ISSN: 0973-7510
E-ISSN: 2581-690X
Paediatric population is the high-risk segment for the infection of COVID-19 due to weak immune status and low compliance to COVID-19 prevention protocols. The first dose of vaccination for the paediatric population is started in the fifth phase of vaccination, after the vaccination was administered to health workers, elderly individuals, and young adults. Present article aims to analyse the status, trends, and challenges in the implementation of the paediatric vaccination for COVID-19 and provide recommendations that could be taken under consideration by healthcare authorities while designing the second and third vaccination protocols for the paediatric population. Relevant articles published by various journals related to paediatric COVID-19 vaccination were searched from the different databases and analysed for the current status of vaccination, trends, challenges, compliance level, implementation hurdles, and other relevant information. Limited research is available in the paediatric domain for the COVID-19 vaccination. Few vaccines are approved for the paediatric population in India, including the Covaxin, ZyCoV-D, Corbevax and Covovax. It is recommended that the vaccination trials should be accelerated by the government agencies to make COVID vaccines available from other indigenous manufacturers. It is also recommended that the COVID-19 prevention protocol should be made in such a manner that children find that interesting and like to follow them.
Paediatric, COVID, Vaccine, ZyCoV-D and Covaxin
COVID-19 (Coronavirus disease 2019) is caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) which led to outbreak of the disease from Wuhan, China on December 31, 2019.1 The first case of COVID-19 in India was reported on January 27, 2020.2 On March 11, 2020, WHO declared it as global pandemic.3
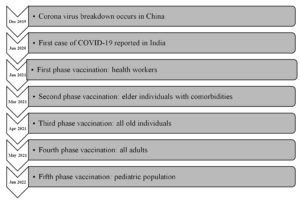
Many measures have been taken to prevent transmission of the disease through prompt diagnosis and treatment. There was an urgent requirement of vaccine to handle this pandemic. So, the development of vaccines was accelerated. The COVID-19 vaccine in India was initially introduced on January 16, 2021, with the first phase of vaccination limited to frontline health workers. All Indians above the age of 60, as well as individuals between the ages of 45 to 59 years with comorbidities, were vaccinated in the second phase, which began on March 1, 2021. Individuals above the age of 45 were eligible for the COVID-19 vaccination in the third phase, which started on April 1, 2021. Vaccinations were administered to all adults aged 18 and more in the fourth phase of vaccination, which started on May1, 2021. On January 3, 2022, the fifth phase of COVID-19 vaccination for children aged 15 to 18 years began.4 India started precautionary doses of vaccine for healthcare and frontline worker and those aged more than 60 years with comorbidities from January 10, 2022. The expert group has expanded the vaccination programme to the age group 12 to 14 years from March 16, 2022. Initially Corbevax was given to the children of age group 12 to 14 years of age. Present article focused on the fifth phase of vaccination i.e., paediatric population. We analyse the status of COVID-19 in paediatric population, global and Indian scenario of the paediatric vaccination, highlighted the approved vaccine for children immunization, and provide the important recommendations that could be taken under consideration by healthcare authorities while designing the second and third vaccination protocols for the paediatric population (Figure).
Relevant articles published by various journals related to pediatric COVID-19 vaccination were searched from the different databases including the NCBI, PubMed, Google Scholar, Scopus and Journals websites. Article published in recent four years (2019-2022) were included in the study. Data from the reports of Central Drug Standard Control Organisation (CDSCO), Drug Controller General of India (DCGI) and similar authorized agencies, were included in the study. All the published articles were analysed and based on relevance, data from different article were included in this manuscript. The data is categorized as current status of vaccination, trends, challenges, compliance level, implementation hurdles, and other relevant information.
COVID-19 in Paediatric Population
In children, about 70% of coronavirus infections are asymptomatic thus the morbidity and mortality in pediatric age group is far less. With COVID-19 infection, only mild symptoms were reported in children but some cases revealed serious illness. Children make up around a quarter of all COVID hospitalizations.5 According to data from the National Center for Disease Control, 3.9% of cases occurred in children aged 0-10 years old and 7.9% in those aged 11–20 years old.6 As a result, weighing the benefits and risks of COVID immunization in children is complicated.
Vaccination in children is important to prevent the COVID-19 infection in them, reduce the community transmission which averts the indirect effects of pandemic which the nationwide lockdown & school closures have put on children. Children can infect high-risk or elderly people at home who have not been immunized. Vaccinating children is, therefore, critical for overall family health as well as preventing community transmission.7 Moreover, by attaining herd immunity, we can put the pandemic to halt. To achieve a good level of herd immunity (65-75%) it is of paramount importance that children who account for 20-35% of population get the vaccination. Moreover, following the accelerated dissemination of more transmissible variants, the number of COVID-19 related hospitalization in children have risen dramatically in several countries.8 This emphasizes the need of COVID-19 immunisation in children.
During the Delta wave in the United States, the hospitalization rate per week for children aged 0 to 4 years surged by roughly tenfold. Data from March 2020 to April 2021, revealed that critical care was required in approximately one third of teenagers (12-17 years) who were admitted with COVID-19 and 5% had to undergo assisted ventilation. The rate of hospitalization in the United States was 10.1 times higher in non-vaccinated adolescents when compared with fully vaccinated adolescents aged 12-17 years from June 20 to July 31, 2021. This highlights the importance of vaccines in decreasing the mortality and morbidity related with COVID-19 disease in adolescents.8
If we analyse the data from India, children aged 0 to 10 years accounted for 3.28 % and 3.05 %; while children aged 11 to 20 years accounted for 8.03% and 8.57% in first and second wave respectively.6 Despite the fact that the shares were nearly the same in both COVID-19 waves, the second wave included nearly twice as many children as the first, resulting in a rise in the total number of children requiring hospitalization and critical care facility.
Children with comorbidities had greater probability of severe COVID-19 infection and related mortality than healthy children.9 In systematic review and meta-analysis, the percentage of children with co-morbidities who had severe COVID-19 was 5.1%, compared to 0.2% of children without co-morbidities.10 Malignancy was the most prevalent underlying comorbidity followed by cardiac disease in multi-centric research conducted at five major hospitals throughout India, with 44% of 402 children having some underlying risk factors. The odds ratio for moderate-severe illness in children with the underlying disease was 8.85 in this research.11, 12
Global Paediatric Vaccination Scenario
The US FDA has approved four COVID-19 vaccines under a Biologics License Application (BLA) or an Emergency Use Authorization (EUA), including the 1) Pfizer-BioNTech 2) Moderna 3) Janssen and 4) Novavax.13 Pfizer and Moderna vaccines are mRNA vaccine which encoded from the SARS-CoV-2 prefusion spike glycoprotein. The Janssen vaccine is a recombinant adenovirus type 26 (Ad26) vaccine and Novavax is a recombinant spike protein nanoparticle vaccine.14 For children aged 6 months to 17 years, presently two COVID-19 vaccines (Pfizer and Moderna) and for children 12 years and more, one vaccine (Novavax) is Food and Drug Administration (FDA) approved13 (Table 1).
Table (1):
Immunization schedule of COVID-19 for paediatric age group.13.
| Immunization Schedule of COVID-19 for Paediatric age group | ||||
|---|---|---|---|---|
| Age | Primary dosage | Booster dosage | ||
| NOT Moderate to severely immunocompromised individual | Moderate to severely immunocompromised individual | NOT Moderate to severely immunocompromised individual | Moderate to severely immunocompromised individual | |
| 1. MODERNA: mRNA Vaccine | ||||
| 6 months to 5 years | Monovalent Dose 1 & 2 (4-8 weeks apart) | Monovalent Dose 1 & 2 (4 weeks apart) Dose 2 & 3 (4 weeks apart) | Bivalent Dose 2 & 3 (8 weeks apart) | Bivalent Dose 3 & 4 (8 weeks apart) |
| 6 years to 11 years | Monovalent Dose 1 & 2 (4-8 weeks apart) | Monovalent Dose 1 & 2 (4 weeks apart) Dose 2 & 3 (4 weeks apart) | Bivalent Dose 2 & 3 (8 weeks apart) | Bivalent Dose 3 & 4 (8 weeks apart) |
| 12 years to 17 years | Monovalent Dose 1 & 2 (4-8 weeks apart) | Monovalent Dose 1 & 2 (4 weeks apart) Dose 2 & 3 (4 weeks apart) | Bivalent Dose 2 & 3 (8 weeks apart) | Bivalent Dose 3 & 4 (8 weeks apart) |
| 2. PFIZER BIONTECH: mRNA Vaccine | ||||
| 6 months to 4 years | Monovalent (dose 1, 2) Dose 1 & 2 (3-8 weeks apart) Dose 2 & 3 (8 weeks apart) | Monovalent (dose 1, 2) Bivalent (dose 3) Dose 1 & 2 (3 weeks apart) Dose 2 & 3 (8 weeks apart) | – | – |
| 5 years to 11 years | Monovalent Dose 1 & 2 (3-8 weeks apart) | Monovalent Dose 1 & 2 (3 weeks apart) Dose 2 & 3 (4 weeks apart) | Bivalent Dose 2 & 3 (8 weeks apart) | Bivalent Dose 3 & 4 (8 weeks apart) |
| 12 years to 17 years | Monovalent Dose 1 & 2 (3-8 weeks apart) | Monovalent Dose 1 & 2 (3 weeks apart) Dose 2 & 3 (4 weeks apart) | Bivalent Dose 2 & 3 (8 weeks apart) | Bivalent Dose 3 & 4 (8 weeks apart) |
| 3. NOVAVAX: Recombinant spike protein nanoparticle vaccine | ||||
| Booster: Bivalent Moderna or Pfizer BioNTech vaccine used for the booster dose | ||||
| ≥ 12 years | Monovalent Dose 1 & 2 (3-8 weeks apart) | Monovalent Dose 1 & 2 (3 weeks apart) | Dose 2 & 3 (8 weeks apart) | Dose 2 & 3 (8 weeks apart) |
Pfizer-BioNTech
The Recommendations have been given by Advisory Committee on Immunization Practices (ACIP) to use Pfizer vaccine in individuals aged 16 years on December 12, 2020, which was then extended to the age range 12 to 15 years on May 10, 2021.15 Pfizer vaccine was authorized in children of 5 years of age and above in October 2021 and age limit was further extended from 6 months to 4 years on June 17, 2022. On 12 October 2022, The Bivalent Pfizer-BioNTech vaccine, is approved by FDA for use as a single booster dose in individuals ≥ 5 years at least two months after completion of primary series of vaccination. Single booster dose of vaccine is extended to individuals at least two months after completing primary series of Pfizer vaccine in age group 12 -15 years on 02 September, 2022 and in age group 5 – 11 years on 12 October, 2022. Till Jan. 2023, there is no booster dose for children aged 6 months to 4 years who received primary series of Pfizer vaccine. Pfizer-BioNTech (Bivalent) vaccine became available on 9 December, 2022 as a third dose of the three-dose primary series following two doses of monovalent Pfizer-BioNTech vaccine.14,15
FDA did analysis of immune response data in a group of paediatric population. Antibody responses in 67 study participants were evaluated after giving the booster dose. The booster dose was given 7 to 9 months after the completion of primary series (2 dose) of Pfizer-BioNtech vaccine. The antibody levels against SARS-CoV2 were analyzed one month after booster dose and were found to be increased as compared to before booster dose. By July 16, 2021, 90.7 percent of the U.S. children between 12–17 years who got the Pfizer vaccine had non-severe adverse events, while 9.3 percent had experienced serious adverse effects, including myocarditis (4.3% ).15 BNT162b2 (Pfizer-BioNTech) vaccination safety data from healthy teenagers aged 12 to 15 years revealed that mild-moderate discomfort at the injection site (86%), fatigue (66%), headache (65%), and fever (20%) were the most prevalent adverse effects.16 The CDC-ACIP evaluated the various findings and concluded that the advantages of COVID-19 immunization to individuals and the public outweigh the risks, and recommended that the vaccine to be used in paediatric age group from 6 months to 17 years.13,16
Pfizer vaccine contains the mRNA, Lipids, cholesterol, tromethamine hydrochloride, sucrose, sodium chloride, monobasic potassium phosphate, and potassium chloride. The Pfizer vaccine is devoid of eggs, preservatives, or latex. mRNA, is the active ingredients in the vaccine that contains the instructions for viral protein that triggers primary immune system response in body which generate the memory to protect body from further infections. Lipids surround and protect the mRNA are the passive ingredients which help in transport of mRNA to the cell. Other passive ingredients including sugars, acids and acid stabilizers work to maintain stability of the of vaccine.17 Dosing and formulation is not based on weight of the beneficiary. The immunisation schedule in children will depend on their age on the day of vaccination. The dosage of monovalent and bivalent Pfizer vaccine is 3 µg, 10 µg and 30 µg each for the aged group between 6 months to 4 years (maroon capped vial), 5years to 11 years (orange capped vial), and 12 years and above (gray capped vial), respectively13 (Table 1).
Moderna Vaccine
FDA-approved as primary series of two-dose monovalent Moderna vaccine with a 4 – 8 weeks gap between doses for most of the people aged between 6 months-5 years, 6-11 years and 12-17 years followed by a booster dose (bivalent) at least 2 months after 2nd dose of primary series.13 People aged 6months-17 years (who are moderately or severely Immunocompromised), a total of 3 doses should be given with a gap of 4 weeks between each dosage followed by a booster dose at least 8 weeks apart after 3rd dose of primary series.13 The dosage of mRNA-1273 monovalent vaccine between 6 months through 5 years, 6-11 years and ≥ 12 years is 25 µg, 50 µg and 100 µg respectively. Whereas, the dosage of mRNA-1273 bivalent booster vaccine is 10 µg, 25 µg and 50 µg for children aged 6 months- 5 years, 6-11 years and ≥ 12 years.13 The ingredients present in the vaccine are messenger ribonucleic acid (mRNA), lipids, dimyristoyl-rac-glycerol, cholesterol, methoxypolyethylene glycol, 1,2-distearoyl-sn-glycero-3-phosphocholine, acid stabilizers (Tromethamine hydrochloride, Tromethamine, Acetic Acid, Sodium Acetate) and sugars.15
Novavax vaccine
FDA authorizes a monovalent Novavax vaccine in children of 12 years and above as primary series of two-dose at least 3-8 weeks apart followed by a booster dose after 8 weeks. The mRNA vaccine (Moderna and Pfizer-BioNTech) is used as a booster dose followed by a primary series of Novavax vaccine.13
Janssen vaccine
This vaccine is given in individuals of ≥ 18 years. Single booster dose is given at least 2 months after completing the primary vaccination. The vaccine consists of recombinant, replication incompetent adenovirus 26, encoding a variant of SARS-CoV-2 Spike protein.13
CoronaVac and BBIBP-CorV
Chinese authorities have licenced these two inactivated vaccines for children aged 3 year to 17 year.18,19
Indian Paediatric Vaccination Scenario
The vaccines approved for combating COVID-19 in India are enumerated in Table 2.20 Union Government has started COVID-19 vaccination for 12-14 years age groups from 16th March 2022 and initiated with Corbevax vaccine.21,22
Table (2):
The COVID-19 Vaccine approved for use in India.20,23.
| Vaccine name | Applicant | Date of approval | Age in years | Dose | Route of administration | Approved |
|---|---|---|---|---|---|---|
| Protein subunit | ||||||
| COVOVAX | M/s Serum Institute of India Pvt. Ltd. | 28.12.2021 | ≥ 18 | 2 (0, 21 days) | Intramuscular | 6 countries |
| 08.03.2022 | ≥ 12 | 2 (0, 21 days) | ||||
| 28.06.2022 | ≥ 7 to < 12 | 2 (0, 21 days) | ||||
| CORBEVAX | M/s Biological E Limited | 28.12.2021 | ≥ 18 | 2 (0, 28 days) | Intramuscular | 2 countries |
| 21.02.2022 | ≥ 12 | 2 (0, 28 days) | ||||
| 26.04.2022 | ≥ 5 to < 12 | 2 (0, 28 days) | ||||
| 03.06.2022 | ≥ 18 | Booster dose: 1 dose after primary vaccination with COVAXIN and COVISHIELD | ||||
| DNA vaccine | ||||||
| ZyCoV D | M/s Cadila Healthcare Limited | 20.08.2021 | ≥ 12 | 3 (0, 28,56 days) | Intradermal
|
1 country |
| 26.04.2022 | ≥ 12 | 2 (0, 28 days) | ||||
| mRNA vaccine | ||||||
| MODERNA | M/s Cipla Ltd. (Importer) | 29.6.2021 | ≥ 18 | 2 (0, 28 days) | Intramuscular | 88 countries |
| GEMCOVAC-19 | Gennova Biopharmaceuticals ltd. | 28.06.2022 | ≥ 18 | 2 (0, 28 days) | Intramuscular | 1 country |
| Viral vector vaccine | ||||||
| Sputnik light | M/s Dr. Reddy’s Lab. Ltd. (Importer) | 05.02.2022 | ≥ 18 | 1 | Intramuscular | 26 countries |
| M/s Hetero Biopharma Ltd | 16.03.2022 | ≥ 18 | 1 | |||
| Sputnik V | M/s Dr. Reddy’s Lab. Ltd. (Importer) | 12.04.2021 | ≥ 18 | 2 (Day 0- comp I Day 21- comp II) | Intramuscular | 74 countries |
| M/s Panacea Biotec Ltd | 02.07.2021 | ≥ 18 | 2 (Day 0- comp I Day 21- comp II) | |||
| M/s Hetero Biopharma Ltd | 07.10.2021 | ≥ 18 | 2 (Day 0- comp I Day 21- comp II) | |||
| Janssen (Ad26-COV2-S) | M/s Johnson & Johnson Pvt. Ltd. (Importer) | 07.08.2021 | ≥ 18 | 1 | Intramuscular | 113 countries |
| M/s Biological E Limited | 18.08.2021 | ≥ 18 | 1 | |||
| COVISHIELD | M/s Serum Institute of India Pvt. Ltd. | 27.01.2022 | ≥ 18 | 2 (4 to 6 weeks apart) | Intramuscular | 49 countries |
| iNCOVACC | M/s Bharat Biotech | 06.09.2022 | ≥ 18 | 2 (0, 28 days) | Intranasal | 1 country |
| Inactivated SARSCoV-2 Vaccine | ||||||
| COVAXIN | M/s Bharat Biotech | 24.12.2021 | ≥ 12 to 18 | 2 (0, 28 days) | Intramuscular | 14 countries |
| 27.01.2022 | ≥ 18 | 2 (0, 28 days) | Intramuscular | |||
| 26.04.2022 | > 6 to <12 | 2 (0, 28 days) | Intramuscular | |||
Following recommendations by Subject Expert Committee (SEC), CDSCO and DCGI on April 26, 2022 has approved the emergency use authorization for following vaccines: Corbevax for children between age group 5-12 years, Covaxin for children between age group 6-12 years and ZyCoV-D for children of age 12 years or above.23,24 On 28 June 2022, Covovax vaccine was approved for emergency use in children ≥ 7 years of age20 (Table 3).
Table (3):
COVID-19 vaccines that have been licensed for paediatric use in India.
Vaccine name |
Platform |
Age |
Dose |
Route |
Storage |
|---|---|---|---|---|---|
COVAXIN (BBV152) |
Inactivated vaccine |
6-12 years |
2
(0, 26 days) |
Intramuscular |
2-80C |
CORBEVAX |
Recombinant protein subunit vaccine |
5-12 years |
2
(0, 28 days) |
Intramuscular |
2-80C |
COVOVAX |
Recombinant nanoparticle vaccine |
7-12 years |
2
(0, 21 days) |
Intramuscular |
2-80C |
ZyCoV D |
DNA vaccine |
>12 years |
2
(0, 28 days) |
Intradermal |
2-80C |
The Covaxin was developed by Bharat Biotech in collaboration with National Institute of Virology (NIV) and Indian Council of Medical Research (ICMR). It is a inactivated SARS-CoV-2 virus with an imidazoquinoline class compound adsorbed to alum to boost immunogenicity.24 The Bharat Biotech COVID-19 vaccine contains 6 µg of inactivated SARS-CoV-2 antigen aluminum hydroxide gel (250 µg), TLR 7/8 agonist (15 µg), 2-phenoxyethanol (2.5 mg), and phosphate buffer saline (0.5 ml) per dosage.25 Participants in the phase 3 study of the Covaxin (BBV152) vaccination were 18 years old or above. Vaccine effectiveness against COVID-19 of any severity was 78% and 93% against severe disease after dose 2. Vaccine effectiveness was 79% in those under the age of 60, and 68% in those 60 years and beyond. The vaccine has a 64% effectiveness against asymptomatic SARS-CoV-2 infection.26 The phase II/III trials in healthy children and adolescents (2-18 years) have shown robust safety, reactogenicity and immunogenicity.
The ZyCoV-D vaccination for COVID-19 is the world’s first and India’s first DNA-based COVID-19 vaccine for children and adults aged 12 and above. This vaccine was manufactured in collaboration with the Department of biotechnology (DBT) of the Indian government as part of the ‘Mission COVID Suraksha,’ and is being deployed by Biotechnology Industry Research Assistance Council (BIRAC), by DBT. The Zydus Cadila COVID vaccine – ZyCoV-D (three dose series) was authorized by the DCGI on 20 August 2021, for children aged 12 and over.27 It consists of a DNA plasmid Vector pVAX1 carrying spike-S protein of SARS-CoV-2 along with gene coding for signal peptide. This vaccine has an advantage in dealing with logistical and manufacturing limitations compared to previous RNA-based vaccines. This vaccine has effective temperature stability, thus facilitating storage and transportation. ZyCoV-D vaccine can be maintained at 2–8°C for 3 months. In open-vial testing, ZyCoV-D remained stable for 28 days.28 The 1400 participants aged 12 to 17 years old were involved in a phase 3 efficacy trial. In the 12–18 year-old age range, no serious vaccine-related adverse effects were seen. The vaccine’s overall effectiveness was reported to be 66.6%.28 DCGI has provided the approval to ZyCOV-D’s two dose formulation for children aged 12 years or above on April 26, 2022. Originally it was given in 3 dose regimen on day 0, day 28 and day 56. Now, the two-dose regimen is on day 0 and day 28. This two-dose schedule will increase the compliance and decrease time needed for vaccination.20
After Covaxin and Zydus Cadila’s ZyCoV-D, Biological E Limited Corbevax is third domestic Covid-19 vaccine in India. At a 28-day interval, two doses of vaccination (each 0.5ml) are administered intramuscularly.7 From pre-clinical to Phase III clinical trials, DBT and BIRAC, have funded this vaccine candidate. The vaccine was found to be well tolerated, safe and highly immunogenic in Phase III clinical studies involving over 3000 participants between the ages of 18 and 80 at 33 research locations across India.29 In India, at least one dose of COVID vaccine is given to around 66% of its population, the Corbevax vaccine got emergency use authorization. The vaccine is more than 90% effective in the trials in preventing symptomatic infections with the original Wuhan strain of SARS-CoV-2 and more than 80% effective against symptomatic infection with the Delta variant based on published studies.30
COVOVAX
It is a SARS-CoV-2 recombinant (Spike protein) nanoparticle vaccine, manufactured by Serum Institute of India Private Limited. The single dose (0.5 mL) of Covavax vaccine consist of 5 micrograms of SARS-CoV-2 spike protein and is adjuvanted with Matrix-M1. It is given as two dose formulation (day 0 and day 21) by intramuscular route. DCGI has approved Covavax vaccine for restricted use in emergency situation for COVID-19 prevention in children aged 7 years or above on June 28, 2022.13,20,23
iNCOVACC
The Bharat Biotech’s intra nasal vaccine – iNCOVACC is an recombinant adenoviral vector vaccine expressing the spike protein of SARS-CoV-2 virus. CDSCO had approved the restricted use of the iNCOVACC vaccine in emergency situations in the age group of ≥ 18 years for active immunization and as a booster dose following completion of primary schedule of Covishield or Covaxin.20, 23
Side effects of COVID-19 Vaccine
Adverse reactions observed in paediatric age are sore arms, fever, chills, muscle aches, headache and tiredness. In majority, the side effects are usually mild.31 In few cases, myocarditis and pericarditis was reported in young children following the administration of COVID-19 vaccine by Pfizer-BioNTech.32 In Saudi Arabia, a self-administered online survey was used to collect information about side effects experienced by children aged 12 to 18 after receiving the Pfizer vaccine. The 60% of research participants reported at least one adverse effect after receiving the Pfizer vaccine. The 90% of individuals experienced pain or redness at the injection site, 67% claimed fatigue, 59% fever, 59% headache, 55% reported nausea or vomiting, and 21% suffered chest discomfort and shortness of breath as adverse effects (20%). Participants reported joint or bone discomfort less frequently (2%). The side effects observed in female participants were 52% when compared with males (48%). Compared to the first dosage, side effects were more frequent following the second dose.33
Recommendation for Paediatric Vaccination
Vaccination should be prioritized for children with high-risk conditions. The fourth all-India serosurvey revealed 57.2% and 61.6% positivity in the age group of 6-9 years and 10–17-years and efficacy may diminish with time. The risk of re-infection with COVID-19 is twice more common in unvaccinated persons when compared to fully vaccinated individuals. Furthermore, antibody responses were greater in persons hospitalized with COVID-19 like disease who had previously received vaccine compared to those who had previously been exposed to the virus naturally.34
Cold chain management, vaccination delivery skills, adverse events following immunization and biomedical waste disposal should be taken care of. Local and district branches should be active in the dissemination of information, education, and communication activities in regional languages using print media, television and social media. This might be crucial for parents’ acceptance of COVID-19 vaccinations for children.5
The safety and efficacy of COVID-19 vaccinations in children must be revealed to paediatricians and parents. Prior to initiation of any COVID-19 immunization programs for children, study data must be disclosed to paediatrician and parents. The easiest approach to attain maximal immunization coverage is through a school-based vaccination campaign. This should not, however, be made necessary, and parents should be given the option of having their children vaccinated in schools or at their paediatrician clinic. Medical professionals qualified to manage crises, emergency drugs and equipment, a tie-up with the nearest hospital for emergency care, and quick transportation to referral hospitals should all be available at school-based clinics.35
COVID-19 vaccinations and other planned children’s vaccinations should be given either concurrently or at any interval between them. It is suggested that active and passive surveillance method for COVID-19 vaccination related side effects should be established. This should involve monitoring for any possible relationship between COVID-19 vaccinations and multisystem inflammatory syndrome in children (MIS-C), as well as any other negative effects seen over time. When compared to adults, children’s immune response to vaccination is markedly different. Younger children have robust active immune response, which might lead to increased immunological reactions and, most likely, reactogenicity. In post-marketing surveillance, the relationship, if any, between dysregulated immunological responses, such as MIS-C, and vaccination should be extensively investigated.6
Vaccination may be delayed in children with acute diseases until they have recovered clinically. Immunodeficiencies caused by medications or illnesses do not exclude children from receiving the COVID-19 vaccination. Studies on the length of protection and effectiveness against variations should be started. This information will be required to make booster dosage recommendations. The government’s top priority should be the development of safe and efficacious COVID-19 vaccines for children.35
With an entrenched and time-tested vaccination network, India boasts the world’s biggest immunization programme for children. These might be used in the COVID-19 immunization campaign. India has significant vaccine production capabilities, along with surgical disposables (such as syringes, vials, stoppers, gauze pieces etc.) as well as adequate vaccine storage and transportation. India’s vaccination potential has been demonstrated with daily vaccinations of 7-10 million adults. India has inoculated over a billion people with at least one dose of the COVID-19 vaccine in just nine months. During a pandemic, it is even more critical that finite resources are used judiciously while balancing the values of equality. The choice to vaccinate healthy paediatric population would be contingent on the accessibility of one or more appropriate vaccinations in sufficient numbers to immunize our country’s vulnerable population.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
GKM, SS conceptualized and designed the study. GKM, SS and BY performed acquisition, analysis and interpretation of data. BY, NT, GKM wrote and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Team E. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19). China, 2020. China CDC Weekly. 2020;2(8):113-122.
Crossref - Andrews MA, Areekal B, Rajesh KR, et al. First confirmed case of COVID-19 infection in India: A case report. Indian J Med Res. 2020;151(5):490-492.
Crossref - Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Bio Medica: Atenei Parmensis. 2020;91(1):157-160.
- Chowdhury SR, Motheram A, Pramanik S. Covid-19 vaccine hesitancy: trends across states, over time. Ideas for India Accessed August. 2021;16. https:// www.ideasforindia.in/topics/governance/covid-19- vaccine-hesitancy-trends-across-states-over-time.html [Last assessed 24/02/2022]
- Zhou L, Wu Z, Li Z, et al. One Hundred Days of Coronavirus Disease 2019 Prevention and Control in China. Clin Infect Dis. 2021:72(2):332-339.
Crossref - Kasi SG, Dhir SK, Shah A, et al. Coronavirus disease 2019 (COVID-19) vaccination for children: Position statement of Indian academy of pediatrics advisory committee on vaccination and immunization practices. Indian Pediatr. 2022;59(1):51-57.
Crossref - Thuluva S, Paradkar V, Turaga K, et al. Selection of optimum formulation of RBD-based protein sub-unit covid19 vaccine (Corbevax) based on safety and immunogenicity in an open-label, randomized Phase-1 and 2 clinical studies. MedRxiv. 2022.
Crossref - Delahoy MJ, Ujamaa D, Whitaker M, et al. Hospitalizations associated with COVID-19 among children and adolescents-COVID-NET, 14 states, March 1, 2020-August 14, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(36):1255-1260.
Crossref - Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246-256.
Crossref - Jat KR, Sankar J, Das RR, et al. Clinical profile and risk factors for severe disease in 402 children hospitalized with SARS-CoV-2 from India: Collaborative Indian Pediatric COVID Study Group. J Trop Pediatr. 2021;67(3):fmab048.
Crossref - Pande N, Save S, Kondekar A, Sawant V, Rathi S, Malik S. Clinical profile of children with sars-cov-2 infection from a dedicated covid-19 hospital in india. Curr Pediatr Res. 2021;25(7):697-703.
- Kasi SG, Dhir SK, Shivananda S, et al. Breastfeeding and Coronavirus Disease 2019 (COVID-19) Vaccination: Position Statement of Indian Academy of Pediatrics Advisory Committee on Vaccination and Immunization Practices. Indian Pediatr. 2021;58(7):647-649.
Crossref - Centers for Disease Control and Prevention (CDC). summary document for interim clinical considerations for use of COVID-19 vaccines currently authorized in the United State. https://www.cdc.gov/vaccines/ covid-19/downloads/summary-interim-clinicalconsiderations. pdf. [Last assessed 24/02/2022]
- Rosenblum HG, Wallace M, Godfrey M, et al. Interim recommendations from the Advisory Committee on Immunization Practices for the use of bivalent booster doses of COVID-19 vaccines-United States, October 2022. MMWR Morb Mortal Wkly Rep. 2022;71(45):1436-1441.
Crossref - World Health Organization. Interim recommendations for use of the Pfizer-BioNTech COVID-19 vaccine, BNT162b2, under emergency use listing. https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-vaccines-SAGE_recommendation-BNT162b2-2021.1 [Last assessed 24/02/2022]
- Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices-United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977-982.
Crossref - Tanno LK, Berard F, Beaudoin E, Didier A, Demoly P. Sars-cov-2 vaccination and anaphylaxis: recommendations of the french allergy community and the Montpellier world health organization collaborating center. Vaccines. 2021;9(6):560.
Crossref - Han B, Song Y, Li C, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: a double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(12):1645-1653.
Crossref - Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated COVID-19 vaccine, BBIBP-CorV, in people younger than 18 years: a randomised, double-blind, controlled, phase 1/2 trial. Lancet Infect Dis. 2022;22(2):196-208.
Crossref - India-COVID19 Vaccine tracker. https://covid19.trackvaccines.org. [Last assessed 24/02/2022]
- COVID-19 Vaccination for 12-14 yrs cohort to begin from tomorrow, National Vaccination Day, in all States/UTs March 4, 2022 Press release: C. https://www.pib.gov.in/PressReleasePage COVID19 Vaccination for 12-14 yrs cohort to begin from tomorrow, National Vaccination Day, in all States/UTs. [Last assessed 24/02/2022]
- Corbevax, Biological E’s COVID-19 vaccine, gets DCGI’s emergency use authorisation for children aged 5-12. India News. April 26, 2022 available on https://www.firstpost.com/india/corbevax-biological-es-covid-19-vaccine-gets-dcgis-emergency-use-authorisation-for-children-aged-5-12-10600811.html. [Last assessed 24/02/2022]
- Central Drugs Standard Control Organization. COVID-19 vaccines approved in the country. Press release 2022.https://cdsco.gov.in [Last assessed 24/02/2022]
- Ganneru B, Jogdand H, Daram VK, et al. Th1 skewed immune response of whole virion inactivated SARS CoV 2 vaccine and its safety evaluation. iScience. 2021;24(4):102298.
Crossref - Sarkale P, Patil S, Yadav PD, et al. First isolation of SARS-CoV-2 from clinical samples in India. Indian J Med Res. 2020;151(2-3):244-250.
Crossref - Ella R, Reddy S, Blackwelder W, et al. Efficacy, safety, and lot to lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): a double-blind, randomised, controlled phase 3 trial. MedRxiv. 2021.
Crossref - DBT-BIRAC supported ZyCoV-D developed by Zydus Cadila Receives Emergency Use Authorization. Aug 20, 2021; Press release. https://www.pib.gov.in/PressReleasePage.aspx?PRID=1747669 [Last assessed 24/02/2022]
- Khobragade A, Bhate S, Ramaiah V, et al. Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): the interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India. Lancet. 2022;399(10332):1313-1321.
Crossref - PIB.gov. Press release: Department of Biotechnology mission COVID Suraksha Supported Biological E limited Novel COVID 19 vaccine candiate- CORBEVAX receives DCGI approval for emergency use authorozation December 29, 2021. https://pib.gov.in/Pressreleaseshare.aspx?PRID=1786152.
- Kuehn BM. New COVID-19 Vaccine Aims to Increase Global Vaccine Access. JAMA. 2022;327(7):614.
Crossref - Thompson LA, Rasmussen SA. Children and COVID-19 vaccines. JAMA Pediatr. 2021;175(8):876.
Crossref - Centers for Disease Control and Prevention. Clinical Considerations: Myocarditis and Pericarditis after Receipt of mRNA COVID-19 Vaccines among Adolescents and Young Adults. 2021.
- Alamer E, Alhazmi A, Qasir NA, et al. Side Effects of COVID-19 Pfizer-BioNTech mRNA Vaccine in Children Aged 12-18 Years in Saudi Arabia. Vaccines. 2021;9(11):1297.
Crossref - Cavanaugh AM, Spicer KB, Thoroughman D, Glick C, Winter K. Reduced risk of reinfection with SARS-CoV-2 after COVID-19 vaccination-Kentucky, May-June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(32):1081-1083.
Crossref - Kumar J, Meena J. COVID-19 Vaccine in Children: Where Do We Stand? Indian Pediatrics. 2021;58(2):194-195.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.