ISSN: 0973-7510
E-ISSN: 2581-690X
Tomato (Lycopersicon esculentum Mill.) production is severely affected by bacterial wilt, a destructive disease caused by Ralstonia solanacearum, which poses a significant challenge to growers worldwide. This study aimed to develop powdered formulations of Bacillus velezensis MSU01 and evaluate their efficacy in controlling the disease and enhancing plant growth. Laboratory evaluation using the agar well diffusion assay demonstrated strong antagonistic activity of B. velezensis MSU01 against R. solanacearum, with an inhibition zone of 13.8 mm. Three powder formulations-talcum-based, corn starch-based, and kaolin-based were prepared and evaluated for physicochemical properties, bacterial viability during storage, seed germination, and disease control efficacy. All formulations showed excellent solubility and stable pH (7.03-8.67), with initial moisture contents ranging from 0.20%-2.17%. After 60 days of storage at room temperature, the kaolin-based formulation maintained the highest viable cell count (6.30 ± 0.30 log CFU/g), while the corn starch-based formulation showed the highest moisture content (5.24 ± 0.30%). The corn starch-based formulation most effectively reduced bacterial wilt, lowering disease incidence to 10.00 ± 0.00% and severity to 5.33 ± 4.16%, outperforming the chemical control (streptomycin). Seed germination rates were highest with the talcum-based formulation (96.67 ± 5.77%), comparable to the positive control and significantly greater than the negative control. Although shoot and root lengths did not differ significantly among treatments, all B. velezensis MSU01 formulations enhanced plant growth compared to the pathogen-only treatment. Overall, the corn starch-based formulation showed the greatest potential as a sustainable and efficient biocontrol agent for managing bacterial wilt in tomato.
Bacillus velezensis MSU01, Bacterial Wilt, Powdered Bioformulation, Seed Germination, Sustainable Agriculture
Tomato (Lycopersicon esculentum Mill.) is considered among the most widely cultivated and economically important vegetable crops worldwide, valued for its nutritional content and versatile culinary use.1 However, tomato production faces significant challenges from various diseases, with bacterial wilt caused by Ralstonia solanacearum being among the most destructive. This soilborne pathogen invades plant vascular tissues, causing accelerated wilting followed by plant death, often resulting in complete yield loss when environmental conditions are favorable.2 Managing bacterial wilt is difficult due to the pathogen’s broad host range, genetic diversity, and its persistence in soil and water. Traditional control measures, for example, crop rotation, chemical treatments, and resistant cultivars, have shown limited effectiveness or are not environmentally sustainable.3 Consequently, there is an increasing need for alternative and eco-friendly disease management approaches.
Utilizing beneficial microorganisms for disease control has emerged as a promising strategy to combat soil- and seed-borne pathogens, with Bacillus velezensis receiving particular attention due to its strong antagonistic activity, endospore-forming capability, and efficient root colonization.4 As a plant growth-promoting rhizobacterium (PGPR), B. velezensis has been proven to suppress a range of phytopathogens, including R. solanacearum, Fusarium oxysporum, and Xanthomonas oryzae pv. oryzae (Xoo), by the generation of antimicrobial metabolites, including surfactin, iturin, and fengycin.5,6 In particular, strain N1 demonstrated promising results against Xoo, with formulation 2 (comprising 10% skimmed milk, 20% maltodextrin, 0.06% sodium alginate, and 5% tapioca starch) reducing disease severity by 45.87% in greenhouse trials.7 Similarly, strains Y6 and F7 exhibited strong antagonism toward R. solanacearum and F. oxysporum via lipopeptide-mediated mechanisms that impacted pathogen motility and biofilm formation.8 Moreover, strain D not only colonized tobacco roots effectively but also disrupted R. solanacearum cell structure through antibacterial metabolites, leading to significant disease suppression in field trials.9 Developing stable and effective bioformulations is essential for the practical use of biocontrol agents, with carriers such as talc, starch, and alginate being investigated to enhance bacterial viability and facilitate application.10 However, despite its biocontrol potential, recent reports have identified possible pathogenicity of B. velezensis in certain crops such as peach, onion, and potato, indicating that crop-specific risk assessments are essential prior to its widespread use.11
This research was conducted to develop and evaluate various formulations of B. velezensis MSU01 for the biological management of bacterial wilt in tomato. The antagonistic activity, physicochemical properties, storage stability, and in planta efficacy of these formulations were assessed to determine their potential as environmentally friendly alternatives to chemical pesticides.
Determination of the antagonism of B. velezensis MSU01 against R. solanacearum by the agar well diffusion method
The antagonistic effect of B. velezensis MSU01 against R. solanacearum, the pathogen causing tomato bacterial wilt, was evaluated using the agar well diffusion assay according to a protocol adapted from Sawatphanit.7 R. solanacearum was cultured on nutrient agar (NA) plates incubated at 30 °C for 24 h, after which the bacterial cells were harvested and resuspended in sterile distilled water to achieve a final concentration of approximately 108 CFU/mL. The bacterial suspension was then evenly spread onto fresh NA plates using the swab plate technique. Plates were left at room temperature until the surface dried. Separately, B. velezensis MSU01 was also cultured on NA plates for 24 h at 30 °C. The bacterial cells were harvested and suspended in sterile distilled water to a concentration of 108 CFU/mL. On each R. solanacearum inoculated plate, a single 6 mm diameter well was created in the center using a sterile cork borer. A 20 µL aliquot of the B. velezensis MSU01 suspension was added into the well. Sterile distilled water was used as negative control, while streptomycin (20 g/20 L) served as positive control. All treatments were conducted in triplicate. Following incubation at 30 °C for 24 h, the diameter of the inhibition zones surrounding each well was measured to determine antagonistic activity.
Development of powdered formulations of B. velezensis MSU01
The powdered formulations of Bacillus velezensis MSU01 were developed following a modified protocol from Sutthisa et al.12,13 Initially, B. velezensis MSU01 was streaked onto nutrient agar (NA) plates and incubated at room temperature (approximately 28-30 °C) for 24 h. A single colony was then transferred to 100 mL of nutrient broth (NB) and incubated under shaking conditions at 150 rpm at room temperature for 24 h. Subsequently, 10% (v/v) of the initial culture was transferred into 500 mL of NB and incubated under identical shaking conditions for 48 h. Following incubation, the bacterial culture was centrifuged at 7,000 rpm for 10 min to collect the cells. The harvested cell pellet was then washed twice with a 0.85% (w/v) sodium chloride solution. The washed cells were resuspended in sterile distilled water and bacterial suspension was standardized to approximately 108 CFU/mL by determining the optical density at 600 nm (OD600 = 0.2). Three powdered bioformulations were prepared using different carriers: talcum powder (Formula 1), corn starch (Formula 2), and kaolin (Formula 3), as shown in Table 1. Each formulation also contained calcium carbonate (CaCO3), carboxymethyl cellulose (CMC), and glucose in fixed proportions. Each formulation mixture was autoclaved at 121 °C for 15 min to sterilize it, and subsequently cooled to room temperature. After cooling, the sterile powder base (900 g) was mixed thoroughly with 100 mL of the prepared B. velezensis MSU01 cell suspension. The mixtures were then dried at 55 °C for 36 h. The resulting powders were packed in sterile foil packets and maintained at room temperature for subsequent analysis of efficacy and shelf life.
Table (1):
Ingredients and proportions of powdered formulations of antagonistic microorganisms
Formula |
Ingredients |
Ratio (%) |
|---|---|---|
1 |
Talcum:CaCO3:CMC: Glucose |
83.75:15:1:0.25 |
2 |
Corn starch:CaCO3:CMC: Glucose |
83.75:15:1:0.25 |
3 |
Kaolin:CaCO3:CMC:Glucose |
83.75:15:1:0.25 |
Survival of B. velezensis MSU01 in powdered formulations
The viability of B. velezensis MSU01 in the three powdered formulations were assessed via the dilution plate count method. Subsamples were randomly taken from each formulation and serially diluted in sterile distilled water. Serial dilutions at 10-3, 10-4, and 10-5 were selected for subsequent analysis. From each dilution, 0.1 mL was plated in triplicate onto nutrient agar (NA). Incubation was carried out at 28-30 °C (room temperature) for 24-48 h. The number of colony-forming units (CFU) was determined and noted. The viability of B. velezensis MSU01 was evaluated at three storage time points: day 0 (immediately after drying), day 30, and day 60 at room temperature. The results were expressed as log CFU/g of formulation.
Determination of moisture content in powdered formulations
The moisture content of each Bacillus velezensis MSU01 powdered formulation was determined using the gravimetric method. A known weight of each powder sample was placed into a pre-weighed aluminum foil cup and oven-dried at 105 °C for 5 h. The dried samples were cooled to room temperature in a desiccator before being reweighed. Moisture content was determined based on the weight loss before and after drying.14 Moisture content percentage was determined by the following formula:
Moisture content (%) = [(W2 – W1) – (W3 – W1) / (W2 – W1)] × 100
Where weight W1 = aluminum foil cup weight (g)
W2 = weight of bioproduct before drying in aluminum foil cup (g)
W3 = weight of aluminum foil cup + bioproduct after baking (g)
Water Solubility of Bacillus velezensis MSU01 Powdered Formulations
The water solubility of each B. velezensis MSU01 powdered formulation was determined following the method adapted from Sutthisa et al.14 One gram of each powdered formulation was added to 1 liter of distilled water. A magnetic stirrer was used to mix the solution at 200 rpm, and the time required for complete dissolution was recorded. Water solubility was categorized into five levels based on the time taken for complete dispersion of the powder in water: level 1: dissolves within 1-5 min, level 2: dissolves within 6-10 min, level 3: dissolves within 11-30 min, level 4: dissolves within 31-60 min and level 5: does not dissolve; forms clumps or floats on the water surface. Each formulation was tested in triplicate, and the average dissolution time was used to classify the solubility level.
Evaluation of the efficacy of B. velezensis MSU01 powdered formulations for inhibiting R. solanacearum and promoting tomato plant growth
The efficacy of powdered formulations of B. velezensis MSU01 in suppressing bacterial wilt caused by R. solanacearum and promoting tomato plant growth was evaluated as follows:
Seed preparation
Tomato seeds (cv. Sida) were surface sterilized using 5% sodium hypochlorite for 5 min, followed by triple rinsing with sterile distilled water. Seeds were dried on sterile tissue paper within an aseptic conditions.
Pathogen suspension preparation
R. solanacearum was cultured and adjusted to an approximate concentration of 106 CFU/mL, the cell suspension was standardized by setting the optical density (OD) at 600 nm to 0.2.
Treatment setup
Each treatment involved mixing 30 sterilized tomato seeds with 1 g of each biological formulation and 1 mL of sterile distilled water. The seeds were then placed on sterile culture plates lined with moist, sterile filter paper. Five seeds were placed per plate. After seeding, 5 mL of the R. solanacearum suspension was added to each plate. The treatments were as follows:
T1: Bio-formulation 1 (Talcum-based) + R. solanacearum
T2: Bio-formulation 2 (Corn starch-based) + R. solanacearum
T3: Bio-formulation 3 (Kaolin) + R. solanacearum
T4: Streptomycin at 20 g/20 L (Chemical positive control) + R. solanacearum
T5: R. solanacearum (Pathogen control)
T6: dH2O (Negative control)
Each treatment was replicated three times. Plates were maintained under room temperature conditions, and disease symptoms were observed daily for 14 days.
Disease Assessment
Disease incidence was calculated as
Disease incidence (%) = (Number of diseased tomato plants / Total number of plants) × 100
Disease severity was evaluated using a six-grade scale adapted from Phiri et al.15 : level 1: no symptoms, level 2: yellow spots on 1%-20% of leaves, level 3: yellowing on 21%-40% of leaves, level 4: Yellowing on 41%-60% of leaves, level 5: Browning and wilting on 61%-80% of leaves and level 6: Wilting on 81%-100% of leaves. The Disease Severity Index (DSI) was determined using the formula below:
DSI = 100 × Σ (Number of plants in each category × Rating score) / [(Total number of plants observed) × (maximal disease index)]
Plant growth promotion assessment
Seed germination was recorded on days 5 and 14 after treatment. At day 14, tomato seedlings were carefully removed, and both stem height and root length were measured. Stem height was determined by measuring from the root–shoot junction to the shoot tip, and root length was measured from the root base to the tip of the longest root. Measurements were taken from all seedlings in each treatment group.
Assessment of the in vitro antagonistic effect of B. velezensis MSU01 on R. solanacearum using the agar well diffusion assay.
Using the agar well diffusion method, the antagonistic effect of B. velezensis MSU01 on R. solanacearum, the pathogen responsible for tomato bacterial wilt, was evaluated. The results showed that B. velezensis MSU01 exhibited a clear inhibition zone measuring 20.8 mm in diameter, indicating effective suppression of the pathogen.
Development of powdered formulations of Bacillus velezensis MSU01
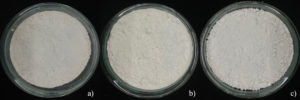
Three powdered formulations of B. velezensis MSU01 were developed using different carrier materials including talcum-based, corn starch-based and kaolin-based. After the drying process, all formulations appeared as fine white powders. However, physical differences were observed: Formula 1 remained a free-flowing fine powder, Formula 2 formed into large lumps, and Formula 3 formed into small lumps (Figure 1). All formulations were packaged in aluminum foil bags and maintained at room temperature for subsequent testing.
Figure 1. Powdered formulations of Bacillus velezensis MSU01: (a) talcum-based, (b) corn starch-based, and (c) kaolin-based formulations
Survival of Bacillus velezensis MSU01 in powdered formulations during storage
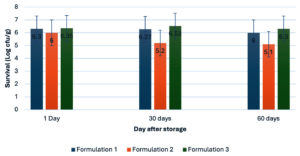
The survival of B. velezensis MSU01 in the three powdered formulations was assessed during a 60-day storage period at room temperature (Figure 2). At day 1, all formulations exhibited high viable counts, ranging from 6.00 to 6.35 log CFU/g. After 30 days, the kaolin-based formulation (Formula 3) showed the highest viable count (6.53 ± 0.20 log CFU/g), followed by the talcum-based (Formula 1) and corn starch-based (Formula 2) formulations. By day 60, the kaolin-based formulation maintained the highest survival (6.30 ± 0.30 log CFU/g), while the corn starch-based formulation exhibited the lowest viable count (5.10 ± 0.85 log CFU/g).
Figure 2. Survival of B. velezensis MSU01 in powdered formulations after storage at room temperature
Physicochemical properties of Bacillus velezensis MSU01 powdered formulations
The physicochemical characteristics including moisture content, water solubility, and pH of three powdered formulations of B. velezensis MSU01 (talcum-based, corn starch-based, and kaolin-based) were evaluated at 1 and 60 days of storage at room temperature (Table 2). On day 1, the talcum-based formulation (Formula 1) had the lowest moisture content (0.20 ± 0.10%), followed by the kaolin-based formulation (Formula 3) at 0.50 ± 0.50%, while the corn starch-based formulation (Formula 2) recorded a higher initial moisture content of 2.17 ± 1.04%. After 60 days, the corn starch-based formulation retained the highest moisture level (5.24 ± 0.30%), whereas the talcum- and kaolin-based formulations remained relatively dry at 0.49 ± 0.03% and 0.56 ± 0.14%, respectively. Although numerical variations in moisture content were noted, they were not statistically significant (p > 0.05).
Table (2):
Water solubility, pH, and moisture content of Bacillus velezensis MSU01 powdered formulations
| Bio-product Formula | Dissolution level | pH | Moisture content (%) | |
|---|---|---|---|---|
| 1 Day after storage | 60 Days after storage | |||
| 1: Talcum-based | 1 | 8.67 ± 0.15ns | 0.20 ± 0.10ns | 0.49 ± 0.03ns |
| 2: Corn starch-based | 1 | 7.17 ± 0.21 | 2.17 ± 1.04 | 5.24 ± 0.30 |
| 3: Kaolin-based | 1 | 7.03 ± 0.15 | 0.50 ± 0.50 | 0.56 ± 0.14 |
ns: no statistically significant difference
In terms of water dissolution, all formulations exhibited excellent solubility (Level 1), achieving complete dissolution within 1-5 min of stirring at 200 rpm. The pH measurement indicated that the talcum-based formulation had the highest pH (8.67 ± 0.15), while the corn starch- and kaolin-based formulations showed slightly lower pH values of 7.17 ± 0.21 and 7.03 ± 0.15, respectively. Similar to the moisture content results, there were no statistically significant differences in pH among the formulations (p > 0.05).
Efficacy of B. velezensis MSU01 powder formulation on disease incidence and severity
Application of B. velezensis MSU01 powder formulations markedly decreased the incidence and severity of bacterial wilt in tomato plants caused by R. solanacearum, relative to the negative control. Among the treatments, the corn starch-based formulation was the most effective, recording the lowest disease incidence (10.00 ± 0.00%) and disease severity (5.33 ± 4.16%), both significantly lower than all other treatments. The talcum-based and kaolin-based formulations showed moderate effectiveness, with disease incidence values of 56.67 ± 25.17% and 50.00 ± 17.32%, and disease severity values of 47.33 ± 15.53% and 47.33 ± 16.77%, respectively. The positive control (streptomycin) also reduced disease incidence (40.00 ± 20.00%) and severity (34.00 ± 14.00%), but was less effective than the corn starch-based formulation (Table 3). In contrast, the negative control (inoculated with R. solanacearum only) exhibited the highest disease incidence (80.00 ± 20.00%) and disease severity (80.00 ± 20.00%). The findings suggest that B. velezensis MSU01 formulated with corn starch is the most effective option for reducing bacterial wilt symptoms in tomato plants.
Table (3):
Efficacy of B. velezensis MSU01 powder formulation on disease incidence and severity
Powder formulation |
Disease incidence (%) |
Disease severity (%) |
|---|---|---|
Talcum-based + R. solanacearum |
56.67 ± 25.17ab |
47.33 ± 15.53d |
Corn starch-based + R. solanacearum |
10.00 ± 0.00ab |
5.33 ± 4.16b |
Kaolin-based + R. solanacearum |
50.00 ± 17.32ab |
47.33 ± 16.77d |
Positive control (Streptomycin 20 g/20 L) + R. solanacearum |
40.00 ± 20.00ab |
34.00 ± 14.00c |
Negative control (R. solanacearum) |
80.00 ± 20.00b |
80.00 ± 20.00e |
dH2O |
0.00 ± 0.00a |
0.00 ± 0.00a |
Means followed by different letters (a, b,c,d,e) indicate significant differences based on the Least Significant Difference (LSD) method
Efficacy of powdered formulation of B. velezensis MSU01 on seed germination, stem height and root length of tomato
The application of B. velezensis MSU01 powder formulations positively influenced tomato seed germination and plant growth compared to the negative control. All powder formulations (talcum-, corn starch-, and kaolin-based) and the positive control (Streptomycin) significantly increased germination percentages, ranging from 90.00 ± 10.00% to 96.67 ± 5.77%, compared to the negative control (70.00 ± 30.00%). The highest germination percentage was observed with the talcum-based formulation (96.67 ± 5.77%) (Table 4). Regarding shoot and root lengths, no significant differences were observed among treatments. Shoot lengths ranged from 3.30 ± 0.20 cm (negative control) to 4.40 ± 0.80 cm (positive control), while root lengths varied from 4.67 ± 0.45 cm (water control) to 8.83 ± 0.99 cm (kaolin-based formulation) (Figure 3). Overall, the powder formulations of B. velezensis MSU01 improved germination rates and maintained comparable shoot and root lengths relative to the positive control and healthy plants, indicating a positive trend in promoting tomato growth and vigor under bacterial wilt conditions.
Table (4):
Efficacy of powdered formulation of B. velezensis MSU01 on tomato growth promotion
Powder formulation |
Germination (%) |
Shoot length (cm) |
Root length (cm) |
|---|---|---|---|
Talcum-based + R. solanacearum |
96.67 ± 5.77a |
4.28 ± 0.23ns |
7.80 ± 1.01ns |
Corn starch-based + R. solanacearum |
93.33 ± 5.77a |
4.07 ± 0.15 |
7.63 ± 0.95 |
Kaolin-based + R. solanacearum |
90.00 ± 10.00a |
3.93 ± 025 |
8.83 ± 0.99 |
Positive control (Streptomycin 20 g/20 L) + R. solanacearum |
90.00 ± 10.00a |
4.40 ± 0.80 |
7.40 ± 0.00 |
Negative control (R. solanacearum) |
70.00 ± 30.00b |
3.30 ± 0.20 |
7.27 ± 0.75 |
dH2O |
90.00 ± 0.00a |
4.10 ± 0.10 |
4.67 ± 0.45 |
Means followed by different letters (a, b) indicate significant differences based on the Least Significant Difference (LSD) method. differences based on the Least Significant Difference (LSD) method
The agar well diffusion assay confirmed the antagonistic activity of B. velezensis MSU01 against R. solanacearum, the bacterium causing bacterial wilt in tomato. Formation of a distinct inhibition zone demonstrated the potent antibacterial activity of the strain. The observed effect is probably attributed to antimicrobial lipopeptides like iturin, fengycin, and bacillomycin, which are widely recognized in B. velezensis strains for their broad-spectrum activity.16 The inhibition zone observed in our study is consistent with previous reports. For example, Chen et al.17 demonstrated that B. velezensis FJAT-46737 effectively controls R. solanacearum growth via antimicrobial lipopeptides, underscoring its potential in managing tomato bacterial wilt. To facilitate field application and extend the shelf life of B. velezensis MSU01, we developed three powdered formulations using different carriers including talcum, corn starch, and kaolin based and assessed their viability and stability over 60 days of storage. All formulations initially exceeded the minimum effective threshold for viable count (>6 log CFU/g), as recommended for microbial biocontrol agents.18 Of the three, the kaolin-based formulation maintained the highest cell viability (6.30 ± 0.30 log CFU/g at day 60), suggesting that kaolin may offer superior protection due to its inert nature, porous structure, and moisture-buffering properties.19 Conversely, the corn starch-based formulation showed a notable decline in viability (5.10 ± 0.85 log CFU/g), potentially due to higher moisture retention, which could accelerate microbial metabolism and reduce shelf stability.20
Formulation appearance and physical traits also varied. The talcum-based formulation remained a fine, free-flowing powder, which facilitates ease of handling and application. In contrast, the corn starch and kaolin-based formulations developed lumps of different sizes, possibly due to the hygroscopic nature of their carriers. Moisture content – an important factor influencing microbial stability was lowest in the talcum-based formulation, followed by kaolin, while corn starch retained the highest moisture (5.24 ± 0.30% at day 60). Although these differences were not statistically significant, they may still impact formulation performance over time.21 All three formulations demonstrated excellent solubility in water (Level 1), making them suitable for field application. pH values of the formulations ranged from neutral to slightly alkaline, with the talcum-based formulation showing the highest pH (8.67 ± 0.15), all within the acceptable range for maintaining microbial stability.22 Among the formulations, the kaolin-based product emerged as the most stable in terms of microbial viability and moisture regulation, while the talcum-based formulation excelled in physical handling characteristics. These outcomes support earlier findings that inert carriers like talc and kaolin offer better shelf life stability compared to organic carriers.22
Evaluation of in planta efficacy revealed that all B. velezensis MSU01 formulations suppressed bacterial wilt to varying degrees. The corn starch-based formulation (Formula 2) achieved the greatest disease control, reducing incidence and severity to 10.00 ± 0.00% and 5.33 ± 4.16%, respectively. Remarkably, this was more effective than both the talcum and kaolin formulations and even outperformed the effect of chemical control (Streptomycin), which exhibited only moderate suppression. These results reinforce previous research demonstrating the effectiveness of B. velezensis strains in controlling R. solanacearum through multiple mechanisms, including the production of antibiotics (e.g., difficidin, fengycin, bacilysin), resource competition, and induction of systemic resistance.23 Interestingly, the corn starch-based formulation’s highest biocontrol efficacy was observed despite its lowest viability at day 60. This implies that biocontrol success may depend not only on survival rates but also on the strain’s metabolic activity and ability to produce bioactive compounds in situ. This aligns with Laishram et al.24 who emphasized that rhizosphere competence and metabolite production are critical determinants of effective biocontrol.
In terms of plant growth promotion, all three formulations supported high seed germination rates (<90%), comparable to the chemical control and significantly higher than the pathogen-inoculated control (70.00 ± 30.00%). The findings indicate that B. velezensis MSU01 decreases pathogen presence in the rhizosphere, leading to improved seedling emergence and vigor.24 While no significant differences in stem height were observed among treatments, root development was most pronounced in the kaolin-based formulation, with an average root length of 8.83 cm. The observed trend is likely due to Bacillus species’ growth-promoting characteristics, such as the production of phytohormones (e.g., IAA), phosphate solubilization, and improved nutrient uptake.25 Collectively, these findings demonstrate that B. velezensis MSU01 is an effective biocontrol agent as well as plant growth enhancer. The choice of carrier material significantly influenced formulation stability and efficacy, with the corn starch-based formulation being most effective in disease suppression and the kaolin-based formulation promoting better root growth. These insights highlight the importance of optimizing formulation components to enhance both the storage stability and field efficacy of microbial bioproducts.
The strong antagonistic activity of B. velezensis MSU01 against R. solanacearum highlights its potential as an effective biocontrol agent for controlling bacterial wilt in tomato. Among the three powdered formulations developed, the kaolin-based formulation maintained the highest microbial viability and moisture stability during storage, while the corn starch-based formulation showed superior disease suppression and seedling protection despite lower viability. All formulations supported high seed germination and promoted plant growth, particularly root development. These findings emphasize the critical importance of carrier selection in optimizing the performance of microbial formulations. Overall, B. velezensis MSU01, especially when formulated appropriately, represents a promising, sustainable alternative to chemical control for tomato bacterial wilt management and plant growth promotion.
ACKNOWLEDGMENTS
The authors are indebted to Mahasarakham University for the support of research equipment and finance. The authors are grateful to Prof. Motoyuki Sumida for English language editing.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was supported by Mahasarakham University, Thailand.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Bihon W, Ognakossan KE, Tignegre JB, Hanson P, Ndiaye K, Srinivasan R. Evaluation of different tomato (Solanum lycopersicum L.) entries and varieties for performance and adaptation in Mali, West Africa. Horticulturae. 2022;8(7):579.
Crossref - Wang Z, Luo W, Cheng S, Zhang H, Zong J, Zhang Z. Ralstonia solanacearum – A soil borne hidden enemy of plants: Research development in management strategies, their action mechanism and challenges. Front Plant Sci. 2023;14: 1141902.
Crossref - Al-Shammary AAG, Al-Shihmani LSS, Fernandez-Galvez J, Caballero-Calvo A. Optimizing sustainable agriculture: A comprehensive review of agronomic practices and their impacts on soil attributes. J Environ Manag. 2024;364:121487.
Crossref - Shafi J, Tian H, Ji M. Bacillus species as versatile weapons for plant pathogens. Biotechnol Biotechnol Equip. 2017;31(3):446 459.
Crossref - Chen XH, Koumoutsi A, Scholz R, Borriss R. More than anticipated-production of antibiotics and other secondary metabolites by Bacillus amyloliquefaciens FZB42. J Mol Microbiol Biotechnol. 2009;16(1-2):14-24.
Crossref - Rabbee MF, Ali MS, Choi J, Hwang BS, Jeong SC, Baek KH. Bacillus velezensis: A valuable member of bioactive molecules within plant microbiomes. Molecules. 2019;24(6):1046.
Crossref - Sawatphanit N, Sutthisa W, Kumlung T. Bioformulation development of Bacillus velezensis strain N1 to control rice bacterial leaf blight. Trends Sci. 2022;19(21):6315.
Crossref - Cao Y, Pi H, Chandrangsu P, et al. Antagonism of two plant-growth promoting Bacillus velezensis isolates against Ralstonia solanacearum and Fusarium oxysporum. Sci Rep. 2018;8:4360.
Crossref - Wang J, Peng Y, Xie S, et al. Biocontrol and molecular characterization of Bacillus velezensis D against tobacco bacterial wilt. Phytopathol Res. 2023;5:50.
Crossref - Harman GE. Myths and dogmas of biocontrol: changes in perceptions derived from research on Trichoderma harzianum T-22. Plant Disease. 2007;84(4):377-393.
Crossref - Rabbee MF, Hwang B-S, Baek K-H. Bacillus velezensis: A Beneficial biocontrol agent or facultative phytopathogen for sustainable agriculture. Agronomy. 2023;13(3):840.
Crossref - Sutthisa W, Nachai P, Yutthasin R. Development of entomopathogenic bacterium Serratia nematodiphila GCSR38 formulation for the control of mango anthracnose disease. J Pure Appl Microbiol. 2025;19(2):1410-1418.
Crossref - Sutthisa W, Srilawong J, Kliangros N, Khankhum S, Charirak P. Development and application of a Trichoderma-PGPR bioformulation to enhance rice growth. J Pure Appl Microbiol. 2025;19(1):296-306.
Crossref - Sutthisa W, Popranom A, Taddeetrakool A, Khankhum S. Development of Trichoderma formulation and application to control durian anthracnose disease. Trends Sci. 2023;21(1):7276.
Crossref - Phiri TM, Bhattarai G, Chiwina KE, et al. An evaluation of bacterial wilt (Ralstonia solanacearum) resistance in a set of tomato germplasm from the United States Department of Agriculture. Agronomy. 2024;14(2):350.
Crossref - Chen XH, Koumoutsi A, Scholz R, Borriss R. More than anticipated – production of antibiotics and other secondary metabolites by Bacillus amyloliquefaciens FZB42. J Mol Microbiol Biotechnol. 2009;16(1-2):14-24.
Crossref - Chen M, Wang J, Liu B, et al. Biocontrol of tomato bacterial wilt by the new strain Bacillus velezensis FJAT-46737 and its lipopeptides. BMC Microbiol. 2020;20(1):160.
Crossref - Bashan Y, de-Bashan LE, Prabhu SR. Hernandez JP. Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998-2013). Plant Soil. 2014;378(1-2):1-33.
Crossref - Choudhary DK, Johri BN. Interactions of Bacillus spp. and plants with special reference to induced systemic resistance (ISR). Microbiol Res. 2009;164(5):493-513.
Crossref - Hu M, Hei R, Guo D, et al. Shelf-life enhancement of bio-inoculants through synergistic effects of encapsulation technology and osmotic protectants. J Environ Chem Eng. 2023;11(5):110996.
Crossref - Vassilev N, Vassileva M, Nikolaeva I. Simultaneous P-solubilizing and biocontrol activity of microorganisms: potentials and future trends. Appl Microbiol Biotechnol. 2006;71(2):137-144
Crossref - Bhardwaj D, Ansari MW, Sahoo RK, Tuteja N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb Cell Fact. 2014;13: 66.
Crossref - Shan X, Dai J, Xu Z, et al. Comprehensive evaluation and mechanisms of Bacillus velezensis AX22 against rice bacterial blight. Biological Control. 2025;207:105820.
Crossref - Laishram B, Devi OR, Dutta R, et al. Plant-microbe interactions: PGPM as microbial inoculants/biofertilizers for sustaining crop productivity and soil fertility. Curr Res Microbl Sci. 2025;8:100333.
Crossref - Petkova M, Marcheva M, Petrova AL, Slavova V, Shilev S. Plant growth-promoting and biocontrol characteristics of four Bacillus strains and evaluation of their effects on wheat (Tr. aestivum L.). Int J Plant Biol. 2025;16(1):1.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.