ISSN: 0973-7510
E-ISSN: 2581-690X
Natural carotenoids from green microalgae exhibit beneficial effects in treating health-related diseases, primarily due to their antioxidant capacity. Therefore, carotenoid-producing microalgae were isolated and characterized from aqueous samples in KwaZulu-Natal (South Africa) under photoautotrophic conditions. Three isolates were characterized comprehensively using light and electron microscopy. In addition, the growth of the three selected microalgae was evaluated under photoautotrophic and photoheterotrophic conditions. Based on the cytological characteristics, the three strains matched the genera Haematococcus, Parachlorella, and Scenedesmus, the latter two additionally confirmed via analysis of the 18S rRNA gene sequence at the genus level. Light and electron microscopy and UV-Vis spectroscopy confirmed pigment production by all three microalgal strains. Both isolate Parachlorella sp. AA1 and Scenedesmus sp. AA2 showed the highest growth rate when cultured with acetate (25 mM) photoheterotrophically, while the isolate Haematococcus sp. AA3 grew best with glucose (50 mM). In addition, all three isolates utilized salicylate as a carbon source under photoheterotrophic conditions, evidently resulting in increased carotenoid production in strains AA1 and AA3. The 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) antioxidant assay confirmed that methanol extracts of all three isolates contained carotenoids that can scavenge radicals, and thin layer chromatography (TLC) analysis showed that β-carotene and astaxanthin were formed by strain AA3 as main carotenoids.
Microalgae, Cytology, Carotenoid Antioxidants, 18S rRNA, Photoautotrophic and Photoheterotrophic growth, Radical Scavenging
Algae are widely distributed and commonly located in various aqueous habitats and soil.1 Most algae perform oxygenic photosynthesis to produce biomass. Still, numerous microalgae can use simple sugars or organic acids as an additional source of energy and carbon with or without illumination. Green microscopic algae have been studied for the production of valuable chemicals such as pigments.2 Such pigments, which have attracted commercial interest, are astaxanthin, β-carotene, and lutein.3 Among the known microalgal producers of the prominent antioxidant carotenoid astaxanthin, Ankistrodesmus braunii (now Chlorolobion braunii), Chlorella zofingiensis (now Chromochloris zofingiensis), Dunaliella salina, Coelastrella rubescens, and Scenedesmus vacuolatus are well-known for producing large quantities of this pigment under mixotrophic growth conditions, albeit Haematococcus pluvialis is considered the most potent producer.3,4 However, cytological changes during the formation of astaxanthin in this microalga vary from other producers, such as Dunaliella spp., Parachlorella spp., Chlorolobion spp, and Scenedesmus spp.5,6 Under favorable cultivation conditions, highly motile biflagellated green cells are characteristic in Haematococcus spp. Unfavorable conditions such as radiation or starvation induce the loss of motility and enlargement of cells, along with the formation of thick cell walls and the characteristic immotile red-colored haematocysts, indicating carotenoid accumulation. The aplanospores of Haematococcus spp. synthesize considerable amounts of astaxanthin, mainly as mono and diesters.7 However, in Scenedesmus spp. varying illumination, nutrient deprivation, and redox stress induce cell colorization from green to brick red, indicating an accumulation of carotenoids protecting the photosynthetic apparatus from photo-oxidative damage under stress conditions such as high light intensity.8 While Chlorella spp. may contain up to 0.7% and Scenedesmus spp. up to 0.3% of its dry weight of the carotenoid astaxanthin, in Haematococcus spp. astaxanthin can reach up to 4% of the dry weight.5,8 Generally, microscopic green algae can be cultured autotrophically in open or closed photobioreactors or heterotrophically in conventional bioreactors when supplemented with additional sources of carbon. The latter cultivation approach enables increased biomass yields and carotenoid production by implementing appropriate growth conditions.9
Thus far, few studies on the isolation and characterization of green microalgae intended for the biotechnological production of beneficial carotenoids with antioxidant potential, like the carotenoid astaxanthin, are available from South Africa. Therefore, this study assessed the antioxidant potential of newly isolated carotenoid-producing microalgae from KwaZulu-Natal, South Africa.
Microalgae isolation and cultivation
Msunduzi river water samples collected in sterile Schott glass bottles (Pietermaritzburg, KwaZulu-Natal, South Africa, S 29°37’27.40″, E 30°22’22.49″) yielded two freshwater green algal isolates. An additional third isolate was obtained from a local bird bath displaying the typical reddish coloration demonstrating a possible occurrence of Haematococcus spp. Defined media, according to Bourrelly,10 Chu,11 and Bristol,12 were used to enrich and isolate microalgae. Decimal dilutions of samples in sterile medium were spread plated onto solidified medium followed by incubation at 20±1°C with constant illumination for 10 days (40 µmol photons × m-2 × s-1). Axenic cultures of three distinct green microalgal isolates were established and maintained via sequential purification, subculturing, and regular monitoring.
Microbiological characterization
The cells of the three axenic green microalgal isolates were analyzed microscopically using an Axioscope A1 (Zeiss, Germany) or a Motic BA 310 (China). Aqueous potassium iodide (0.2 % w/v, 30 minutes) was used to microscopically establish the number of pyrenoids in individual cells of the three isolates.13
Electron Microscopy
Cells were collected from individual microalgal colonies, soaked in millipore water, fixed in glutaraldehyde (3% v/v, Sigma-Aldrich, USA), and washed in a sodium cacodylate buffer (pH 7.4, 50 mM, Sigma-Aldrich, USA), post-fixed in osmium tetroxide (2% w/v, Sigma-Aldrich, USA), and serially dehydrated using ethanol (10/30/50/70/100%) and propylene oxide (Sigma-Aldrich, USA), with final embedding in Spurr (50/50) resin (Sigma-Aldrich, USA).14 Embedded microalgal cells were sectioned (EM UC7 ultramicrotome, Leica, Germany), followed by staining with uranyl acetate (2% w/v), and analyzed (JEOL 1400 TEM, Japan). For SEM analysis, cells of the three isolates were fixed with glutaraldehyde (3% v/v), dehydrated using ethanol as above and carbon dioxide critical point drying (Quorum K850), and analyzed (Zeiss EVOLS 15, Germany) after sputter coating with gold.
Phylogenetic analysis of selected isolates
As isolate AA3 was clearly identified as a member of the genus Haematococcus due to its distinct cellular features and its unique lifecycle, the sequence of the partial 18S rRNA gene of isolate AA1 and AA2 was analyzed after amplification employing two different primer pairs (S35DB, S35DC; S3F24, S3F25) to confirm assignment at the genus level.15,16 The microalgal DNA was extracted from cells using a micro prep kit (D6007, Zymo, USA). The final reaction mix contained 10 µM of each primer, 12.5 µl Taq master mix (Thermo, USA), 10µl nuclease-free water, and 1.5 µl DNA template. Amplification was done using 2 minutes of initial denaturation at 94°C and 35 cycles of 30 seconds denaturation at 94°C, annealing for 60 seconds at 60°C, extension for 60 seconds at 72°C, and finalized using 7 minutes extension at 72°C, with 4°C holding temperature. The size of the amplicons (⁓850bp and ⁓1400bp) was determined using agarose gel electrophoresis and ethidium bromide staining, as reported previously.17 The sequences obtained by bidirectional sequencing of amplicons (CAF, Stellenbosch University, South Africa) were assembled into sequence contigs using Geneious prime.17 The resulting sequences for the two isolates AA1 and AA2 were compared with 18S rRNA gene sequences for closely related Parachlorella and Scenedesmus strains available in GenBank (www.ncbi.nim.nih.gov) using the basic local alignment search tool (BLAST). Clustal W (used to establish sequence alignments) and the neighbor-joining algorithm implemented under Mega X (V10)18 were used to generate phylogenetic trees for strains AA1 and AA2. In addition, the maximum likelihood algorithm implemented under Mega X (V10)18 was used to verify tree topologies. The sequences of Ochromonas danica SAG 933.7 (GU 935657.1) and Chlamydomonas reinhardtii UTEX 90 (AB 511834.1) served as out-group for isolate AA1 and AA2, respectively. The consensus sequences obtained for strains AA1 and AA2 were deposited with GenBank under accession numbers MT 984304 and MT 984303.
Growth of isolates
For photoautotrophic growth, 100 ml Erlenmeyer flasks with 20 ml sterile medium were inoculated to an initial cell density of about 3 × 106 cells/ml. For isolate AA1 and AA2, the defined medium described by Chu11 was used, while isolate AA3 was grown in the defined medium described by Bourrelly.10 The inoculated cultures were incubated under continuous illumination (40µmol photons × m-2 × s-1) at 20 ± 1°C over a period of 14 days with shaking at 100 rpm. 20 µL culture samples were collected at 12-hour intervals for cell counts using a Neubauer counting chamber (Wertheim, Germany) and bright field light microscopy. For photoheterotrophic growth, the sterile defined medium was supplemented with either 25 mM sodium-acetate or 50 mM glucose as a carbon source.
Total carotenoid content was evaluated for the algal cultures by taking 500 µL from each flask at 12 hourly intervals. Algal cells were harvested by centrifuging at 10,000 × g for 5 min, and microalgae cell pellets were washed twice using millipore water. The harvested cells were broken using 0.3 mm glass beads, extracted using 1ml acetone, and centrifuged at 10,000 × g for 5 min. The total carotenoid content in acetone extracts was determined using a Shimadzu UV-VIS spectrophotometer (UV mini1240, Japan) at 470 nm, as described by Lichtenthaler and Wellburn.19
For carotenoid analysis, algal cells at the stationary growth phase were harvested by centrifugation with two times washing (15 mins, 10,000 × g; millipore water was used to wash pellets) from photoautotrophically grown cultures of the three strains (200 ml culture flasks). The harvested biomass was broken using 0.3 mm diameter sterile glass beads, extracted using 1 ml acetone, and centrifuged for another 15 mins (10,000 × g). The extraction process was carried out thrice until cell debris lost coloration. The total carotenoid content in acetone extracts was evaluated using a Shimadzu UV-VIS spectrophotometer (UV 1800, Japan) between 400-700 nm.20
Effects of sodium salicylate on growth and carotenoid production
Sodium salicylate is known to boost the growth and carotenoid accumulation of green algae.21 To determine if salicylate supports the growth and carotenoid production of the three isolates, 100 ml Erlenmeyer flasks containing 20 ml of sterile defined medium supplemented with various concentrations of sodium salicylate (0, 0.5, and 1.5 mM) were inoculated in triplicate to an initial cell density of about 3 × 106 cells/ml. The medium described by Chu11 was used to cultivate AA1 and AA2, while isolate AA3 was grown in the culture medium described by Bourrelly.10 Growth and total carotenoid content were monitored as described above. To determine if the isolates could use added salicylate, cell-free sample volumes (1 ml) obtained during the growth analysis were used to assess the consumption of salicylate during the growth of isolate AA1, AA2, and AA3, respectively. Flasks containing salicylate and algae cells of all three isolates that were heat inactivated (90°C, 1 hour) and flasks without algal cells but salicylate served as abiotic controls. Each algae culture sample, including controls, was diluted 5-fold and subjected to spectral analysis, and the salicylate concentration was established using the molar extinction coefficient at 295 nm reported for this compound (e = 3840 L x mol-1 x cm-1).22
Analytical TLC
For isolate AA3, acetone extracts of cells were spotted on a 5 × 10 cm analytical silica gel thin layer chromatography plate (Merck, Germany). The TLC plates were analyzed in an enclosed chamber in the dark via hexane:acetone (70:30) (v/v) and acetone:petroleum ether (25:75) (v/v) as mobile phase.23,24 Authentic astaxanthin and β-carotene (Sigma-Aldrich, USA) were used as standard.
Determination of antioxidant capacity of cell extracts
The radical scavenging ability of cell extracts generated using methanol was estimated using the stable organic radical 1,1-diphenyl-2-picrylhydrazyl (DPPH), according to Blois.25 A stock solution (5 mM) of DPPH (Sigma-Aldrich) was dissolved in methanol. Ascorbic acid and β-carotene (Sigma-Aldrich, USA) were used as reference antioxidants. A 1 ml assay cuvette contained DPPH (100 µM) and various concentrations of ascorbic acid (0-2.5 mM) dissolved in methanol, cell extracts in methanol ranging from 0-900 µM based on the molar extinction coefficient of β-carotene at 450 nm of 136 400 L × mol ̄1 × cm ̄1.26 Controls containing only methanol and DPPH were used as blank. After 30 min of incubation without illumination, decolorization of the solution containing DPPH from violet to yellow was visually observed, while the absorbance was determined at 517 nm (Shimadzu, UV1800, Japan).25 The effect concentration (EC50) value was estimated as the concentration of ascorbic acid, β-carotene, and the carotenoids in extracts required to scavenge 50% DPPH compared to controls using only methanol.
Phenotypic Characteristics
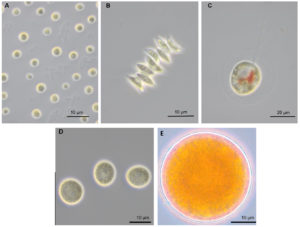
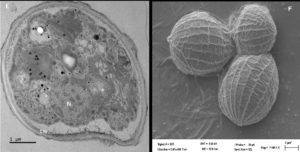
The morphological characterization of the three purified algal isolates by light microscopy showed that cells of strain AA1 were planktonic, ovoidal-shaped, and non-motile, ranging between 3-10µm, which is typical of Parachlorella spp. (Figure 1A). In addition, the cells contained a pyrenoid (Figure 2A) within the chloroplast, again a regular feature of Parachlorella spp. Based on the cell characteristics, strain AA1 was provisionally assigned to the genus Parachlorella.27 Isolate AA2 cells appeared spindle-shaped (8-15µm), non-motile, and planktonic (Figure 1B) with a single pyrenoid and cup-shaped chloroplast (Figure 2B). Cells of strain AA2 were fused together with nipple-shaped projections on the obtuse apices of strain AA2 cells. Isolate AA2 was, therefore, provisionally assigned to the genus Scenedesmus.28 These unique cell features differentiate strain AA2 from the cells of microalgae such as Coelastrum and Coelastropsis that are spherical and compact in shape with intracellular spaces.29 Cells of strain AA3 in young cultures were typically ellipsoidal-shaped, motile biflagellate cells (Figure 1C). In addition, cells contained a central pyrenoid (Figure 2C). Furthermore, the cells of isolate AA3 were undergoing a developmental growth cycle including different cytological stages as reported for the genus Haematococcus such as the formation of a non-motile vegetative cell stage (Figure 1D). As adverse conditions occur, cells of strain AA3 form the characteristic non-motile spherical carotenoid accumulating aplanospores (Figure 1E).30 The ellipsoidal-shaped flagellate motile cells are surrounded by a mucous envelope and are between 13-15 µm in diameter, and hematochrome (e.g., carotenoid) formation was visible in the protoplast (Figure 1C). In addition, the periplasmic space between the protoplast and the cell wall is connected by delicate radiating cytoplasmic strands, features present in isolate AA3 that are distinctive and typical of Haematococcus at the motile stage (Figure 1C).5,30
Figure 1. Typical cell shape of isolates AA1 (A), AA2 (B), and life cycle stages of AA3 (C, D, E) during the exponential (A, B, C) and the late stationary (D, E) growth phase under photoautotrophic conditions
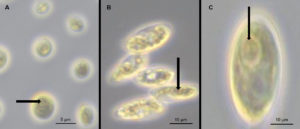
Figure 2. Detection of pyrenoids in cells of isolate AA1 (A), isolate AA2 (B), and isolate AA3 (C) after staining with 0.2% (w/v) aqueous potassium iodide by light microscopy. The arrow highlights the presence of a stained pyrenoid
Ultrastructural analysis
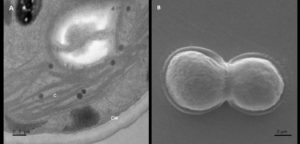
A smooth, double-layered cell wall was observed in isolate AA1 by TEM analysis (Figure 3A). The chloroplast stroma showed a single pyrenoid. These cytological features match the findings of Bock et al.31 for the genus Parachlorella. Scanning electron microscopy showed that isolate AA1 reproduces asexually via cell division (Figure 3B).
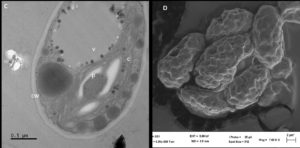
Thin sections of isolate AA2 proved that the cell wall had both an inner polysaccharide and an outer trilaminar layer (Figure 3C). The chloroplast was located near the periphery of isolate AA2 cells, containing the pyrenoid and starch grains scattered in the stroma (Figure 3C). Lipid bodies containing carotenoids were situated near the center of the cells, confirming carotenoid production by strain AA2. The SEM analysis further confirmed asexual reproduction via multiple fission as coenobia of isolate AA2 were formed during the exponential growth phase (Figure 3D), which agreed with an earlier report showing that a single mother cell can reproduce 2-16 coenobia in Scenedesmus spp.28
TEM analysis of thin sections of isolate AA3 revealed a thick electron-dense cell wall encompassing strain AA3 cells, matching a report by Hagen et al.32 Cytological analysis (Figure 3E) additionally confirmed the expected presence of a nucleus with partially condensed chromatin visible. As noted by Buchheim et al.33 Haematococcus spp. produces the carotenoid astaxanthin both in vegetative and late life cycle stages, thereby matching isolate AA3. The SEM analysis indicated asexual reproduction, as autospores of strain AA3 were detected (Figure 3F). The outer cell sheath surfaces showed protuberates and were irregularly wavy. Based on the observed distinct cytological features (e.g., the distinct and characteristic biflagellated motile cells) and the specific and unique lifecycle, strain AA3 was provisionally allocated to the genus Haematococcus.
Figure 3. Analyses of isolate AA1, AA2, and AA3 cells from the exponential growth phase by transmission and scanning electron microscopy. The symbol CW indicates the cell wall, C indicates chloroplasts, and P indicates a pyrenoid in AA1 (A), while the SEM micrograph indicates asexual reproduction by binary fission in isolate AA1 (B). The symbol CW indicates a double-layered cell wall; V indicates a vacuole, C indicates the chloroplast, and P represents a pyrenoid in AA2 (C), while the SEM micrograph shows asexual reproduction by multiple fission of cells in isolate AA2 (D). The symbol N indicates the nucleus with condensed chromatin, CW represents the cell wall with plasmalemma in AA3 (E), while the SEM micrograph indicates possible autospores (F)
Phylogenetic analysis of isolates AA1 and AA2
If morphological characteristics are not distinct and reliable enough, 18S rRNA gene sequence analysis is commonly applied for the phylogenetic characterization of green microalgae to confirm an assignment of isolates to genus level.33 Comparing the resulting sequences (18S rRNA) of the isolated green microalgae (isolate AA1 and AA2) to sequences deposited in GenBank showed the highest resemblance level of more than 99% for isolate AA1 to Parachlorella kessleri and for isolate AA2 to members of the family Scenedesmaceae, with more than 98% sequence similarity to members of the genera Scenedesmus, Coelastrum, and Coelastropsis, which is adequate to assign algal isolates to genus level.28,31 Therefore, isolate AA1 can be assigned tentatively to the genus Parachlorella with 100% sequence resemblance to Parachlorella kessleri strain SAG 211-11c (accession number KM 020114.1). Even though the sequence similarity of strain AA2 to Scenedesmus ovalternus with 98.51% (accession number X 81966.1) was similar to that for strains of the genera Coelastrum and Coelastropsis, isolate AA2 can be assigned tentatively to the genus Scenedesmus based on its distinct cell morphology. Toledo-Cervantes et al.34 highlighted the identification of Scenedesmus spp. based exclusively on the 18S rRNA sequence similarity is not always sufficient and recommends the further use of morphological characteristics. The unique spindle shape of isolate AA2 clearly differentiates it from the genera Coelastrum and Coelastropsis, the cells of which are spherical and compact in shape. According to Hegewald et al.,35 Coelastrum and Coelastropsis possess net-like ridges produced by an inner cellulosic cell wall. This morphological feature was not observed in strain AA2, thereby allowing for the assignment of strain AA2 to the genus Scenedesmus. The phylogenetic relationship of isolates AA1 and AA2 with type strains of Parachlorella kessleri, Scenedesmus ovalternus, and S. raciborskii is demonstrated in supplementary Figures S1 and S2.
Table:
Growth kinetic data for the three isolates AA1, AA2 and AA3 under photoautotrophic and photoheterotrophic growth conditions using acetate, glucose, and salicylate supplemented medium.
| Isolates | Photoautotrophic conditions | Photoheterotrophic conditions | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No supplement Specific growth rate (hr –1) | Generation time (Hrs) | Acetate (25mM) Specific growth rate (hr –1) |
Generation time (Hrs) | Glucose (50mM) Specific growth rate (hr –1) | Generation time (Hrs) | Salicylate (0.5mM) Specific growth rate (hr –1) | Generation time (Hrs) | Salicylate (1.5mM) Specific growth rate (hr –1) | Generation time (Hrs) | |
| Parachlorella sp. strain AA1 | 0.010±0.001 | 69 | 0.020±0.006 | 35 | 0.017±0.003 | 41 | 0.011±0.002 | 63 | 0.014±0.002 | 49 |
| Scenedesmus sp. strain AA2 | 0.011±0.004 | 63 | 0.019±0.005 | 36 | 0.012±0.025 | 58 | 0.012±0.005 | 58 | 0.016±0.045 | 43 |
| Haematococcus sp. strain AA3 | 0.009±0.001 | 72 | 0.015±0.035 | 46 | 0.016±0.005 | 43 | 0.012±0.045 | 58 | 0.010±0.004 | 69 |
Data shown are the means (± range) of two independently performed experiments.
Growth of the three isolates
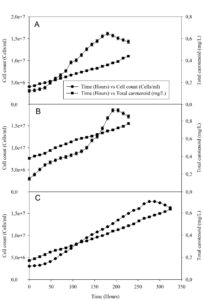
Under photoautotrophic conditions without an organic carbon source, isolate AA1 achieved a growth rate of 0.010 hr-1 with a generation time of 69 hours (Table, Figure 4A). Praveen et al.36 reported a two-fold lower growth rate of 0.005 hr-1 for Chlorella pyrenoidosa (now Auxenochlorella pyrenoidosa) under similar photoautotrophic conditions. Maroneze et al.37 recorded a specific growth rate of 0.023 hr-1 for Scenedesmus obliquus (now Tetradesmus obliquus), which is higher than the result for isolate AA2 (specific growth rate 0.011 hr-1, generation time 63 hours) under photoautotrophic conditions (Table, Figure 4B). Isolate AA3 grew under photoautotrophic conditions at 0.009 hr-1 (generation time 72 hours) (Table, Figure 4C), which is lower than the specific growth rate of 0.025 hr-1 reported previously by Giannelli et al.38 However, these differences are probably due to varying culture conditions, as constant illumination with 70 µmol photons × m-2 × s-1 was used by Giannelli et al.38 Under photoautotrophic conditions, the chlorophyll a concentration increased concomitantly with cell numbers, the highest chlorophyll a concentration of 14.48 µg/ml was observed for isolate AA3, followed by 12.50 µg/ml in isolate AA1, while isolate AA2 recorded the lowest chlorophyll concentration of 11.15 µg/ml (Figures S3.1, S3.2, S3.3). Comparable results were reported for Haematococcus pluvialis, with the chlorophyll a concentration increasing alongside astaxanthin accumulation.39 Similarly, under photoautotrophic conditions, carotenoid yields increased alongside cell numbers in Parachlorella sp. strain AA1, yielding 0.44 mg/L total carotenoids, followed by isolate AA2 with a total carotenoid yield of 0.81 mg/L, while isolate AA3 yielded 0.65 mg/L of total carotenoids (Figure 4). The total carotenoid yields observed for strains AA1 and AA2 were higher than data reported by De Jesus et al.40 for Parachlorella kessleri, while the carotenoid yield of 0.81 mg/L recorded for isolate AA2 was higher than the yields reported for Scendesmus almeriensis (now Tetradesmus almeriensis) under photoautotrophic conditions.41 Leonardi et al.42 reported a carotenoid yield of 0.63 mg/L for Haematococcus pluvialis, matching the result obtained for isolate AA3. Different organic carbon sources, like glucose, glycerol, salicylate, and acetate, are available to boost microalgae growth.7 The three algal isolates from KwaZulu-Natal showed higher growth rates under photoheterotrophic conditions supplemented with 25 mM of acetate compared to photoautotrophic growth without supplement (Table). Under photoheterotrophic conditions with acetate as a carbon source, isolate AA1 had the highest growth rate of 0.020 hr-1 (generation time 35 hours), isolate AA2 of 0.019 hr-1 (36 hours), and isolate AA3 of 0.015 hr-1 (46 hours) (Table, Figure S3.2). Heredia-Arroyo et al.43 reported a higher growth rate of 0.040 hr-1 in a 25 mM acetate-supplemented medium for Chlorella protothecoides (now Auxenochlorella protothecoides), while the growth rate recorded for isolate AA2 with acetate resembles the growth rate of 0.022 hr-1 reported for Scenedesmus obliquus (now Tetradesmus obliquus).44 Thus, acetate enhanced cell growth under moderate light intensity compared to photoautotrophic growth conditions. The enhanced growth rate of isolate AA3 in the presence of 25 mM acetate matches a report by Kobayashi et al.,45 showing similarly that 25 mM acetate enhanced the growth rate in Haematococcus pluvialis along with changes in morphological features. Acetate increased the chlorophyll a concentration to 17.80 µg/ml in isolate AA1 and 18.28 µg/ml in isolate AA2 (Figure S3.2). Acetate, however, reduced the chlorophyll a concentration in strain AA3 (Figure S3.2), with only 7.54 µg/ml chlorophyll a observed for isolate AA3. The results for strain AA1 agreed with the study by Papageorgiou46 for Chlorella pyrenoidosa (now Auxenochlorella pyrenoidosa), while the result for isolate AA2 matches the reported chlorophyll a yield data for Scenedesmus dimorphus (now Tetradesmus dimorphus).47 Growth analysis of photoheterotrophic cultures of different green algae, such as Parachlorella spp., Ankistrodesmus spp., Haematococcus spp., Dunaliella spp., and Scenedesmus spp., demonstrated that growth kinetics are strain dependent and affected by the light intensity.48 Isolate AA1, when grown photoheterotrophically with glucose supplement (0.017 hr-1), achieved a higher growth rate than isolate AA2 (0.012 hr-1) and was similar to that of isolate AA3 (0.016 hr-1) (Table, Figure S3.3). A study on the assimilation of glucose and carbon dioxide in Chlorella vulgaris by Martinez and Orus49 showed that glucose enhanced growth rate and biomass yield, similar to the present study, suggesting that acetate stimulated the vegetative growth of strains AA1, AA2, and AA3, while glucose specifically stimulated the growth of strain AA3. Similar results were reported by Kim et al.50 for Haematococcus pluvialis. A total chlorophyll a content of 22.01 µg/ml was achieved by isolate AA1 when grown in the presence of glucose, which is higher than the 17.15 µg/ml reported by Amin et al.51 for Chlorella vulgaris (Figure S3.3). The highest total chlorophyll content of 22.40 µg/ml was recorded for Haematococcus sp. strain AA3, agreeing with Kim et al.,50 reporting a chlorophyll a content of 19.8 µg/ml for Haematococcus pluvialis.
Effects of salicylate on carotenoid yields
Growth with salicylate (0.5 mM, 1.5 mM) under photoheterotrophic conditions in isolate AA1 led to optimum specific growth rates of 0.011 hr-1 and 0.014 hr-1, matching generation times of 63 and 49 hours (Table). This is lower than the specific growth rate of 0.087 hr-1 reported by Martinez et al.52 for Chlorella pyrenoidosa (now Auxenochlorella pyrenoidosa) in 1.5 mM salicylate-supplemented medium. However, the difference might be due to varying experimental conditions (e.g., illumination). The total carotenoid yield of 1.30 mg/L achieved for isolate AA1 is higher than the total carotenoid concentration of 0.72 mg/L reported by Heo et al.53 for Parachlorella kessleri (Figure S4.1C). For strain AA2, the results showed that 1.5 mM salicylate stimulated better growth (0.016 hr-1, generation time 43 hours) than a control without salicylate (figure S4.2, Table), and the highest carotenoid yield of about 0.99 mg/L was recorded with cultures supplemented with 1.5 mM salicylate (Figure S4.2C). This observation corresponded with Guedes et al.54 reporting a carotenoid productivity of 0.84 mg/L for Scenedesmus obliquus (now Tetradesmus obliquus) but is two-fold higher than the 0.4 mg/L earlier reported by Bao et al.9 for Scenedesmus dimorphus (now Tetradesmus dimorphus). Depending on cultivation conditions, salicylate, similar to other simple aromatic compounds, can serve as a carbon source for microscopic algae, increasing cell growth and inducing carotenoid accumulation.55 For isolate AA3, a low concentration of salicylate (0.5 mM) enabled a specific growth rate of 0.012 hr-1, a generation time of 58 hours, and a carotenoid yield of about 1.20 mg/L; this is more than the growth rate of 0.009 hr-1, the generation time of 72 hours and carotenoid yield of 0.65 mg/L under photoautotrophic conditions (Table, Figure 4C). These results for isolate AA3 agreed with Gao et al.,56 reporting that salicylate stimulated carotenoid accumulation in Haematococcus pluvialis compared to photoautotrophic conditions. Hu et al.21 showed that sodium salicylate at similar concentrations (e.g., 0.5 to 1mM) was removed from active cultures and enhanced cell growth and production of carotenoid in Haematococcus pluvialis, matching results for isolate AA3 as cultures supplemented with 1.5mM salicylate achieved a carotenoid yield of about 1.36 mg/L (Figure S4.3C), thus similar to earlier carotenoid yield data reported by Cifuentes et al.57 for Haematococcus pluvialis.
Figure 4. The growth and carotenoid production of strain AA1 (A), strain AA2 (B), and strain AA3 (C) under photoautotrophic conditions in Chu (A, B) and Bourelly medium (C). All data points shown are the means of two independently performed experiments. The bars shown indicate the range of the mean.
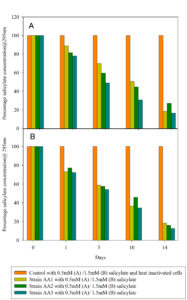
For cultures of isolates AA1, AA2, and AA3 supplemented with 0.5 mM salicylate, the initial concentration of salicylate decreased by ˃82% (AA1), ˃73% (AA2), ˃84% (AA3) after 14 days (Figure 5A). Similarly, the initial concentration of 1.5mM salicylate decreased by ˃82%, ˃84%, and ˃87% for strains AA1, AA2, and AA3 after 14 days incubation (Figure 5B). The control with heat-inactivated algae cells of strains AA1, AA2, and AA3 and salicylate indicated that the salicylate concentration did not change in the absence of metabolically active cells. Therefore, only in the presence of active cultures of strains AA1, AA2 and AA3 was salicylate eliminated from the medium. This finding matches a report of Fu et al.58 wherein salicylate was applied as a carbon source and induced carotenoid production in Chlorella regularis (now Coelastrum microporum). Furthermore, Kovacik et al.59 showed that Scenedesmus quadricauda utilized salicylate as carbon source to produce carotenoids.
Similarly, Scenedesmus sp. strain AA2 decreased the initial salicylate concentration in both 0.5 mM and 1.5 mM salicylate supplemented medium. Hu et al.56 showed that salicylate served as source of carbon for Haematococcus pluvialis, improving cell growth and boosting astaxanthin accumulation, matching our observation that Haematococcus sp. strain AA3 utilized salicylate for cell growth and carotenoid production. Haematocococcus pluvialis has been reported to use carbon sources like glycerol, glucose, and acetate to boost astaxanthin production under photoheterotrophic conditions.44 However, while salicylate was categorized by Gao et al.55 as a phytohormone, inducing increased astaxanthin biosynthesis in Haematococcus pluvialis, our results show that active cells of isolate AA3 utilized more than 80% of available salicylate as a carbon source for biomass and carotenoid production (Figure 5, S4.3). Therefore, salicylate can function as a carbon source to enhance carotenoid biosyntheses like acetate and glucose.
Figure 5. The photoheterotrophic growth of strains AA1, AA2, and AA3 with 0.5mM (A) and 1.5mM salicylate (B) as the sole carbon source over 14 days. The controls contain 0.5mM or 1.5mM salicylate and heat-inactivated cells of strains AA1, AA2, and AA3. The values shown are the means of two independently performed experiments
Pigment Production
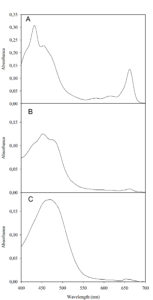
Acetone extracts of cells of isolate AA1 exhibited a spectrum with absorbance maxima of 450 and 665nm (Figure 6A), matching the spectral properties for β-carotene and chlorophyll a in acetone.60 The spectral characteristics of acetone cell extracts of isolate AA2 (Figure 6B) revealed a higher β-carotene (A450 nm) to chlorophyll a (665 nm) ratio than isolate AA1, matching spectral characteristics previously reported by Qin et al.8 for Scenedesmus spp. The spectral characteristics of acetone extracts from cells of isolate AA3 (Figure 6C) revealed low chlorophyll a content based on the absorbance at 665 nm and demonstrated astaxanthin production, as astaxanthin shows an absorption maximum at 474 nm in acetone.20 The absorption spectrum revealed a ratio of about 10 for astaxanthin (474 nm) to 1 for chlorophyll a (665 nm) (Figure 6C). Similar ratios were reported by Kim et al.49 for astaxanthin and chlorophyll a in a strain of Haematococcus pluvialis. The absorption spectrum of TLC-purified astaxanthin from strain AA3 matched the spectrum for authentic astaxanthin (Figure S5A and B) and spectral data reported by Dragos et al.20 for astaxanthin.
Figure 6. The visible absorption spectrum of diluted acetone extracts of late stationary cells of strain AA1 (A), strain AA2 (B), and strain AA3 (C) after photoautotrophic growth
TLC analysis of strain AA3 extracts
Two major bands were detected in strain AA3 acetone cell extracts. Based on the Rf values obtained when using two different mobile phases, both β-carotene and astaxanthin were identified when compared to authentic β-carotene and astaxanthin (Table S1), additionally matching results reported previously.22,23
Evaluation of antioxidant activity
As expected, vitamin C scavenged the DPPH radical with an EC50 value of about 10 µM, which is in agreement with the value of 10.2 µM reported by Mishra et al.61 Burton and Gold62 reported β-carotene as an effective radical quencher with an EC50 of 50 µM for DPPH, matching our experimental results for β-carotene with an EC50 value of 50 µM. Methanolic cell extracts of isolate AA1 could quantitatively decolorize DPPH (100 µM) within 30 mins of incubation, demonstrating that cells of isolate AA1 contain carotenoids possessing antioxidant capacity, with the EC50 value estimated as 330 µM. Comparable results were reported recently63 for Parachlorella kessleri methanol extracts with an estimated EC50 of 215 µM.63 Based on β-carotene content, Scenedesmus sp. strain AA2 methanol cell extracts yielded an estimated EC50 of 280 µM, likewise Haematococcus sp. strain AA3 extracts neutralized DPPH with an estimated EC50 of 264 µM, matching earlier results showing that Haematococcus pluvialis methanol extracts possessed substantial antioxidative capacity.64 The scavenging of DPPH by methanol extracts of strain AA3 and the TLC-purified astaxanthin with an estimated EC50 value of 100 µM is expected as astaxanthin is considered a more efficient radical scavenger than β-carotene; comparable data were reported by Oh et al.65 with an estimated EC50 value of 105 µM for astaxanthin using the DPPH assay.
This study demonstrated that potent carotenoid-producing algal strains representing different algal genera, such as Parachlorella, Scenedesmus, and Haematococcus, could be isolated from local aqueous samples in KwaZulu-Natal, South Africa. All the microalgal isolates obtained in the current study evidently used salicylate under photoheterotrophic conditions as a carbon source and produced carotenoids exhibiting antioxidant properties. Thus, these isolates might have the potential to provide such carotenoids as food supplements to tackle diseases caused by radicals.
Additional file: Additional Table S1 and Figures S1-S5.
ACKNOWLEDGMENTS
The authors would like to acknowledge the financial support received from the National Research Foundation.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Both authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was supported by the National Research Foundation with NRF Grant No 132700, SS.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript and/or in the supplementary files.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
- Schlichting Jr HE. Survival of some fresh-water algae under extreme environmental conditions. Trans Am Microsc Soc. 1974;93(4):610-613.
Crossref - Wang J, Hu X, Chen J, Wang T, Huang X, Chen G. The extraction of β-Carotene from microalgae for testing their health benefits. Foods. 2022;11(4):502.
Crossref - Sathasivam R, Radhakrishnan R, Hashem A, Abd_Allah EF. Microalgae metabolites: A rich source for food and medicine. Saudi J Biol Sci. 2019;26(19):709-722.
Crossref - Wongsnansilp T, Yokthongwattana K, Roytrakul S, Juntawong N. β-carotene production of UV-C induced Dunaliella salina under salt stress. J Pure Appl Microbiol. 2019;13(1):193-200.
Crossref - Wayama M, Ota S, Matsuura H, Nango N, Hirata A, Kawano S. Three-dimensional ultrastructural study of oil and astaxanthin accumulation during encystment in the green alga Haematococcus pluvialis. PloS one. 2013;8(1):e53618.
Crossref - Othman FS, Jamaluddin H, Ibrahim Z, Hara H, Yahya NA, Mohamad KISE. Production of a-linolenic acid by an oleaginous green algae Acutodesmus obliquus isolated from Malaysia. J Pure Appl Microbiol. 2019;13(3):1297-1306.
Crossref - Li Q, Li B, Li J. The dynamic behaviors of photosynthesis during non-motile cell germination in Haematococcus pluvialis. Water. 2022;14(8):1280.
Crossref - Qin S, Liu G-X, Hu Z-Y. The accumulation and metabolism of astaxanthin in Scenedesmus obliquus (Chlorophyceae). Process Biochem. 2008;43(8):795-802.
Crossref - Bao B, Thomas-Hall SR, Schenk PM. Fast-tracking isolation, identification and characterization of new microalgae for nutraceutical and feed applications. Phycology. 2022;2(1):86-107.
Crossref - Bourrelly P. Les Algues d’Eau Douce. Initiation a la systematiques. Tome 11. Les Algues Jaunes et Brunes. Chrysophycees, Pheophycees, Xanthophycees et Diatomees. Boubee and Cie. 1968: 438.
- Chu S. The influence of the mineral composition of the medium on the growth of planktonic algae: part I. Methods and culture media. J Ecol. 1942;30(2):284-325.
Crossref - Bristol BM. On the retention of vitality by algae from old stored soils. New Phytologist. 1919;18(3-4):92-107.
Crossref - Lang NJ. Electron microscopy of the Volvocaceae and Astrephomenaceae. Am J Bot. 1963;50(3):280-300.
Crossref - Spurr AR. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969;26(1-2):31-43.
Crossref - Chekanov K, Lobakova E, Selyakh I, Semenova L, Sidorov R, Solovchenko A. Accumulation of astaxanthin by a new Haematococcus pluvialis strain BM1 from the white sea coastal rocks (Russia). Marine Drugs. 2014;12(8):4504-4520.
Crossref - Katana A, Kwiatowski J, Spalik K, Zakrys B, Szalacha E, Szymanska H. Phylogenetic position of Koliella (Chlorophyta) as inferred from nuclear and chloroplast small subunit rDNA. J Phycol. 2001;37(3):443-451.
Crossref - Claussen M, Schmidt S. Differentiation of Basidiobolus spp. isolates: RFLP of a diagnostic PCR amplicon matches sequence-based classification and growth temperature preferences. J Fungi. 2021;7(2):110.
Crossref - Kumar S, Stecher G, Tamura K. MEGA 10: Molecular evolutionary genetics analysis version 10.0 for bigger datasets. Mol Biol Evol. 2018;35(6):1547-1549.
Crossref - Lichtenthaler HK, Wellburn AR. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans. 1983;603:591-603
Crossref - Dragos N, Bercea V, Bica A, Druga B, Nicoara A, Coman C. Astaxanthin production from a new strain of Haematococcus pluvialis grown in batch culture. Ann Rom Soc Cell Biol. 2010;15(2):353-361.
- Hu Q, Song M, Huang D, Hu Z, Wu Y, Wang C. Haematococcus pluvialis accumulated lipid and astaxanthin in a moderate and sustainable way by the self-protection mechanism of salicylic acid under sodium acetate stress. Front Plant Sci. 2021;12:763742-763742.
Crossref - Ungar G, Damgaard E, Wong W. Determination of salicylic acid and related substances in serum by ultraviolet spectrophotometry. Proc Soc Exp Biol Med. 1952;80(1):45-47.
Crossref - Khanafari A, Saberi A, Azar M, Vosooghi G, Jamili S, Sabbaghzadeh B. Extraction of astaxanthin esters from shrimp waste by chemical and microbial methods. J Environ Health Sci Eng. 2007;4(2):93-98.
- Jaime L, Rodriguez-Meizoso I, Cifuentes A, Santoyo S, Suarez S, Ibanez E, Senorans FJ. Pressurized liquids as an alternative process to antioxidant carotenoids extraction from Haematococcus pluvialis microalgae. Food Sci Technol. 2010;43(1):105-112.
Crossref - Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199-1200.
Crossref - Craft NE, Soares JH. Relative solubility, stability, and absorptivity of lutein and beta-carotene in organic solvents. J Agric Food Chem. 1992;40(3):431-434.
Crossref - Skaloud P, Nemcova Y, Pytela J, Bogdanov NI, Bock C, Pickinpaugh SH. Planktochlorella nurekis gen. et sp. nov. (Trebouxiophyceae, Chlorophyta), a novel coccoid green alga carrying significant biotechnological potential. Fottea. 2014;14(1):53-62.
Crossref - Kessler E, Schafer M, Hummer C, Kloboucek A, Huss V. Physiological, biochemical, and molecular characters for the taxonomy of the subgenera of Scenedesmus (Chlorococcales, Chlorophyta). Botanica Acta. 1997;110(3):244-250.
Crossref - Lukavsky J. Coelastrum pascheri sp. n., a new green alga from lakes of the Bohemian Forest. Biologia. 2006;61(20):485- 490.
Crossref - Pegg C, Wolf M, Alanagreh LA, Portman R, Buchheim MA. Morphological diversity masks phylogenetic similarity of Ettlia and Haematococcus (Chlorophyceae). Phycologia. 2015;54(4):385-397.
Crossref - Bock C, Krienitz L, Proeschold T. Taxonomic reassessment of the genus Chlorella (Trebouxiophyceae) using molecular signatures (barcodes), including description of seven new species. Fottea. 2011;11(2):293-312.
Crossref - Hagen C, Siegmund S, Braune W. Ultrastructural and chemical changes in the cell wall of Haematococcus pluvialis (Volvocales, Chlorophyta) during aplanospore formation. Eur J Phycol. 2002;37(2):217-226.
Crossref - Buchheim MA, Sutherland DM, Buchheim JA, Wolf M. The blood alga: phylogeny of Haematococcus (Chlorophyceae) inferred from ribosomal RNA gene sequence data. Eur J Phycol. 2013;48(3):318-329.
Crossref - Toledo-Cervantes A, Solorzano GG, Campos JE, Martinez-Garcia M, Morales M. Characterization of Scenedesmus obtusiusculus AT-UAM for high-energy molecules accumulation: deeper insight into biotechnological potential of strains of the same species. Biotechnol Rep. 2018;17:16-23.
Crossref - Hegewald E, Bock C, Krienitz L. A phylogenetic study on Scenedesmaceae with the description of a new species of Pectinodesmus and the new genera Verrucodesmus and Chodatodesmus (Chlorophyta, Chlorophyceae). Fottea. 2013;13(2):14.
Crossref - Praveen K, Abinandan S, Natarajan R, Kavitha M. Biochemical responses from biomass of isolated Chlorella sp., under different cultivation modes: non-linear modelling of growth kinetics. Braz J Chem Eng. 2018;35:489-496.
Crossref - Maroneze MM, Zepka LQ, Lopes EJ, Perez-Galvez A, Roca M. Chlorophyll oxidative metabolism during the phototrophic and heterotrophic growth of Scenedesmus obliquus. Antioxidants. 2019;8(12):600.
Crossref - Giannelli L, Yamada H, Katsuda T, Yamaji H. Effects of temperature on the astaxanthin productivity and light harvesting characteristics of the green alga Haematococcus pluvialis. J Biosci Bioeng. 2015;119(3):345-350.
Crossref - Lababpour A, Lee C-G. Simultaneous measurement of chlorophyll and astaxanthin in Haematococcus pluvialis cells by first-order derivative ultraviolet-visible spectrophotometry. J Biosci Bioeng. 2006;101(2):104-110.
Crossref - De Jesus PdCC, Mendes MA, Perpetuo EA, Basso TO, do Nascimento CAO. Extracellular carotenoid production and fatty acids profile of Parachlorella kessleri under increased CO2 concentrations. J Biotechnol. 2021;329:151-159.
Crossref - Di Caprio F, Visca A, Altimari P, Toro L, Masciocchi B, Iaquaniello G, Pagnanelli F. Two stage process of microalgae cultivation for starch and carotenoid production. Chem Eng Trans. 2016;49:415-420.
- Leonardi RJ, Ibanez MV, Morelli MN, Heinrich JM. Evaluation of the phototrophic growth of Haematococcus pluvialis under outdoor lighting conditions inside a bubble column reactor at a laboratory scale. Algal Research. 2022;66:102800.
Crossref - Heredia-Arroyo T, Wei W, Hu B. Oil accumulation via heterotrophic and mixotrophic growth of Chlorella protothecoides. Appl Biochem Biotechnol. 2010;162(7):1978-1995.
Crossref - Combres C, Laliberte G, Reyssac JS, De la Noue J. Effect of acetate on growth and ammonium uptake in the microalga Scenedesmus obliquus. Physiologia Plantarum. 1994;91(4):729-734.
Crossref - Kobayashi M, Kurimura Y, Tsuji Y. Light-independent, astaxanthin production by the green microalga Haematococcus pluvialis under salt stress. Biotechnol Lett. 1997;19(6):507-509.
Crossref - Papageorgiou G. Light-induced changes in the fluorescence yield of chlorophyll a in Vivo: II. Chlorella pyrenoidosa. Biophys J. 1968;8(11):1316-1328.
Crossref - Soares J, Loterio RK, Rosa RM, et al. Scenedesmus sp. cultivation using commercial-grade ammonium sources. Ann Microbiol. 2018;68(1):35-45.
Crossref - Sansawa H, Endo H. Production of intracellular phytochemicals in Chlorella under heterotrophic conditions. J Biosci Bioeng. 2004;98(6):437-444.
Crossref - Martinez F, Orus MI. Interactions between glucose and inorganic carbon metabolism in Chlorella vulgaris strain UAM 101. Plant Physiology. 1991;95(4):1150-1155.
Crossref - Kim JY, Lee C, Jeon MS, Park J, Choi Y-E. Enhancement of microalga Haematococcus pluvialis growth and astaxanthin production by electrical treatment. Bioresour Technol. 2018;268:815-819.
Crossref - Amin M, Chetpattananondh P, Khan M, Mushtaq F, Sami S. Extraction and quantification of chlorophyll from microalgae Chlorella sp. IOP Conference Series: Materials Science and Engineering. 2018:012025.
Crossref - Martinez ME, Camacho F, Jimenez J, Espinola J. Influence of light intensity on the kinetic and yield parameters of Chlorella pyrenoidosa mixotrophic growth. Process Biochem. 1997;32(2), 93-98.
Crossref - Heo J, Shin D-S, Cho K, Cho D-H, Lee YJ, Kim H-S. Indigenous microalga Parachlorella sp. JD-076 as a potential source for lutein production: Optimization of lutein productivity via regulation of light intensity and carbon source. Algal Research. 2018;33:1-7.
Crossref - Guedes AC, Amaro HM, Pereira RD, Malcata FX. Effects of temperature and pH on growth and antioxidant content of the microalga Scenedesmus obliquus. Biotechnol Progress. 2011;27(5):1218-1224.
Crossref - Kozlova TA, Hardy BP, Krishna P, Levin DB. Effect of phytohormones on growth and accumulation of pigments and fatty acids in the microalgae Scenedesmus quadricauda. Algal Research. 2017;27:325-334.
Crossref - Gao Z, Meng C, Zhang X, et al. Induction of salicylic acid (SA) on the transcriptional expression of eight carotenoid genes and astaxanthin accumulation in Haematococcus pluvialis. Enzyme Microb Technol. 2012;51(4):225-230.
Crossref - Cifuentes AS, Gonzalez MA, Vargas S, Hoeneisen M, Gonzalez N. Optimization of biomass, total carotenoids and astaxanthin production in Haematococcus pluvialis flotow strain steptoe (Nevada, USA) under laboratory conditions. Biol Res. 2003;36(3-4):343-357.
Crossref - Fu L, Li Q, Chen C, et al. Benzoic and salicylic acid are the signaling molecules of Chlorella cells for improving cell growth. Chemosphere. 2021;265:129084.
Crossref - Kovacik J, Klejdus B, Hedbavny J, Backor M. Effect of copper and salicylic acid on phenolic metabolites and free amino acids in Scenedesmus quadricauda (Chlorophyceae). Plant Sci. 2010;178(3):307-311.
Crossref - Del Campo JA, Garcia-Gonzalez M, Guerrero MG. Outdoor cultivation of microalgae for carotenoid production: current state and perspectives. Appl Microbiol Biotechnol. 2007;74(6):1163-1174.
Crossref - Mishra K, Ojha H, Chaudhury NK. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem. 2012;130(4):1036-1043.
Crossref - Burton GW, Ingold K. β-Carotene: an unusual type of lipid antioxidant. Science. 1984;224(4649):569-573.
Crossref - Tiong IKR, Nagappan T, Wahid MEA, et al. Antioxidant capacity of five microalgae species and their effect on heat shock protein 70 expression in the brine shrimp Artemia. Aquac Rep. 2020;18:100433.
Crossref - Ceron MC, Garcia-Malea MdC, Rivas J, et al. Antioxidant activity of Haematococcus pluvialis cells grown in continuous culture as a function of their carotenoid and fatty acid content. Appl Microbiol Biotechnol. 2007;74(5):1112-1119.
Crossref - Oh S, Kim YJ, Lee EK, Park SW, Yu HG. Antioxidative effects of ascorbic acid and astaxanthin on arpe-19 cells in an oxidative stress model. Antioxidants. 2020;9(9):833.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.