ISSN: 0973-7510
E-ISSN: 2581-690X
The tomato plant is usually infected with various pathogens such as pests, bacteria, and different mycoflora. In this investigation, Tomato plant cultivar Beeli was pathogenized with Fusarium oxysporum f.sp. Lycopersicon (FOL1) fungi. The FOL1 fungus was controlled by inoculating the pathogenized Tomato plants with each one of the biocontrol microorganisms, such as Arbuscular mycorrhiza (AM), Trichoderma harzianum (T. harzianum), and microbial blend, named as Effective Microorganisms (EM). Consequently, the effect of these biocontrol microorganisms on the amount of chlorophyll, proteins, and defense enzymes of the Tomato plant was estimated. The results showed that the AM, T. harzianum fungi, and “EM” gave similar ameliorative effects. However, there are regulated increasing content of chlorophyll, proteins, and the activities of many protecting compounds such as acid invertase peroxidase. Moreover, these important plant defense mechanisms have a vital role in oxidizing phenolic compounds, which could increase antimicrobial activity. Altogether, the results demonstrate that the protein and chlorophyll are increased differently in all treatments. The protein level is the highest in FOL1 + EM treatment and the ML showed the highest level of chlorophyll.
Fusarium oxysporum f.sp. Lycopersicon, Mycorrhiza (Local isolate), Trichoderma harzianum, Effective Microorganisms
The Tomato (Solanum Lycopersicum L.) is one of the most important vegetable crops, either fresh or through manufacturing processes at the global level. Tomatoes are one of the most important vegetable crops grown in greenhouses in Saudi Arabia. The tomato crop is one of the important vegetable crops whose cultivation spreads in different areas, especially in new lands. It achieves high profitability that covers the costs and services of agriculture since tomatoes are included in most foods. Tomatoes grow in many types of sandy to heavy clay lands and prefer sandy lands when the goal of cultivation is to produce an early crop or when the growing season is short. It is a significant source of vitamin C, potassium, folic acid, and carotenoids, for example, lycopene. 1
Plant pathogens make genuine misfortunes or harm crops worldwide and diminish the quality and amount of horticultural items. These misfortunes represent a significant danger to worldwide food creation yearly. 2, 3, 4
Tomatoes are usually subjected to many bacterial and fungal pests from emergence to harvest. Fusarium is responsible for significant reductions in tomato quality and yield among these diseases incited every year. Because the impact of tomato diseases cannot be predicted from one year to the next, certain precautions must be taken every year to ensure maximum fruit production with minimum Fusarium wilt occurrence5 reported that Fusarium wilt is one of the most genuine sicknesses of tomato all through the globe. This infection is brought about by Fusarium oxysporum f. sp. lycopersici (Sacc.), prompting natural financial misfortunes6 becomes perhaps the most pervasive and harmful illness any place tomatoes are developed thoughtfully because the microbe can persevere endlessly in pervaded soil.7
Biological controlling agents can replace chemical agents in controlling pathogenic insects, weeds, microbes, and several bio fungicides based on antibiotic metabolic, sites and hydrolytic enzymes. Application of biological control against parasitic fungi has been reported worldwide using antagonistic fungi and bacteria isolated from coastal soils. 8,9
Effective Microorganisms (EM) are a liquid mixed culture made up of lactic acid bacteria, photosynthesis bacteria, and yeast. They are the basis for all EM products created by fermentation. Organic substances (such as herbs, sugar cane, molasses, etc.) are converted by enzymes or microorganisms during fermentation. The addition of microorganisms enables meanings to be created that would be very difficult or even impossible to produce chemically.
This study aimed to investigate the curative effect against Fusarium oxysporum f.sp. lycopersici using Mycorrhiza (Local isolate), Trichoderma harzianum, and Effective Microorganism. Also, to observe the impact of these biocontrol microorganisms on the amount of chlorophyll, protein, and defense enzyme.
Chlorophyll content assay
Chlorophyll was extracted in 90% acetone, according to Lichtenthaler. 10 Sub-samples of shoots (about 12 g each) were placed in a chilled homogenizer with a bit of magnesium carbonate, then distilled water was added to pure chilled acetone to make the volume approximately 250 ml of 90% (v/v) acetone solution. The water contents of the shoot were estimated from previous determinations of similar samples. The mixture was transferred to a weighed beaker, and the homogenizer was washed out into the cup with 90% acetone. The total extract (c. 300 ml) was considered, and 30ml of the extract was poured out into another weighed beaker while stirring vigorously. The ratio of sub-samples to the total weight of extract gives an aliquot factor. The sub-sample was centrifuged at 2500 r. p. m. for 15 min depending on the nature of the extract) or filtered through a number 4 sintered glass filter. The optical density of these pigments was obtained at 665 mµ (D665 and D645) and calculated below:
µg chl. a = (11.6 D665 -1.3 D645). (ν/Ɩ).
D665= absorbance (optical density) reading at665 mµ.
D645= absorbance (optical density) reading at645 mµ.
ν= volume of acetone in ml.
Ɩ= length of spectrophotometer cell in cm.
Total protein assay.
The Kjeldahl method was used to determine the total nitrogen content according to AOAC.11 and then the crude protein by multiplying with a factor of 6.25. Crude protein was determined by adding about 25 ml H2SO4 to a constant weight in a crucible placed in an oven at 100°C for 3hr and then cooled to 50°C. Sample was transferred to a volumetric flask. The volume was completed to 100 ml with distilled water (DW) 5ml was placed in marc cam distillation, and 10 ml of 40% 1N NaOH was added gradually. Resift in a conical flask with 25ml of boric acid and indicter (methyl red and bromogresol), at least 75ml. Then titrate with HCL N 0.02%
Nitrogen =T×14×0.02×20×100/weight of sample ×1000
Crude protein = (% total nitrogen × 6.25).
Total soluble phenol content assay (TSP)
TSP content in tomato seedlings was extricated using the method of Hsu.12 5 g of each sample was blended in with 80 mL methanol, sifted, and the filtrate was diluted to finish the volume to 100 ml and utilized for the resulting examination. Slinkard and Singleton13 indicated that 200 microlitres of the stock arrangement were blended in with 1.4 mL distilled water and 0.1mL of 50% (1N) Folin-Ciocalteau phenol reagent. Then, 0.3 mL of 20% (w/v) sodium carbonate was added. The response combination was permitted to stand for 2 hours. After momentarily vortexing, optical density was determined at 765 nm. Total soluble phenol content was standardized against tannic acid, and absorbance values were changed over to mg of phenol per gram of fresh weight tissue.
Polyphenol oxidase activity (PPO) assay
PPO activity was determined according to Houssien et al.14 The fresh weight of 0.25 g of tomato seedlings was homogenized. One ml of the supernatant was blended with 2 ml of borate cradle, 1 ml P-aminobenzoic corrosive 1% (alc. Arrangement), and 1 mL 1% catechol. The response combination was incubated for one h at 40°C. After momentarily vortexing, the absorbance was determined at 575 nm. PPOA was expressed as Ab 575/ min/ g of fresh weight of seedlings.
Peroxidase (POD) activity assay
POD was determined by the method of Hussien et al.14 0.25g sample was homogenized and centrifuged for 15 min at 4000 pm. The enzyme activity was expressed as Ab 470/ min/ g of fresh weight of seedlings.
Acid invertase (AIV) activity assay
According to the method,15 an activity was estimated as 0.25 g tomato shoots seedlings were drenched in super cold ethyl acetic acid derivation for 20 min. And we lastly washed in super cold distilled water. Samples were incubated in a water bath at 30°C for 60 min. The acid invertase activity was expressed as a total sugar.16
Determination of phenylalanine ammonia-lyase (PAL) activity
PAL activity was determined according to Dunn et al.,17 in which tomato seedlings shoots were homogenized in 0.1M sodium borate buffer containing ethanol and Polyvinyl Pyrroprolidin. The mixture was incubated in a water bath at 40°C, and the optical density was determined at 290 nm.
Statistical analysis
Two-way analysis of variance (ANOVA) was used to study the data. Mean separations were carried out, and differences at P < 0.05 were considered significant.18
Effect of Different inocula on the Protein Content, Chlorophyll, Peroxidase, Polyphenol Oxidase, and Soluble Phenol in Tomato cultivar Beeli:
In Table 1, it was observed that the protein contents of the pathogenized plants varied widely, i.e., ranging between 1.2-2.01 %. The tomato plant pathogenized with a combination of FOL1 + EM has the highest protein content (2.01%), while the one pathogenized with only FOL1 was the lowest (1.21%). Moreover, the protein content of all pathogenized plants exceeded that of the control un- pathogenized plants. However, the data suggest that inoculation improved plant protein contents, particularly the EMTM inoculum or the local mycorrhiza.
Table (1):
Effect of Different inoculum on the Protein Content, Chlorophyll, Peroxidase, Polyphenol Oxidase and Soluble Phenol in Tomato cultivar Beeli.
Treatments |
Protein (%) |
Chlorophyll (mg/g fresh leaves) |
Peroxidase (Ab. 470/ min/ g ) |
Polyphenol oxidase (Ab. 575/ min/ g ) |
Soluble phenol (mg/g fresh leaves) |
|---|---|---|---|---|---|
Control |
0.59g |
35.21b |
0.68a |
0.33bc |
0.74d |
FOL1 |
1.21f |
24.69d |
0.71a |
0.34b |
1.15 a |
ML |
1.81b |
35.44a |
0.30d |
0.36a |
0.81c |
FOL1 + ML |
1.54c |
14.44e |
0.38c |
0.31d |
0.70d |
FOL1 + ML + MG |
1.34e |
11.06 f |
0.29d |
0.31d |
0.70d |
FOL1 + Th |
1.51d |
8.19g |
0.41b |
0.33bc |
0.89b |
FOL1 + EM |
2.01a |
33.18c |
0.69a |
0.32cd |
0.74d |
±SEM |
7.35 |
0.04 |
0.01 |
8.09 |
0.03 |
FOL1 = Fusarium oxysporum f.sp. lycopersici ; ML = Mycorrhiza (Local isolate) ; MG = Mycorrhiza (Glomus mosseae) ; Th = Trichoderma harzianum ; EM = Effective Microorganisms@TM.
* Values are means ± standard error. Significant differences (P<0.05 using Tukey post-hoc test) among treatments in the same column are indicated by different letters.
*The legends(letters): Decrease from a to g
Similarly, Chlorophyll content was suppressed in the pathogenized plants (24.69 mg/g) but significantly improved in the presence of the local mycorrhiza or EMTM, which contained 35.44 mg/g 33.18 mg/g, respectively. The chlorophyll content of all other samples was significantly less than that of the control/un-homogenized plant.
The activity of Peroxidase enzyme of the pathogenized tomato plant: The FOL1 and EMTM inocula did not affect peroxdase activity (0.71 and 0.69 min/g) respectively) with non-significant difference compared to control un-pathogenized plant (0.68 min/ g (Ab. 470)). However, the other inoculums used (like ML, FOL1 + ML, FOL1 + ML + MG and FOL1 + Th) significantly impaired the enzyme activity (0.30, 0.38, 0.29, 0.41 min/g, respectively).
The activity of polyphenol oxidase enzymes of the pathogenized plants was not altered. Likewise, the introduction of inoculum also did not change the enzyme’s activity.
Furthermore, the data suggest that the plant soluble phenol content was raised after patronization (1.15 mg/g) and control plants (0.74 mg/g). However upon the administration of different inoculums have significantly restored the phenol content, such as: FOL1 + ML (0.70 mg/g), FOL1 + ML + MG (0.70 mg/g), and FOL1 + EM (0.74 mg/g), as indicated in Table (1). Additionally, the treatment of FOL1 + Th (0.89 mg/g) was not able to significantly decrease the phenol contents compared with the control.
Table (2):
Phenylalanine Ammonia Lyase Assay on Tomato cultivar Beeli Inoculated with Fusarium oxysporum f. sp. lycopersici (FOL1) in the Presence of Mycorrhizae and Trichoderma harzianum, at 6th Weeks.
Treatments |
Ab290/15min./g |
Ab290/30min./g |
Ab290/60min./g |
Ab290/90min./g |
|---|---|---|---|---|
Control |
1.36d |
1.57c |
1.30ab |
1.29ab |
FOL1 |
1.37d |
1.61ab |
1.49a |
0.49d |
ML |
1.47b |
1.63a |
1.34ab |
1.33a |
FOL1 + ML |
1.42c |
1.63a |
1.11bc |
1.05bc |
FOL1 + ML + MG |
1.37d |
1.62a |
1.08bc |
1.02bc |
FOL1 + Th |
1.43c |
1.45d |
1.03c |
1.01c |
FOL1 + EM |
1.54a |
1.57bc |
1.18bc |
1.15abc |
±SEM |
0.01 |
0.02 |
0.12 |
0.12 |
The activity of the phenylalanine ammonia-lyase enzyme
Table (2) shows that the activity of the phenylalanine ammonia-lyase (PAL) enzyme was similar in both control and FOL1- pathogenized plants after the first 15 minutes of the assay (1.36 Ab290/g). However, all inoculation treatments succeeded in boosting the activity of this enzyme, particularly in plants treated with EMTM. In all treatments, the activity ty of PAL reached a peak at 30 minutes from starting of the assay and then dropped gradually. At the end of the assay, all treatments exhibited PAL values significantly higher than in the FOL1- pathogenized plants.
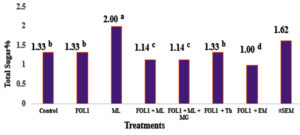
Fig. 1. Acid Invertase Content of Tomato var. Beeli Inoculated with Fusarium oxysporum f. sp. lycopersici (FOL1) in The presence of Mycorrhizae and Trichoderma harzianum at 6th Week from Transplanting.
FOL1 = Fusarium oxysporum f.sp. lycopersici; ML = Mycorrhiza (Local isolate); MG = Mycorrhiza (Glomus mosseae); Th = Trichoderma harzianum; EM = Effective Microorganisms.
* Values are means ± standard error. Significant differences (P<0.05 using Tukey posthoc test) among treatments in the same column are indicated by different letters. *The legends(letters): Decrease from a to g.
Fig. 1 indicates the content of the defense enzyme acid invertase at six weeks (expressed as total sugar content) gave low activities in all inoculation treatments of the pathogenized plants except those inoculated with T. harzianum. In the unpathogenized plants inoculated with the local mycorrhiza, however, the content of this enzyme was significantly higher than in the control and all inoculated pathogenized treatments.
Fusarium oxysporum f.sp. lycopersici (FOL) are predominantly soil-borne microorganisms of a broad scope of green and agronomic harvests, which cause disastrous vascular wilts, rots, and damping-off sicknesses.19 Likewise, Fusarium poisons are viewed as the greatest20, 21, 22, 23. For instance, FOL causes the vascular wilt of tomatoes, resulting in a remarkable reduction in the crop output.24 Tomato wilt is one of the major diseases of tomatoes caused by FOL Borisade et al.25 The current scenario demands a better understanding of the disease more than ever, hence the quest for sustainable disease management strategies.
In the current study, we implored an in vitro model to investigate the FOL1 pathogenesis in tomato var. Beeli. Subsequently, the antimicrobial biological compounds (such as Mycorrhiza (Local isolate), Mycorrhiza (Glomus mosseae), Trichoderma harzianum, and Effective Microorganisms were explored as a potential antidote.
If cultures of beneficial microorganisms are effective after inoculation into the soil, their initial populations must be at a certain critical threshold level. This helps to ensure that the amount of bioactive substances produced by them will be sufficient to achieve the desired positive effects on crop production and crop protection.
Our data suggest the protein contents of all pathogenized plants exceeded that of the control. Measurement of total protein content provides a robust tool to understand the variation in cellular protein expression during the host-pathogen communication.26 Compared to the control, an increase in total protein content was possible attributes to the pathogenicity and plant defense mechanism.27
The measurement of the chlorophyll content indicates the plant’s health, rate of photosynthesis, and resistance to biotic and abiotic stress.28 In our settings the FOL1 pathogenicity (24.69 mg/g), FOL1 + ML (14.44 mg/g), FOL1 + ML + MG (11.06 mg/g) and FOL1 + Th (8.19 mg/g) significantly decrease the chlorophyll content compared to the control (35.21 mg/g).
However, the ML treatment (35.44 mg/g) did not alter the content. Moreover, FOL1 + EM (33.18 mg/g) restored the content largely. EM (biofertilizer – a mixture of potentially beneficial microorganisms) maintained the leaf photosynthetic activity and increased bean plant seed yield.29 Likewise, EM rescued the chlorophyll content in salinity affected Arabidopsis.30
Apparently, the peroxidase activity remained unaltered after FOL1 pathogenicity and FOL1 + EM treatment compared to the control. However, FOL1 in combination with ML, MG, and Th significantly suppressed the enzyme activity.
Contrary to our findings, peroxidase is known to be the key enzyme of the defense-related pathway in plants.31 Besides, FOL1 pathogenized plants (1.15 mg/g) exhibited significantly increased levels of soluble phenols compared with the control (0.74 mg/g). However, additions of ML, MG, Th to FOL1 -infected plants significantly reversed the phenols level. The increased phenols level indicates that the plant is undergoing and/or combating stress.32
Further, we investigated the effect of PAL activity, yet another well-known enzyme related to plant immunity against stress,33 in inoculated plants compared to control at different time points (i.e., 15, 30, 60, and 90 minutes post-inoculation). The level of PAL in all inoculations, but FOL1 +EM, remained comparable to control. The peak activity was observed at 30 minutes, yet the levels remained comparable to the control in all inoculations. The levels dropped at 60 minutes and kept on dropping at 90 minutes. The PAL levels stayed lower than the control at 60 and 90 minutes in most of the inoculations. However, seemingly FOL1 and Mycorrhiza (Local isolate) solo inoculations demonstrated higher levels at all time points compared with control.
Altogether, our findings suggest that Tomato cultivar Beeli subjected to a fungal (Fusarium oxysporum f.sp lycopersici.) infection mediated stress elicited plant immune response. Evidently, the pathogenic effects were counteracted and rescued in most cases through the application of numerous generic and commercial biocontrol and biofertilizers. This information substantially contributes to the understanding of the underlying, FOL1 mediated immune/rescue mechanism in Tomato plants, and assists the development of novel markers for disease management strategies.
In conclusion, inoculation (pathogenicity) of tomato plant cultivar Beeli with FOL1 fungus improved plant protein contents, particularly the EM inoculum or the local mycorrhiza. In addition, chlorophyll content was decreased in pathogenized plants but was significantly improved in the presence of the local mycorrhiza or EM. The amounts of chlorophyll, proteins, and, the activities of the defense enzymes such as acid invertase, peroxidase, polyphenol oxidase, and phenylalanine ammonia-lyase are important plant defense mechanisms through their role in the oxidation of phenolic compounds.
ACKNOWLEDGMENTS
The authors would like to thank Deanship of Research Deanship at the University of Hail for financial support of the project number BA-2030.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors contributed to the study’s conception and design. NI, RB, AE, and AJ performed the experiments. NA, RB, AE, and HE analyzed the data. All authors read and approved the manuscript.
FUNDING
This work was supported by the Deanship of Research of the University of Hail, Project No: BA-2030.
ETHICS STATEMENT
Not applicable.
AVAILABILITY OF DATA
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
- Rashida P, Hafiz ARS, Faqir MA, Masood SB, Imran P, Sarfraz A. Tomato (Solanum lycopersicum) Carotenoids and Lycopenes Chemistry; Metabolism, Absorption, Nutrition, and Allied Health Claims-A Comprehensive Review. Critical Reviews in Food Science and Nutrition, 2015;55(7): 919–929.
Crossref - Singh HB.Management of plant pathogens with microorganisms. Proceedings of Indian National Science Academy, 2014;80 (2):443–454
Crossref - O’Brien PA. Biological control of plant diseases. Australian Plant Pathology, 2017;46(4):293-304.
Crossref - Kasun MT, Dinushani A, Daranagama AJ, Phillips L, Sagarika DK, Itthayakorn P. Fungi vs. Fungi in Biocontrol: An Overview of Fungal Antagonists Applied Against Fungal Plant Pathogens. Front. Cell. Infect. Microbiol., 2020;30.
Crossref - Charoenporn C, Kanokmedhakul S, Lin FC, Poeaim S, Soytong K. Evaluation of bio-agent formulations to control Fusariumwiltoftomato. African Journal of Biotechnology, 2010;9(36):5836-5844. http://www.academicjournals.org/AJB
- Halima ZH, Shaker I, Al-Dulaimi B. Biological management of Fusarium wilt on tomato caused by Fusarium oxysporum f. sp. lycospersici by some plant growth-promoting bacteria. bioRxiv, 2020;
Crossref - Agrios GN. Plant Pathology. 4th ed. Elsevier Academic Press. New York, USA, 2004; 635. https://booksite.elsevier.com/samplechapters/9780120445653/0120445654.pdf.
- Singh VK, Singh AK, Kumar A. Disease management of tomato through PGPB: current trends and future perspective. 3 Biotechnology,2017;7:255
Crossref - Kamil Z, Rizk M, Mustafa S. Isolation and Identification of Rhizophere Soil Chitinolytic Bacteria and their Potential in Antifungal Biocontrol. Global Journal of Molecular Science, 2007;24(13):2339-2349 http://www.idosi.org/gjms/gjms2(2)/4.pdf
- Lichtenthaler HK. Chlorophylls and Carotenoids: Pigments of Photosynthetic Bio-membranes. Methods in enzymology, 1987;148:350-382.
Crossref - AOAC. Official Methods of analysis. 8th Edition, Association of Official Analytical Chemists. Washington DC, 2010.
- Hsu CL, Chen W, Weng YM, Tseng CY. Chemical composition, physical properties and antioxidant activities of yam flours as affected by different drying methods. Food Chemistry, 2003;83:85-92.
Crossref - Slinkard K, Singleton VL. Total phenol analysis: automation and comparison with manual methods. American Journal of Enology and Viticulture, 1977;28: 49-55 http://www.sciepub.com/reference/125515
- Houssien AA, Ahmed SM, Ismail AA. Activation of tomato plant defense response against Fusarium wilt disease using Trichoderma harzianum and salicylic acid under greenhouse conditions. Research Journal of Agriculture and Biological Sciences, 2010;6(3): 328-338 http://www.aensiweb.net/AENSIWEB/rjabs/rjabs/2010/328-338.pdf.
- Hwang BK, Heitefuss R. Sugar composition and acid invertase activity in spring barley plants in relation to adult – plant resistance to powdery mildew. Phytopathology, 1986;67:365-369.
- Schneider F. Sugar Analysis, Official and tentative methods recommended by the International Commission for Uniform Methods of Sugar Analysis, Peterborough, 1979; pp. 41-73.
Crossref - Dunn DC, Duncan LW, Romeo JT. Changes in arginine, PAL activity and nematode behavior in salinity stressed citrus in Honour of Professor GH Neil Towers 75th birthday Photochemistry, 1998; 49: 413-417.
Crossref - Statsoft Inc. Statistica for Windows (Data Analysis Software System), Version 8.0. 298 p. Computer Program Manual, Quick Reference. Statsoft. Inc., 2008.
- Bodah ET. Root rot diseases in plants: a review of common causal agents and management strategies. Agri. Res. Tech. 2017;5(3):555661.
- Venkataramana M, Navya K, Chandranayaka S, Priyanka SR, Murali HS, Batra HV. Development and validation of an immunochromatographic assay for rapid detection of fumonisin B1 from cereal samples. J. Food Sci. Technol., 2014;1-9.
- Divakara ST, Santosh P, Aiyaz M, Ramana MV, Hariprasad P, Nayaka SC, Niranjana SR. Molecular identification and characterization of Fusarium spp. associated with sorghum seeds. J. Sci. Food Agric. 2014; 94(6):1132-1139.
- Kalagatur NK, Mudili V, Siddaiah C, Gupta VK, Natarajan G, Sreepathi MH, Putcha VL. Antagonistic activity of Ocimum sanctum L. essential oil on growth and zearalenone production by Fusarium graminearumin Maize grains. Front Microbiol. 2015;6:892.
- Kumar KN, Venkataramana M, Allen JA, Chandranayaka S, Murali HS, Batra HV. Role of Curcuma longa L. essential oil in controlling the growth and zearalenone production of Fusarium graminearum. LWT-Food Sci. Technol. 2016;69:522-528
- Asha, BB, Nayaka SC, Shankar ACU, Srinivas C, Niranjana SR. Selection of effective bio–antagonistic bacteria for biological control of tomato wilt caused by Fusarium oxysporum f Sp. Lycopersici. BioscanIntquart.. J. Life Sci. 2011;6:239-244.
- Borisade OA, Uwaidem YI, Salami AE. Preliminary report on Fusarium oxysporum f.sp. Lycopersici (Sensulato) from some tomato producing agroecological areas in Southwestern Nigeria and susceptibility of F1- resistant tomato hybrid (F1-Lindo) to infection. Ann. Res. Rev. Biol. 2017;18(2):1-9
- Sun J, Fernandez-Cid A, Riera A, Tognetti S, Yuan Z, Stillman B, Speck C, Li H. Structural and mechanistic insights into Mcm2-7 double-hexamer assembly and function. Genes Dev. 2014;28(20):2291-303.
Crossref - De Sain M, Rep M. The Role of Pathogen-Secreted Proteins in Fungal Vascular Wilt Diseases. Int. J. Mol. Sci., 2015; 16:23970-23993.
Crossref - Pavlovik D, Nikolic B, Durovic S, Waisi H, Andelikovic A, Marisavljevic D. Chlorophyll as a Measure of Plant Health: Agro-Ecological Aspects. Pestic. Phytomed. (Belgrade), 2014;29:21-34.
- Iriti M; Scarafoni A, Pierce S, Castorina G, Vitalini S. Soil Application of Effective Microorganisms (EM) Maintains Leaf Photosynthetic Efficiency, Increases Seed Yield and Quality Traits of Bean (Phaseolus vulgaris L.) Plants Grown on Different Substrates. Int. J. Mol. Sci, 2019;20: 2327.
Crossref - Kalaji HM, Jajoo A, Oukarroum A, et al. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol Plant, 2016;38: 102.
Crossref - Singh H, Dixit S, Singh P, Verma PC. Differential peroxidase activities in three different crops upon insect feeding. Plant Signal Behav. 2013;8:e25615
Crossref - Shalaby S, Horwitz BA. Plant phenolic compounds and oxidative stress: integrated signals in fungal-plant interactions. Curr Genet. 2015;61(3):347-57.
Crossref - Kim DS, Hwang BK. An important role of the pepper phenylalanine ammonia-lyase gene (PAL1) in salicylic acid-dependent signalling of the defence response to microbial pathogens. J Exp Bot. 2015;65(9):2295-2306.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.