ISSN: 0973-7510
E-ISSN: 2581-690X
Lactobacillus plantarum MTCC 9503 and Lactobacillus acidophilus NCDC 291 are known bacteriocin producers. Bacteriocin produced by them was quantified. The former produced 10,000 AU/ml and the latter produced 1000 AU/ml of bacteriocin. The effect of bacteriocin preparation on spore viability, spore germination and mycelial growth of laboratory isolates Aspergillus, Penicillium, Fusarium and Alternaria was investigated. 10,000, 15,000 and 20,00 AU of bacteriocin was used for in vitro studies. Plantarum bacteriocin was more effective than acidophilus bacteriocin in reducing spore viability. Spore germination in presence of bacteriocin was observed at 12h compared to 6h in the control run. Mycelial growth was significantly reduced in presence of different concentrations of bacteriocins of both the strains.
Bacteriocins, in vitro antifungal activity, spore viability, spore germination, mycelial growth
Conventional food preservation techniques are asepsis, removal of microorganisms, use of high temperature, use of low temperature, drying, use of chemical preservatives, irradiation and modified atmosphere packagaing. Newer methods include use of high hydrostatic pressure, ozonization, high pulsed electric field, etc. They are not used industrially because of cost of processing especially because India processes a dismal 2-3% of what is produced. Also, some nutrients as water soluble vitamins are sensitive to food processing techniques. Production of ‘minimally processed food’ with little or no chemical additives is a challenge for food industry today. Bacteriocins hold promise on this account.
Bacteriocins are ribosomally synthesized proteins produced by lactic acid bacteria as well as some non-lactic acid bacteria. They are antimicrobial in nature, a property which can be exploited in food biopreservation. They are effective against food borne pathogens as well as food spoilage organisms1. This property can be exploited to make food microbiologically safe and also prevent food spoilage. Bacteriocin producer can be inoculated in the food or the antimicrobial cane be purified and used as food additive. Moreover, lactic acid bacteria are probiotic in nature which boost immunity.
One third of the food produced for human consumption is lost globally, which amounts to about 1.3 billion tons per year2. India losses nearly 40-50% of produce post-harvest due to poor infrastructure, high average temperature which favors microbial growth, etc. Microbes are major agent of food spoilage. Besides bacteria, Aspergillus, Penicillium, etc are very well documented food spoilage molds. The present work was planned to ascertain antimycotic effect of bacteriocin preparation from L plantarum and L acidophilus on laboratory isolates of Aspergillus, Penicillium, Fusarium and Alternaria. Effect of different concentrations of bacteriocin preparation was studied on spore viability, spore germination and fungal growth, dry weight basis.
Lactobacillus plantarum and L acidophilus are very well known bacteriocin producers. They were the subject of the present investigation. Growth kinetics of these bacterial strains was recorded to determine the stage of growth for maximum bacteriocin production (data not shown). Bacteriocin was preparation3. Antimicrobial assay using well diffusion technique was performed using bacteriocin prepared from broth cultures corresponding to different phases of growth identified earlier4. Bacteriocin later quantified5,6 Related work by the author was earlier published in which bacteriocin prepared by adsorption-desorption method.
Pure cultures of laboratory isolates causing food spoilage were prepared on potato dextrose medium and used to determine effect of bacteriocin on spore viability, spore germination and mycelial growth of these isolates. Spore viability experiment was performed7 using different concentrations of bacteriocin preparation were used- 10,000 AU to 20,000 AU. Spore suspension containing 107 spores/ml was prepared by scrapping off spores from the surface of mold growth on agar plates using an ordinary brush. This mixture of spore suspension and bacteriocin was incubated at 25-28°C. 0.1 ml of the portion was plated every six hours for a total time period of 24h. Standard plate count (cfu) was recorded.
Effect of presence of bacteriocin on spore germination was recorded8. Known number of spores were added to broth medium. Bacteriocin preparation of AU ranging from 10,000 to 20,000 were added to this spore suspension. It was vortexed for one minute. 0.15 ml was pipetted onto a sterile glass slide which was then incubated at 25-28°C for nearly 20h. The slides were examined microscopically every six hours. Direct count of 300 germinated spores per slide was recorded and percentage spore germination was calculated.
In order to study the effect of bacteriocin on mold growth, Potato Dextrose Broth was inoculated with approximately 106 spores/ml of mold strain and also with active bacteriocin of concentration from 10,000 to 20,000 AU. After incubation for 72 h at 30°C the growth of the fungi was ascertained by dry weight recordings9,10.
A control without bacteriocin was also run for these three parameters. 0.1g of commercially available Nisin was dissolved in 10 ml of 0.02M HCl and stored at -10°C was quantified using Well Diffusion Assay. Nisin was also tested for antifungal efficacy using the methods described earlier.
Bacteriocin preparation from Lactobacillus plantarum MTCC 9503 contained 10,000 AU/ml whereas that of L acidophilus contained 1000 AU/ml. Commercially available Nisin contained 100,000 AU/ml. Antimycotic efficacy of these preparations was determined as per methods outlined above. Initially, bacteriocin concentration of 1000-5000 AU was tried for preliminary examination and found ineffective. Bacteriocin concentration of 10,000, 15,000 and 20,000 was used for all experiments. Statistical analysis of the observations was done using CPCS1.
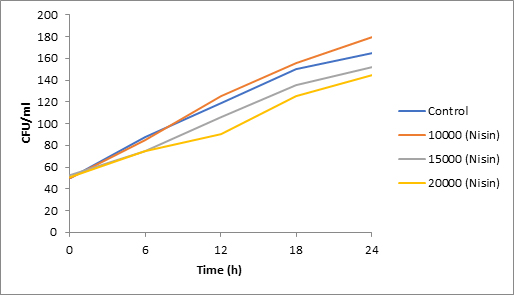
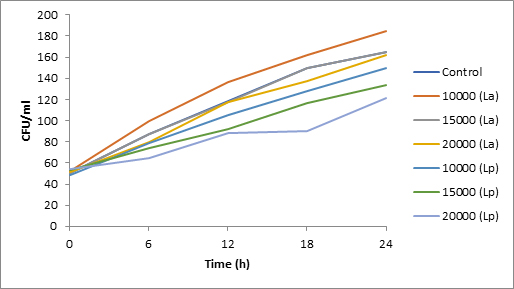
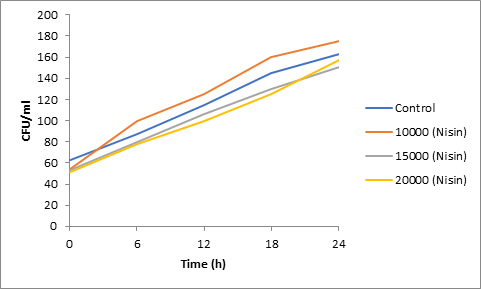
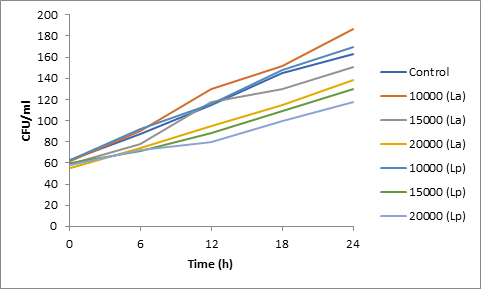
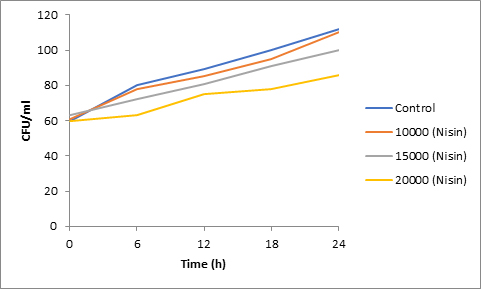
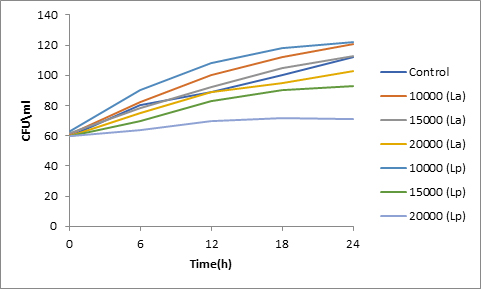
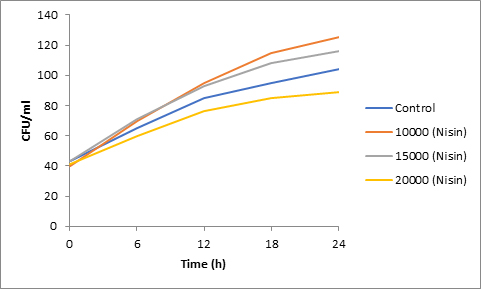
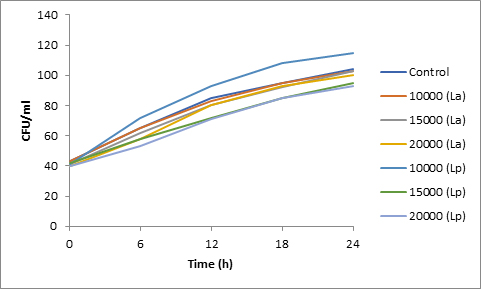
Results of spore viability are presented in Figures 1-6. The difference in spore viability of control and trial runs increased with increase in time period of incubation. Treatment with 15,000 and 20,000 AU of Nisin resulted in decrease of spore viability of all four molds isolates- Aspergillus, Penicillium, Fusarium and Alternaria. 20,000 AU/ml of Nisin decreased spore viability of Aspergillus by 12%, Alternaria by 14.4%, Fusarium by 23.2% after 24h. Penicillium spore viability however decreased by only 3.3%. Acidophilus preparation was ineffective against Aspergillus. After 24 h of incubation, it decreased spore viability of Penicillium by 16.4%, Fusarium by 8%, Alternaria by only 3.8% at a concentration of 20,000 AU. Plantarum preparation was more effective in decreasing spore viability of all four molds. 15,000 AU decreased Aspergillus spore viability by 18.7%, Penicillium by 21.2%, Fusarium by 16.9% and Alternaria by 8.7%. 20,000 AU was expectantly more effective in Aspergillus and Fusarium. This benefit was marginally higher in Penicillium and Alternaria. 10,000 AU of plantarum bacteriocin was more effective than 20,000 AU/ml of acidophilus bacteriocin.
Bacteriocin demonstrated their ability to delay spore germination in in vitro studies. Significant decrease in spore germination was observed in test run as compared to the control. Spore germination of all four molds was observed after 6h of incubation in the control run. Treatment with 20,000 Nisin delayed it to 12h. Plantarum bacteriocin at 15,000 AU and 20,000 AU delayed spore germination to 12h. Significant decrease was observed at 10,000 AU but only after 24h of incubation. Acidophilus bacteriocin at 20,000 AU delayed spore germination to 12h only in Fusarium and Alternaria. As in spore viability observations, planatrum preparation was more effective than acidophilus.
Table (1):
Effect of bacteriocin on % spore germination in Aspergillus.
| Time (h) |
L plantarum (AU) | L acidophilus (AU) | Nisin (AU) | Control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10,000 | 15,000 | 20,000 | 10,000 | 15,000 | 20,000 | 10,000 | 15,000 | 20,000 | ||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 20 | 0 | 0 | 23 | 24 | 20 | 22 | 12 | 0 | 37 |
| 12 | 36 | 30 | 24 | 45 | 47 | 39 | 40 | 20 | 32 | 53 |
| 18 | 48 | 42 | 34 | 61 | 71 | 49 | 31 | 34 | 28 | 73 |
Table (2):
Effect of bacteriocin on % spore germination in Penicillium.
| Time (h) |
L plantarum (AU) | L acidophilus (AU) | Nisin (AU) | Control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10,000 | 15,000 | 20,000 | 10,000 | 15,000 | 20,000 | 10,000 | 15,000 | 20,000 | ||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 13 | 0 | 0 | 21 | 20 | 21 | 19 | 15 | 0 | 23 |
| 12 | 36 | 46 | 24 | 43 | 36 | 35 | 24 | 35 | 27 | 61 |
| 18 | 46 | 51 | 36 | 54 | 40 | 48 | 46 | 39 | 31 | 79 |
Table (3):
Effect of bacteriocin on % spore germination in Fusarium.
| Time (h) |
L plantarum (AU) | L acidophilus (AU) | Nisin (AU) | Control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10,000 | 15,000 | 20,000 | 10,000 | 15,000 | 20,000 | 10,000 | 15,000 | 20,000 | ||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 34 | 0 | 0 | 29 | 28 | 0 | 9 | 19 | 0 | 36 |
| 12 | 44 | 46 | 47 | 46 | 37 | 27 | 16 | 21 | 12 | 51 |
| 18 | 48 | 45 | 40 | 52 | 41 | 39 | 31 | 38 | 11 | 70 |
Table (4):
Effect of bacteriocin on % spore germination in Alternaria.
| Time (h) |
L plantarum (AU) | L acidophilus (AU) | Nisin (AU) | Control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10,000 | 15,000 | 20,000 | 10,000 | 15,000 | 20,000 | 10,000 | 15,000 | 20,000 | ||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 23 | 0 | 0 | 31 | 19 | 0 | 13 | 12 | 0 | 34 |
| 12 | 32 | 44 | 41 | 42 | 33 | 21 | 24 | 26 | 5 | 51 |
| 18 | 49 | 46 | 43 | 49 | 41 | 32 | 36 | 41 | 19 | 73 |
Table (5):
Aspergillus growth in presence of bacteriocin (dry weight basis).
| Time days |
L plantarum(AU) | L acidophilus(AU) | Nisin (AU) | Control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10,000 | 15,000 | 20,000 | 10,000 | 15,000 | 20,000 | 10,000 | 15,000 | 20,000 | ||
| 4 | 2.10± 0.11 |
2.12± 0.06 |
2.07± 0.04 |
2.46± 0.08 |
2.08± 0.04 |
1.99± 0.07 |
2.12± 0.06 |
1.77± 0.03 |
1.55± 0.23 |
3.56± 0.21 |
| 8 | 3.56± 0.16 |
2.92± 0.03 |
3.73± 0.20 |
3.10± 0.05 |
2.95± 0.03 |
2.86± 0.05 |
2.84± 0.05 |
2.47± 0.01 |
2.62± 0.05 |
3.89± 0.06 |
Table (6):
Penicillium growth in presence of bacteriocin (dry weight basis).
| Time days |
L plantarum(AU) | L acidophilus(AU) | Nisin (AU) | Control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10,000 | 15,000 | 20,000 | 10,000 | 15,000 | 20,000 | 10,000 | 15,000 | 20,000 | ||
| 4 | 2.27± 0.03 |
1.90± 0.05 |
1.82± 0.09 |
2.37± 0.03 |
1.96± 0.03 |
1.77± 0.03 |
2.06± 0.03 |
1.90± 0.05 |
1.68± 0.05 |
2.48± 0.04 |
| 8 | 3.26± 0.04 |
2.81± 0.06 |
2.39± 0.04 |
3.09± 0.05 |
2.87± 0.01 |
2.43± 0.00 |
2.90± 0.05 |
2.42± 0.02 |
2.43± 0.02 |
3.90± 0.04 |
Table (7):
Fusarium growth in presence of bacteriocin (dry weight basis).
| Time days |
L plantarum(AU) | L acidophilus(AU) | Nisin (AU) | Control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10,000 | 15,000 | 20,000 | 10,000 | 15,000 | 20,000 | 10,000 | 15,000 | 20,000 | ||
| 4 | 1.22± 0.01 |
1.21± 0.07 |
1.06± 0.04 |
1.38± 0.03 |
1.18± 0.04 |
1.09± 0.01 |
1.16± 0.03 |
1.21± 0.05 |
1.09± 0.05 |
1.55± 0.03 |
| 8 | 1.82± 0.02 |
1.52± 0.01 |
1.41± 0.02 |
1.94± 0.05 |
1.65± 0.01 |
1.25± 0.03 |
1.62± 0.03 |
1.69± 0.01 |
1.50± 0.05 |
2.07± 0.03 |
Table (8):
Alternaria growth in presence of bacteriocin (dry weight basis).
| Time days |
L plantarum(AU) | L acidophilus(AU) | Nisin (AU) | Control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10,000 | 15,000 | 20,000 | 10,000 | 15,000 | 20,000 | 10,000 | 15,000 | 20,000 | ||
| 4 | 1.93± 0.03 |
1.63± 0.01 |
1.47± 0.02 |
1.91± 0.06 |
1.88± 0.05 |
1.46± 0.02 |
1.77± 0.01 |
1.37± 0.02 |
1.37± 0.02 |
2.19± 0.01 |
| 8 | 2.74± 0.02 |
2.22± 0.06 |
2.26± 0.02 |
2.99± 0.06 |
2.50± 0.20 |
1.99± 0.01 |
2.65± 0.01 |
2.23± 0.02 |
2.37± 0.14 |
3.44± 0.00 |
The effect of bacteriocin from L plantarum and L acidophilus on fungal growth was determined and the results are presented in Table 5-8. The growth was recorded on 4th and 8th day in terms of dry weight measurements dry weight measurements. Significant reduction in growth of all molds was recorded at both four days and eight days of incubation using CPCS1 statistical software.
After eight days of incubation 10,000 AU of Nisin reduced growth of Aspergillus by 27%, Penicillium by 26%, Fusarium by 22% and Alternaria by 23%. Aspergillus growth was reduced to a greater extent after only four days by both plantarum and acidophilus bacteriocins. The extent of reduction was however less at eighth day in incubation. Plantarum reduced Penicillium growth by 28 % (15,000 AU) and 39% (20,000 AU); Fusarium by 27% (15,000AU) and 32% (20,000AU); Alternaria by 20% (10,000AU), 35% (15,000AU) and 34% (20,000AU) at eighth day of incubation. 20%, 24% reduction in dry weight of Aspergillus was observed using 10,000 and 15,000 AU of acidophilus bacteriocin respectively. Similarly, growth reduction was observed in Fusarium and Alternaria. Growth of all the four mold genera was inhibited up to 42% by using 20,000 AU of bacteriocin which demonstrates their ability to restrict fungal spoilage.
Bacteriocin activity to inhibit bacterial strains is very well documented. Utility of these antimicrobials as antimycotic agents has also been reported by many workers. Lactobacillus acidophilus‘ antifungal activity was reported11-13. Strains of L plantarum designated as DW1, DW3 and DW4 successfully inhibited growth of yeast as Rhodotorula, Candida, Hansenula, Pichia, Saccharomyces14. Combination of these two lactic acid bacterial strains influenced growth of Aspergillus flavus15. Actively growing cells were however more effective than antimicrobial preparation. Fermented food isolates have demonstrated antifungal activity16. Lactic acid bacteria were isolated from Eko, Fufu, Iru and Ogi. 68% of the isolates possessed antifungal activity against Penicillium citrinum, Aspergillus niger and Aspergillus flavus. Spore germination as well as mycelial growth were both inhibited17.
Bacteriocin like peptide pentocin TV35b, produced by Lactobacillus pentosus had a fungistatic effect. Penicillium expansum IDM/FS2 and Fusarium graminaerum IDM 623 were inhibited by Lactobacillus sp, Leuconostoc sp18. Lactobacillus plantarum CGMCC 11856 was reported to inhibit Aspergillus flavus spore viability19. Antifungal activity of L plantarum ATCC 8014 against Penicillium sp has been reported by other workers as well20. Effect of Lactobacillus on fungal spore germination of Aspergillus. Percentage spore germination in control was recorded to be 80% compared to 48% in presence of bacteriocin.
Bacteriocins can be an important tool in production of ‘minimally processed food’ which contain little or no use of chemical additives. They do not cause allergies. Being probiotic in nature lactic acid bacteria aid in maintaining gut microflora. Application of bacteriocin to prevent mold growth can prevent spoilage of food and counter the problem of mycotoxin production in food. Bacteriocins can ensure food safety. Despite the many benefits extended there is little industrial use of bacteriocins. Major deterrents include low yield and high cost of down-stream processing. Increasing gene dosage of genetic determinants of bacteriocins can enhance fermentative production. Legal and biosafety hurdles must be dealt with to facilitate their safe commercial use in food and also feed.
Despite discovery of newer methods of food processing there are many challenges for the food processing industry. One of them is substitution of chemical additives which are associated with increased incidence of food allergies. Bacteriocins can play this role as biopreservatives. Bacteriocin producing Lactobacillus plantarum and Lactobacillus acidophilus were both used in the present study to ascertain their antifungal efficacy. Plantarum bacteriocin was more effective compared to acidophilus bacteriocin preparation. Spore viability, spore germination and mycelial growth were all inhibited at higher concentration of 15,000 AU in in vitro studies. Further work on enhancing their antimicrobial spectrum must be pursued. Alternatively, bacteriocins can be used as a component in hurdle concept of food preservation.
ACKNOWLEDGMENTS
Research grant in the form of major research project by UGC, New Delhi is gratefully acknowledged.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
- Settani, L. and Corsetti, A. Application of bacteriocins in vegetable food biopreservation. Intl. J. Food. Microbiol. 2008; 121, 123-138.
- Gustavsson, J., Cederberg, C. and Sonesson, U. Global food losses and food waste. Swedish Institute for Food and Biotechnology, Gothenburg, Sweden, 2011.
- Cabo, M.l., Murade, M.A., Gongalez, M.P. and Pastoria, L. A method for bacteriocin quantification. J. Appl. Microbiol. 1999; 87, 907-914.
- Ogunbanwo, S.T., Sanni, A.I. and Onilude, A.A. Characterization of bacteriocin produced by Lactobacillus plantarum F1 and Lactobacillus brevis OG1. Afr. J. Biotechnol. 2003; 2, 219-227.
- Schillinger, U. and Lucke, F.K. Antibacterial activity of Lactobacillus sake isolated from meat. Appl. Environ. Microbiol. 1989; 55, 1901-1906.
- Barefoot, S.F. and Klanhammer, T.R. Detection and activity of lactacin B, a bacteriocin produced by Lactobacillus acidophilus. Appl. Environ. Microbiol. 1983; 45, 1808-1815.
- Gourama, H. and Bullerman, L.B.Inhibition of growth and aflatoxin production of Aspergillus flavus by Lactobacillus species. J. Food Protect. 1995; 58, 1249-1256.
- Coxon, D.T., Ogundana, S.K.and Dennis, C. Antifungal phenathrenes in yam tubers. Phytochem. 1982; 21,1389-1392.
- Neilson, L., Huss, H.H. and Gram, L. Inhibition of L monocytogenes on cold smoked salmon by nisin and carbon monoxide atmosphere. Intl. J. Food Microbiol. 1997; 38, 217-227.
- Roy, U., Batish, V.K., Grover, S. and Neellkantan, S. Production of antifungal substance by Lactococcus lactis subspp lactis CHD-28.3. Intl. J. Food Microbiol. 1996; 32, 27-34.
- Batish, V.K., Lal, R. and Grover, S. Screening lactic acid starter cultures for antifungal activity. Cul Dairy Prod J. 1989; 24, 22-25.
- Batish, V.K., Lal, R. and Grover, S. (1990) Studies in environmental and nutritional factors on production of antifungal substance by Lactobacillus acidophilus R. Food Microbiol. 1990; 7, 199-206.
- Batish, V.K., Roy, U., Lal, R. and Grover, S. Antifungal attributes of lactic acid bacteria- a review. Critical Rev. Biotechnol. 1997; 17, 209-225.
- Prachyakij, P., Schnurer, J., Charernjiratrakul, W. and Kantachote, D. (2007). Selection and identification of lactic acid bacteria that inhibit yeast contaminants isolated from fermented plant beverages. J. Sci. Technol. 2007; 29, 211-218.
- Gourama, H. and Bullerman, L.B. Anti-aflatoxigenic activity of Lactobacillus casei pseudoplantarum. Intl J Food Microbiol.1997; 34, 131–143.
- Adebayo, C.O. and Aderiya, B.I. Antibacterial activity of bacteriocin from indigenous fermented foods. Intl. J. Res. 2008; 4, 25-32.
- Okkers, D.J., Dicks, L.M.T., Silvester, M., Joubert, J.J. and Odendaal, H.J. Characterization of pentocin TV35b a bacteriocin like peptide isolated from Lactobacillus pentosus with a fungistatic effect on Candida albicans. J. Appl. Microbiol. 1999; 87, 726-734.
- Lavermicocca, P., Valerio, F., Evidente, A., Lazzaroni, S., Corsetti, A. and Gobbetti M. Purification and characterization of novel antifungal compounds by sourdough Lactobacillus plantarum 21B. Appl. Environ. Microbiol. 2000; 66, 4084-4090.
- Xu, M.G., Chen, J.F., Martin, F., Zhao, M.W., Eriani, G. and Wang, E.D. Leucyl-tRNA synthetase consisting of two subunits from hyper-thermophilic bacteria Aquifex aeolicus. J. Biol. Chem. 2002; 44, 969-974.
- Yu, K.H., Kang, S.N. and Park, S.Y. Physiochemical characteristics of L acidophilus KH-1 isolated from faeces of breast fed infant. J. Food Sci. Nutr. 2005; 10, 333-339.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.