ISSN: 0973-7510
E-ISSN: 2581-690X
Wound healing is a complex, multi-phase process involving numerous cellular and molecular mechanisms. Chronic wounds, exacerbated by conditions such as diabetes or bacterial biofilms, pose significant clinical challenges, increasing healthcare costs. This study aimed to develop biogenic nano-hydrogels (BgNH) incorporating silver nanoparticles synthesized from medicinal plant extracts. The efficacy of these hydrogels was tested in wound healing, antibiofilm, and antivirulence assays, particularly targeting Pseudomonas aeruginosa. Results revealed that BgNH exhibited significant antibiofilm activity, with 96% inhibition and 89.93% biofilm dispersal, while demonstrating minimal cytotoxicity at lower concentrations. Structural analysis confirmed an amorphous, porous matrix facilitating controlled nanoparticle release. In vivo studies demonstrated a 25.23% improvement in wound healing compared to untreated controls, highlighting BgNH’s potential as a therapeutic wound dressing with both antimicrobial and healing-promoting properties. This study concludes that BgNH offers a promising alternative to conventional wound care treatments, with further research needed to optimize formulations for clinical use.
Biogenic Nano Hydrogel, Silver Nanoparticles, Antibiofilm Activity, Wound Healing
Wound healing is a complex, multi-phase process involving diverse cell types, cytokines, mediators, and vascular responses. Initially, vasoconstriction and platelet aggregation halt bleeding, succeeded by an influx of neutrophils and other inflammatory cells releasing cytokines to promote angiogenesis, thrombosis, and epithelialization. Fibroblasts subsequently lay down extracellular matrix components, essential for scaffolding.1 This is followed by the proliferative phase, characterized by granulation tissue formation and neovascularization, and finally, the maturation phase where the wound gains maximum tensile strength.2
Chronic wounds, often influenced by systemic conditions such as diabetes or local factors like bacterial biofilms, fail to heal within the typical 4 to 6 weeks, leading to increased morbidity and healthcare costs.3 Integrating nanomaterials, especially silver nanoparticles, into wound dressings has shown promise due to their antimicrobial and anti-inflammatory properties.4 Hydrogels incorporating silver nanoparticles offer a novel approach, combining biocompatibility with enhanced antimicrobial activity, potentially overcoming challenges posed by multidrug-resistant infections.5 This study explores the development and characterization of biogenic nano-hydrogels, evaluating their efficacy in wound healing and anti-biofilm activity.
Initially twenty medicinal aqueous extracts were screened in our laboratory, identifying five quorum quenching agents: Garcinia cambogia, Terminalia chebula, Trachyspermum ammi, Zingiber officinale, and Agaricus bisporus. These were used to synthesize biogenic silver nanoparticles (BSNPs), labeled GcNPs, TcNPs, TaNPs, ZoNPs, and AbNPs. Pseudomonas aeruginosa YU-V10, a multidrug-resistant urinary catheter pathogen, was tested for antibiofilm formulation. A combination of two BSNPs (Biogenic Silver Nanoparticles) increased biofilm inhibition by 7.23% (80.7% to 87.93%) which was carried out based on Synergy measurement by Checkerboard design.6 Using a three-BSNP combination BgNF formulation was developed, which significantly enhanced the antibiofilm activity. Biogenic Nano Formulation (BgNF) efficacy as an antibiofilm agent was tested in hydrogel-based wound dressings against Pseudomonas aeruginosa.
In this study, we investigated the synthesis and characterization of biogenic nano-hydrogels (BgNH), incorporating silver nanoparticles derived from medicinal plant extracts. We evaluated the efficacy of BgNH in wound healing and antibiofilm activity, specifically targeting Pseudomonas aeruginosa, a common pathogen in chronic wounds.
Synthesis of Biogenic Nano Hydrogel (BgNH)
The Biogenic Nano Hydrogel (BgNH) was synthesized through a two-step methodology. First, 2% (w/v) solutions of sodium alginate and gelatin were prepared by dissolving the polymers at 90 °C and 70 °C, respectively. These solutions were then homogeneously mixed at 50 °C using a magnetic stirrer. Following this, the biogenic nano formulation (BgNF) was incorporated into the polymer blend.7
To cross-link the polymers and form the hydrogel matrix, 0.0125% (v/v) glutaraldehyde was added, and the mixture was poured into casting plates to dry at room temperature, yielding thin hydrogel sheets.8
Second, these dried sheets were cured by immersion in a solution containing 5% (w/v) calcium chloride (CaCl2) and 0.1% (w/v) glycine for 30 minutes to enhance their mechanical strength and stability. The cured sheets were then rinsed with distilled water and dried to obtain the final BgNH product.
XRD analysis
XRD analysis using the Rigaku Ultima instrument of BgNH was performed at the College of Technology, Osmania University. XRD analysis was done at current 30 mA, voltage 40 kV, X-ray tube target Cu, scan range 10.0-80.0 degrees, scan mode-continuous scan, scan speed -2.000 deg/min, sampling pitch -0.0200 deg. This analysis was done to identify whether the hydrogel was crystalline or amorphous in nature.9
Spectroscopic analysis of BgNH
BgNH of 1 cm2 area was suspended in 1 ml of the aqueous medium and anlysed for the increase in absorption at OD350 nm using UV-visible spectrophotometer 119-Systronics. The increase in OD was recorded after every 1 hr time interval.10
Cytotoxicity assay
HeLa cells were cultured in a 96-well plate at a seeding density of 10,000 cells per well in 200 µL of Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS). The cells were incubated at 37 °C in a humidified atmosphere with 5% CO2 for 24 hours to allow for cell attachment and growth. To evaluate the cytotoxic effects of biogenic silver nanoparticles (BSNPs) and the biogenic nano formulation (BgNF), the culture medium was replaced with fresh DMEM containing varying concentrations of BSNPs and BgNF (10-50 µg/mL). Each concentration was tested in triplicate to ensure statistical validity. The treated cells were incubated for an additional 24 hours under the same conditions.11
Following treatment, the medium was removed and replaced with DMEM containing 0.5 mg/mL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). The cells were then incubated in the dark for 4 hours to allow for the formation of formazan crystals. After the incubation period, the MTT-containing medium was carefully aspirated, and 100 µL of dimethyl sulfoxide (DMSO) was added to each well to dissolve the formazan crystals. The plate was incubated in the dark for an additional 5 minutes to ensure complete solubilization.
Absorbance was measured at 570 nm using a microplate reader, with cell viability being directly proportional to the absorbance value. The concentration range tested (10-50 µg/mL) was selected based on previous studies that demonstrated dose-dependent cytotoxicity of similar nanoparticles.12,13 The results were analyzed to determine the half-maximal inhibitory concentration (IC50) of BSNPs and BgNF, providing insights into their potential therapeutic window and cytotoxicity profile.
Wound Healing activity of BgNH by excision model
The test system used in this study involved Rattus norvegicus (Wistar rats), which were selected for their suitability in wound healing models. The animals were 6 to 8 weeks old at the time of treatment, with body weights ranging from 180 to 200 grams. Only male rats were used in the study, all of which were sourced from CPCSEA-registered vendors to ensure compliance with ethical standards. A total of 18 animals were divided into three experimental groups, with each group consisting of six male rats. This grouping allowed for appropriate comparison across different treatment conditions.14
To induce wounds, the dorsal skin of Wistar rats was shaved and animals were anesthetized with Phenobarbital. Circular excision wounds (2 cm × 2 cm diameter) were created using toothed forceps, an excision blade, and pointed scissors, with wound size measured. Negative controls ensured no adverse effects from unknown variables. Test and reference components were topically applied once daily, with wound area manually traced and photographed daily for 21 days. The positive control group remained untreated, and all wounds were covered with surgical gauze. Wound area was calculated using a formula, and histological examination was performed on surviving animals’ wound areas. The percentage of wound healing was calculated based on initial and final areas drawn on glass slides.15,16
% wound healing = [Wound area day 0 – Wound area days n / Wound area day 0] × 100
Where,
n is number of days after wound excision (n = days 3, 6, 9 etc.).
BgNH Synthesis and Structural Characterization
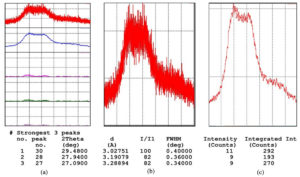
XRD analysis confirmed that BgNH exhibited an amorphous structure, crucial for its flexible application in wound dressings. UV-visible spectroscopy revealed the characteristic peaks of silver nanoparticles, confirming their successful incorporation into the hydrogel matrix (Figure 1).
Figure 1. XRD analysis of BgNH. (a) XRD patterns showing multiple scans of BgNH sample. (b) Enlarged view of the most intense diffraction peak with corresponding 2θ, d-spacing, and FWHM. (c) Intensity vs. counts plot used for integrated intensity analysis
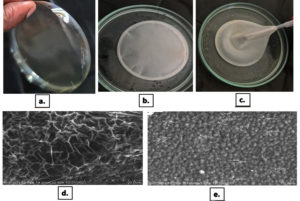
To understand the surface topography and visualize entrapped BSNPs within the hydrogel images were captured in the Scanning Electron Microscope. For this 1 cm2 segment of BgNH coated with gold surface staining was used for capturing the SEM images. The images when observed revealed a network of pores with BSNPs entrapped within them. The size of the particles entrapped in the pores showed variations as a number of particles agglomerated. Due to this a definite uniform structure was not revealed. The size variations of BSNPs embedded in BgNH were observed to be in the range of 266 nm-601 nm [266 nm, 281 nm, 302 nm, 251 nm, 286 nm, 557 nm, 601 nm, 391 nm, 444 nm, 522 nm, 566 nm, 515 nm, 586 nm, 589 nm, 849 nm, 398 nm, 472 nm, 449 nm, 416 nm] (Figure 2). From the results observed it indicates that the surface morphology of the hydrogel has no characteristic organization. The random organization of particles and non-uniform pores shows that the hydrogel has no crystalline structure. UV characterization to analyze the diffusion of AgNPs and X-ray Diffraction studies for structural organization were performed.
Figure 2. SEM analysis of BgNH. (a) Photograph of air-dried silver nano xerogel showing its physical transparency and rigidity; (b & c) Images depicting silver nano hydrogel in hydrated form during preparation and handling. (d) SEM image of the silver nano hydrogel surface (×2500 magnification), showing fibrous network structure with measurements. (e) High-resolution SEM image (×3000 magnification) highlighting the nanoparticulate distribution and surface topography
Cytotoxicity assay
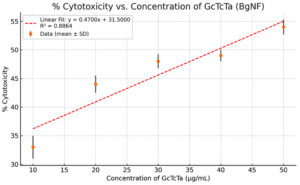
The BgNH formulation exhibited minimal cytotoxicity at concentrations below 50 µg/mL, indicating its safety for therapeutic application. The GcTcTa-based nanoformulation (BgNF), derived from Garcinia, Terminalia, and Trachyspermum extracts, demonstrated a dose-dependent increase in cytotoxicity, rising from approximately 33% at 10 µg/mL to about 54% at 50 µg/mL. The interpolated IC50 value was slightly above 40 µg/mL, falling within the acceptable range for topical wound healing applications. Additionally, low standard deviations across replicates confirmed the reproducibility and reliability of the results (Figure 3).
Anti-biofilm activity of BgNH
It was essential to determine the antibiofilm activity of BGNH to use it in wound dressings or any other medical applications. Therefore, the efficacy of the BNF incorporated BgNH were evaluated for its anti-biofilm potential on two pathogens Chromobacterium violaceum and Pseudomonas aeruginosa at 32 °C and 37 °C, respectively. The hydrogels were incubated for 4 days enabling the development of biofilm in 20 ml Nutrient broth in petri dishes under aseptic conditions with the test pathogen. Controls were set up with hydrogels devoid of AgNPs along with the test samples. BgNH and control (hydrogel without BNF) were incubated with actively growing culture suspensions.
1 cm2 of hydrogels subjected for biofilm formation were later analyzed under scanning electron microscope. The BgNH showed no significant biofilm formation for either of the organisms. The anti-bacterial activity was evident from the clear broth in which the BgNH was incubated. While the anti-biofilm effect of BgNH was evident from the SEM images (Figure 4 and Figure 5). Since hydrogels are three dimensional crosslinked hydrophilic networks that have nano particle loading capacity, it can be used for coating materials where the formation of biofilm could happen. Biogenic nano hydrogel used in current study can be characterized as multi-functional hydrogel with bactericidal, anti-virulent and anti-biofilm activities. This gives prospective application of biogenic nano hydrogel to be used as anti-infective hydrogel coatings on medical devices and wound dressings.
Figure 4. SEM image of C. violaceum 4212 biofilm inhibited by BgNH. (a) SEM image of Chromobacterium violaceum 4212 biofilm formed on a plain hydrogel surface (without biogenic silver nanoparticles), showing dense bacterial aggregation and biofilm matrix. (b) SEM image showing significant biofilm inhibition of C. violaceum 4212 on biogenic silver nanoparticle-embedded hydrogel (BgNH). The image reveals a cleaner surface with sparse bacterial presence, indicating antibiofilm activity
Figure 5. SEM image of P. aeruginosa YU-V10 biofilm Inhibited by BgNH. (a) SEM image showing dense and well-structured biofilm of Pseudomonas aeruginosa YU V10 formed on the hydrogel surface without biogenic silver nanoparticles (AgNPs). Biofilm matrix and clustered bacterial cells are visibly prominent. (b) SEM image displaying significant biofilm inhibition of P. aeruginosa YU V10 on biogenic silver nanoparticle-embedded hydrogel (BgNH). The reduction in bacterial colonization and the absence of structured biofilm indicate the antibiofilm efficacy of BgNH
Wound healing activity
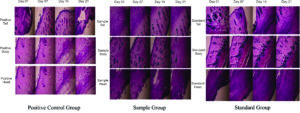
Histological analysis revealed that BgNH-treated rats (Sample group) exhibited significantly enhanced wound healing across all anatomical regions (tail, body, and head) when compared to Positive and Standard control groups. As shown in Figure 6, progressive improvement was evident across Day 0, 7, 14, and 21, with the Sample group demonstrating well-organized dermal architecture, pronounced granulation tissue formation, and advanced re-epithelialization, particularly by Day 21. Quantitatively, BgNH treatment led to a 25.23% greater wound closure than controls. Additionally, the nanohydrogel exhibited potent antibiofilm activity, achieving 96% inhibition and 89.93% dispersal of Pseudomonas aeruginosa biofilms. These findings highlight the dual functionality of BgNH in promoting tissue regeneration while combating bacterial biofilms-making it a strong candidate for managing chronic, infected wounds.
Figure 6. Histological Evaluation of Wound Healing at Different Time Points (H&E Staining). Each row is labelled based on treatment group and anatomical site (Head, Body, and Tail), while each column indicates the time points of observation (Day 0, Day 7, Day 14, and Day 21). The three treatment groups included in the figure are:
Positive Control Group
Sample Group (Biogenic formulation-treated)
Standard Group
For each anatomical region (Head, Body, Tail), histological images at four different time points (Day 0, Day 7, Day 14, and Day 21) are shown in succession from left to right
The present study highlights the promising therapeutic potential of biogenic nano-hydrogels (BgNH) in the management of chronic wounds, especially those infected with Pseudomonas aeruginosa, a biofilm-forming and multidrug-resistant pathogen. BgNH exhibited a dual mode of action-potent antibiofilm activity coupled with enhanced wound healing capability-making it a valuable candidate for addressing limitations of current wound care strategies.17,18
Biofilms formed by P. aeruginosa are known to impede wound healing by creating a protective barrier that shields bacteria from antibiotics and host immune responses.19 The BgNH formulation effectively inhibited 96% of biofilm formation and achieved 89.93% dispersal of pre-formed biofilms. This efficacy can be attributed to the incorporation of silver nanoparticles (AgNPs) within the hydrogel matrix. These nanoparticles, synthesized using medicinal plant extracts, are known for their broad-spectrum antimicrobial activity and ability to interfere with quorum sensing, a key mechanism in biofilm development.20-22 The porous structure of the hydrogel facilitated a sustained release of AgNPs, ensuring prolonged antimicrobial action.23
Beyond its antimicrobial capabilities, BgNH significantly accelerated wound healing. In vivo experiments demonstrated a 25.23% improvement in wound closure compared to untreated controls. This enhanced healing effect can be attributed to the hydrogel’s ability to maintain a moist wound environment, minimize inflammation, and provide a scaffold for cellular proliferation and migration.24 Moreover, the reduction in microbial burden by AgNPs helped to mitigate chronic inflammation, a major impediment to tissue regeneration.25
Structural characterization revealed the amorphous nature of the hydrogel, as confirmed by XRD analysis. This morphology supports controlled, gradual release of AgNPs, preventing burst release and reducing the risk of cytotoxicity.26 The balance between sustained antimicrobial activity and tissue compatibility is a critical feature for chronic wound management.
Cytotoxicity studies further confirmed the biocompatibility of BgNH. Assays performed on HeLa cells showed minimal toxicity at concentrations between 10-50 µg/mL, with an IC50 value exceeding the therapeutic concentration range. This aligns with previous research indicating that biogenically synthesized AgNPs, particularly those derived from plant extracts, tend to exhibit lower cytotoxicity compared to chemically synthesized counterparts.27 The natural polymer matrix-comprising sodium alginate and gelatin-also contributed to the biocompatibility, as these materials are well-documented for supporting cell adhesion and growth while minimizing adverse tissue responses.28
Conventional wound treatments often target either infection or tissue regeneration, but rarely both. In contrast, BgNH integrates antimicrobial and regenerative functionalities within a single platform. Its design ensures sustained release of therapeutic agents and a conducive healing environment, addressing the multifactorial challenges of chronic, biofilm-associated wounds.29
Despite these promising outcomes, further work is warranted. Optimization of the nanoparticle release profile could allow for stage-specific healing support. Moreover, studies evaluating the efficacy of BgNH against a wider array of pathogens-including gram-positive bacteria and fungi-would broaden its clinical applicability, especially in polymicrobial infections such as diabetic foot ulcers and burn wounds.30,31
Finally, while in vitro cytotoxicity results were favorable, long-term in vivo safety studies are essential. These should assess the potential accumulation of AgNPs in tissues and evaluate systemic toxicity to ensure a robust safety profile for clinical use. Such investigations will be crucial in transitioning BgNH from a promising experimental formulation to a viable clinical therapeutic.32
Biogenic nano-hydrogels (BgNH) incorporating silver nanoparticles show great promise as a multifunctional therapeutic for chronic wounds. The hydrogel’s strong antibiofilm properties, coupled with its ability to enhance wound healing, make it a valuable alternative to conventional wound care treatments. Further research is required to optimize BgNH formulations and assess long-term safety for clinical use.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the Department of Microbiology, Bhavan’s Vivekananda College of Science, Humanities and Commerce, for their support and for providing research seed funding that enabled the successful completion of this project. The authors also appreciate the encouragement and resources provided by the college management.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
SA and JS conceptualized the study and planned the experiments. SA performed the experimental work. SA and JS wrote, reviewed and approved the final manuscript for publication.
FUNDING
This study was supported by the Bhavan’s Vivekananda College for Science, Humanities and Commerce, Sainikpuri, for the animal studies through the Research Seed Money vide grant number 600/BVC/MRP-Seed Money/2022-23.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Animal Ethics Committee (IAEC), Jeeva’s Life Sciences, Telangana, India, vide IAEC Number CPCSEA/IAEC/JLS/19/02/23/171.
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314-321.
Crossref - Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49(1):35-43.
Crossref - Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736-1743.
Crossref - Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27(1):76-83.
Crossref - Zhao X, Wu H, Guo B, Dong R, Qiu Y, Ma PX. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials. 2017;122:34-47.
Crossref - Orhan G, Bayram A, Zer Y, Balci I. Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis. J Clin Microbiol. 2005;43(1):140-143.
Crossref - Skopinska-Wisniewska J, Górecka M, Kostrzewa P, Ginalska G. Hybrid polymeric materials with embedded biogenic nanoparticles for biomedical applications. Mater Sci Eng C Mater Biol Appl. 2024; 150:114666.
Crossref - He J, Feng Y, Wang Y, et al. Injectable self-healing hydrogels for tissue engineering and wound healing applications. Mater Sci Eng C Mater Biol Appl. 2023;139:113539.
Crossref - Wang QQ, Liu Y, Zhang HM, et al. Structural characterization and mechanical properties of hydrogels with tunable hierarchical porous structures. Carbohydr Polym. 2019;208:34-44.
Crossref - Ghaffari M, Dehghan G, Baradaran B, et al. Green synthesis and characterization of silver nanoparticles using Diospyros lotus extract and their antibacterial activity. Micron. 2019;117:88-94.
Crossref - SS, More SR, Kumbhar BV, et al. Evaluation of cytotoxicity and cellular uptake of biogenic silver nanoparticles in mammalian cell lines. J Drug Deliv Sci Technol. 2025;82:105412.
Crossref - Moradhaseli S, Johari-Ahar M, Roudkenar MH, et al. Cytotoxic effect of silver nanoparticles on mouse embryonic fibroblast and MCF-7 cell lines. Iran J Biotechnol. 2013;11(3):173-179.
Crossref - Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2):55-63.
Crossref - Sheba LA, Thomas R, Nair SV, et al. Evaluation of wound healing efficacy of biogenic silver nanoparticles in Wistar rats: histopathological and biochemical studies. Int J Nanomedicine. 2023;18:1231-1244.
Crossref - Quazi A, Haldar S, Das S, et al. Evaluation of wound healing activity of silver nanoparticle-loaded hydrogel in animal models. J Drug Deliv Sci Technol. 2022;67:102910.
Crossref - Vargas Martínez F, Luna Martínez H, González C, et al. Silver nanoparticles incorporated in natural polymer-based hydrogel for enhanced wound healing. Int J Pharm. 2024;634:122890.
Crossref - Pereira LO, Oliveira JP, Silva PN, et al. Biogenic silver nanoparticle-loaded hydrogel with dual antibacterial and wound healing properties. Int J Biol Macromol. 2023;233:123456.
Crossref - Kimura Y, Tanaka R, Nakamura T, et al. Multifunctional nanocomposite hydrogels for biofilm disruption and accelerated wound healing. Acta Biomater. 2025;170:112-125.
Crossref - Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95-108.
Crossref - Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27(1):76-83.
Crossref - Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73(6):1712-1720.
Crossref - Morones JR, Elechiguerra JL, Camacho A, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16(10):2346-2353.
Crossref - Hu R, Wang Y, Li X, et al. Porous hydrogel-loaded silver nanoparticles for sustained antimicrobial activity and enhanced wound healing. Carbohydr Polym. 2024;316:121123.
Crossref - Boateng JS, Matthews KH, Stevens HN, Eccleston GM. Wound healing dressings and drug delivery systems: A review. J Pharm Sci. 2008;97(8):2892-2923.
Crossref - Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49(1):35-43.
Crossref - Wang QQ, Liu Y, Zhang HM, et al. Structural characterization and mechanical properties of hydrogels with tunable hierarchical porous structures. Carbohydr Polym. 2019;208:34-44.
Crossref - Moradhaseli S, Johari-Ahar M, Roudkenar MH, Nikkhah M, Zarei L. Cytotoxic effect of silver nanoparticles on mouse embryonic fibroblast and MCF-7 cell lines. Iran J Biotechnol. 2013;11(3):173-179.
Crossref - Balakrishnan B, Mohanty M, Umashankar PR, Jayakrishnan A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials. 2005;26(32):6335-6342.
Crossef - Percival SL, McCarty SM, Lipsky B. Biofilms and wounds: an overview of the evidence. Adv Wound Care (New Rochelle). 2015;4(7):373-381.
Crossref - Bisht GS, Chhillar AK, Gupta A, Prasad R. Antimicrobial and antibiofilm activity of silver nanoparticles synthesized from Candida utilis against multidrug-resistant pathogens. 3 Biotech. 2021;11(4):178.
Crossref - Lin T, Chen Y, Zhang Z, et al. Multifunctional silver nanoparticle-loaded hydrogel for the treatment of polymicrobial infected wounds. Bioact Mater. 2024;29:112-124.
Crossref - Zhang L, Wang Y, Zhao X, et al. Translational potential of silver nanoparticle-based hydrogels for clinical wound management: current status and future perspectives. Acta Biomater. 2024;170:98-111.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.