
Crossref - Patten CL, Glick BR. Regulation of indoleacetic acid production in Pseudomonas putida GR12-2 by tryptophan and the stationary-phase sigma factor RpoS. Can J Microbiol. 2002;48(7):635-642.
Crossref - Theunis M, Kobayashi H, Broughton WJ, Prinsen E. Flavonoids, NodD1, NodD2, and nod-box NB15 modulate expression of the y4wEFG locus that is required for indole-3-acetic acid synthesis in Rhizobium sp. strain NGR234. Mol Plant Microbe Interact. 2004;17(10):1153-1161.
Crossref - Asghar H, Zahir Z, Arshad M, Khaliq A. Relationship between in vitro production of auxins by rhizobacteria and their growth-promoting activities in Brassica juncea L. Biol Fertil Soils. 2002;35:231-237.
Crossref - Glick BR. The enhancement of plant growth by free-living bacteria. Can J Microbiol. 1995;41(2):109-117.
Crossref - Yadav J, Verma JP, Tiwari KN. Effect of plant growth promoting Rhizobacteria on seed germination and plant growth Chickpea (Cicerarietinum L.) under in vitro conditions. Biological Forum – An International Journal. 2010;2(2):15-18.
- Adesemoye AO, Torbert HA, Kloepper JW. Enhanced plant nutrient use efficiency with PGPR and AMF in an integrated nutrient management system. Can J Microbiol. 2008;54(10):876-886.
Crossref - Moenne-Loccoz Y, Naughton M, P. Higgins J. Powell, B. O’Connor, F. O’Gara. Effect of inoculum preparation and formulation on survival and biocontrol efficacy of Pseudomonas fluorescens F113. J Appl Microbiol. 1999;86:108-116.
Crossref - Kishore GK, Pande S, Podile AR. Biological Control of Late Leaf Spot of Peanut (Arachis hypogaea) with Chitinolytic Bacteria. Phytopathology. 2005;95(10):1157-1165.
Crossref - Singh S, Gupta G, Khare E, Behal KK, Arora NK. Effect of enrichment material on the shelf life and field efficiency of bioformulation of Rhizobium sp. and P-solubilizing Pseudomonas fluorescens. Science Research Reporter. 2014;4(1):44-50.
- Hussein HS, Shaarawy HH, Hussien NH, Hawash SI. Preparation of nano-fertilizer blend from banana peels. Bull Natl Res Cent. 2019;43:26.
Crossref
© The Author(s) 2021. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.