ISSN: 0973-7510
E-ISSN: 2581-690X
Giardia lamblia is an intestinal protozoan parasite; hence, to diagnose the cyst, it is necessary to find a reliable, simpler and less expensive diagnostic method in the laboratory. Therefore, in order to choose a more sensitive, cheaper and applicable diagnostic method in the laboratory; three methods, namely, sucrose gradient, wet mount and formalin-ether in fecal samples from BALB/c mice infected with the protozoan Giardia, were studied. In this diagnostic laboratory study, 200 stool samples from infected mice (BALB/c) with Giardia were studied. Stool specimens from three direct smear method, formalin-ether and sucrose were prepared. A total of 200 specimens for each method were prepared and examined by microscope. Furthermore, it enhanced the accuracy check at each stage; the feces of healthy mice were used as negative controls for comparison. Stool examination revealed G. lamblia cysts in mice infected BALB/c from 200 samples for each method. In the direct method, formalin – ether and sucrose gradient, 101 (55.00%), 166 (83.00%) and 180 (94.00%) cases were positive respectively. Therefore, the sucrose method has demonstrated the highest sensitivity (94.00%), compared to other methods. According to the results of this study, sucrose is a sensitive method for high-throughput and cost-effective diagnostic method, than previous methods. Therefore, sucrose method is suggested suitable and can be used in place of formalin-ether. Given their potency, the sucrose gradient may be recommended to undergo futher studies.
BALB/c, Giardia lamblia, Diagnostic method, Formalin – ether, Sucrose gradient.
Giardiasis is a parasitic infection associated with diarrhea, stomach cramps, upset stomach, and excessive gas. Prompt and accurate diagnosis of intestine protozoa, particularly in excrement samples is important in parasitology. Giardia lambelia is one of the important human intestine protozoa. There are two forms of trophozoite and cyst in the life cycle of this parasite, and they are transmitted through fecal-oral method1,2. Protozoa can be transmitted to the next host via resources such as polluted water and food, directly and indirectly. Disease diagnosis is as a result of parasite, by virtue of clinical symptoms in persons who had symptoms, as well as tests of excrement samples of the persons who had clinical symptoms and no symptom3,4. Test methods such as wet mount, condense methods with ether formation, saturated saltwater, tin sulphate and sucrose gradient, small intestine biopsy, various serology, and molecule methods are usually employed for the diagnosis of G. lamblia,. Normally, microscopic tests of excrement samples in giardiasis involved preparing wet mount, condense methods of samples and constant coloring methods. The common method of diagnosing Giardia in the laboratories in Iran is wet mount. However, in rare cases, sedimentation with formalin-ether used for diagnosis is required for an experienced person, since it is not easily observed under lamp, particularly when the rate of the parasites is low in the excrement samples5.
Since the diagnosis methods used for excrement samples in the diagnosis laboratories and in the studies are so sensitive; in the diagnosis studies of determining the parasitology methods used, negative and positive report value should be considered in addition to sensitivity and specificity. Therefore, it is necessary to find a confident, simple and low cost method for the diagnosis of Giardia. Therefore, in responding to the choice of a more sensitive, low cost and performable method in the diagnosis laboratory, three methods, namely sucrose gradient, wet mount and formalin-ether in excrement samples of BALB/c mice contaminated with Giardia were investigated. These methods are applicable in the diagnosis laboratories and require less time, along with complex and developed equipments6,7.
In this laboratory diagnosis study, for the diagnosis of samples contaminated with Giardia, 200 excrement samples of male BALB/c mice were chosen, with 20g of human samples contaminated with Giardia at the ratio of 2:10. The contaminated rats were examined prior to the study, and their contaminations were confirmed 11 days after receiving Giardia. Subsequently, excrement samples were studied by means of these three methods, wet-mount, condense method of formalin – ether and sucrose. The excrement samples of the mice contaminated with the Giardia lamblia after preparation were delivered to the research group of parasitology section of Medical Faculty of Medical Sciences University of Mazandaran, and investigated with light microscope. For the test, any excrement samples of BALB/c mice prepared with wet-mount, formalin ether and sucrose methods (in total 600 samples, 200 specimens of any method) and in any stage with the sample related to the excrement of the healthy rat were used as negative control. A few sampling had specificity labels, these labels were used not to mistake the mice with other mice, and for the researcher to identify the required type of mice (healthy or polluted) and prepared with the 3 methods during test. In this study, following the preparation of samples with the three mentioned methods, the infection of the parasite in the samples was investigated with light microscope.
Microscopic test of excrement samples prepared by wet mount
Normally, microscopic test of excrement samples is carried out using wet mount. In this method, about 2mg of excrement was mixed with a drop of physiology serum on a lamp, and a neat G. lamblia was put over it. In this stage and subsequent stages, after the preparation of developed G. lamblia, the infection of the parasite in samples was investigated with light microscope.
Condense method of Formalin ether
This method was carried out by means of test parasite kits. 3.5ml of the test parasite solution was added to the above kit, and then the sample was placed in the kit and centrifuged at a speed of 100g. Finally, the liquid obtained was measured in the conic section of the kit, and sediment of G. lamblia was used for microscopic test8,9.
Purification method with sucrose gradient
In this method, the excrement samples were diluted with 1 to 12L of distilled water and 20 cc of the distilled excrement was poured in a plate containing glass peril and shaken for 5 min to form a soupy solution. Thereafter, the suspension glossed. Subsequently, 5ml of water was added to the obtained sediment, and 3.85ml of sucrose was slowly added to the mentioned solution. The obtained solution was centrifuged at 600g for 10min, and transformed by pasture pipe with the liquid that gathered in the middle layer, and G. lamblia sediment prepared for microscopic test. After examining the parasites, the data obtained were analyzed using SPSS software version 17. The sensitivity and specificity was calculated with report value in any method based on related formulas2.
Investigating the excrement of healthy mice by wet mount, formalin-ether and sucrose methods showed that, no method reported false positive, therefore, the specificity and positive report value of the three methods calculated was 100%. In addition, investigating the excrement samples of BALB/c mice contaminated with G. lamblia showed that, of the 200 prepared specimens for any method, wet mount, formalin–ether and sucrose methods, reported positive cases of 101 (55.00%), 166 (83.00%) and 180 (94.00%) respectively. Therefore, the sucrose method has demonstrated the highest sensitivity (94.00%), compared to other methods. Furthermore, to determine the abilities of diagnosis used in parasitology, Positive and Negative Predictive Value were calculated, the results showed the higher value of sucrose method when compared with other methods. In addition, the results of this study showed that, the use of purification method increased the possibility of Giardia cyst although not a routine diagnostic method (Table and Figure 1).
Table (1):
Diagnosis specificity, positive cases number, sensitivity, specificity, three methods of wet mount, formalin-ether and sucrose of 200 excrement samples contaminated with the cyst of G. lamblia in excrement samples of BALB/c mice.
| Cases Number | Sensitivity with Confidence interval(CI):95% | Positive Predictive Value | ||||
|---|---|---|---|---|---|---|
| Positive | Negative | Total | ||||
| wet mount | 101 | 99 | 200 | 55.00% (47.82% to 62.02%) | 52.63% | |
| formalin-ether | 166 | 34 | 200 | 83.00% (77.06% to 87.93%) | 62.64% | |
| sucrose | 180 | 20 | 200 | 94.00% (89.75% to 96.86%) | 65.51% | |
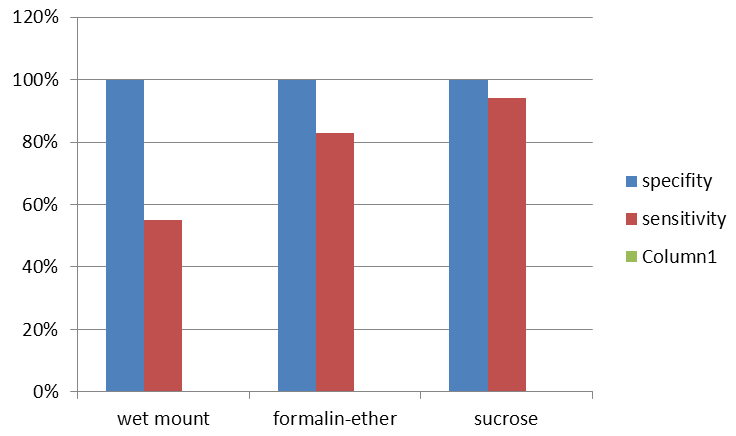 Fig. 1: Comparing the amount of specificity, sensitivity, three methods of wet mount, formalin-ether and sucrose in the diagnosis of Giardia in the excrement samples of BALB/c mice.
Fig. 1: Comparing the amount of specificity, sensitivity, three methods of wet mount, formalin-ether and sucrose in the diagnosis of Giardia in the excrement samples of BALB/c mice. Parasite diseases are one of the important health-economic problems of most countries of the world. Diagnosis parasite pollutions (protozoa and helminth) with suitable, inexpensive, safe and good diagnosis power were considered by experts of this field. Therefore, various studies were carried out globally, for the introduction and evaluation of exact methods of parasite pollutions diagnosis10-12. Determining the amount of parasite infection in the best condition is relative, and caution in diagnosing this infection depends on the method of test, skill and care in preparation stage, readiness and microscopic study of the samples and repeated number of times the test is performed. As a result, owing to the different use of diagnosis methods or method of performing them, the rate of outbreak in the studies were different from each other, and were constantly lower from the real amount of infection in the society.
Since it is important to employ diagnosis methods in treatment and diagnosis centers in various parts of the world, various studies were carried out to access the suitable diagnosis method of intestine parasite infections. Concerning the studies carried out in India in the year 2003, 17 studies of 80 common studies for parasite infection were positive for method of wet mount, 35 studies for formalin–ether, and 45 studies for the method of formalin-estone.13 Furthermore, formalin–eston showed the highest amount of sensitivity in diagnosis of intestine protozoa. In this study, of the 200 prepared samples for any method, formalin-ether and sucrose methods were reported to be positive with values of 166 and 180 respectively. A cross sectional study was conducted from May to June 2013 in Ethiopia. Single stool sample was processed for direct, Formol ether concentration (FEC) and Kato Katz methods. The sensitivity and negative predictive value (NPV) of diagnostic tests were calculated in terms of the “Gold” standard method (the combined result of the three methods altogether). In the study, the Kato Katz technique outperformed the other two methods but the true values for sensitivity, specificity and diagnostic values are not known. Moreover, it is labor intensive and not easily accessible. Hence, it is preferable to use FEC technique to complement the Wet mount test. In an attempt to achieve the easier and more sensitive faecal examination, in other study the ability of formol- detergent (FD) technique for detection of intestinal parasites was compared with the direct examination and the formalin – ether (FE) sedimentation technique. In this cross – sectional (descriptive – analitic) study faecal specimens were collected from 501 people of the Bilaghan and Sarvadar Villages in the vicinity of karaj city and examined by direct, FE and FD technique. This study privides evidence that FD technique is an important tool for the diagnosis of Giardia lamblia and is suitable and cheap for performing under field condition and should be considered in situations where resources are scarce. FD technique is available that is preferred to FE method. Ether is relatively expensive and explosive. The direct method is suggested for immediate answer and the motion of G. lamblia trophozoite.14,15 In this study, evaluation of sucrose method for diagnosis of G. lamblia proved to be more effective, when compared with the other methods of wet mount and formalin–ether. In the study 600 samples was examined with three methods of wet mount, formalin –ether, formalin–ethyl acetate. The sensitivity of the three methods was reported as 32, 73.3 and 64.7 respectively, and their Negative Predictive Value were 32.9, 55.6 and 48.5 respectively while Positive Predictive Value in three methods calculated as 100%. Acetone is preferred to ether due to more stability, less flammability and safer and cheaper. In our study, Positive Predictive Value of three direct methods of wet mount, formalin ether, and sucrose were 52.63%, 52.63% and 65.51% respectively (CI:95%). Therefore, the results of formalin-ether method in two studies were similar, with difference in the direct quantity method. It may be due to the method of preparing sample, and the experience of the researcher. Various studies from 1979 to 1996, pointed to the relative superiority of formalin-ethyl acetate method over formalin-ether in the diagnosis of intestine parasites . In other study detection of intestinal protozoa by using different methods was done .This study showed concentration techniques were significantly higher (P < 0.05) detection rates of parasites compared with direct smear microscopy.16-18 This study was carried out for the evaluation and completion of previous studies, and for finding suitable and applicable method in the diagnosis laboratories. In the diagnosis of the infected and non-infected samples with G. lamblia, the sensitivity of the direct methods, formalin-ether and sucrose method was 55.00%, 83.00% and 94.00%respectively. The results showed that sucrose method had the highest sensitivity in the diagnosis of G. lamblia. However, in studies conducted in and out of the country, the methods of formalin-ether and formalin-acetate were reported to be more sensitive.19,20 The method employed for the diagnosis of intestine parasite diseases should have the ability to be used for the diagnosis of nucleolus, larvae worm, trophozoite, unicellular cyst, not be time consuming, and should have minimum infection in the workplace. Formalin-ether is a difficult and expensive method, and also in the case of not observing safety issues, may lead to risks for health of employees21,22. In this study, sucrose method is a new method that is more sensitive than previous sediment method due to higher amount of its positive report value. In addition, this method is less expensive than formalin method and it is economical. Therefore, in supplementary studies, employing this method in cyst diagnosis, intestine unicellular trophozoite, and different forms of eggs and larvae worm parasites is necessary.
ACKNOWLEDGMENTS
We would like to thank of Research Deputy of Mazandaran University of Medical Sciences for the financial Support this work [Code: 1605].
- Saebi E. Text Book of Clinical Parasitilogy, protozoal diseases in Iran. 5th ed. Tehran: Aeij. (2011) p: 97-117.
- Elmi T. Gholami sh. Azadbakht M. ziaei H. Effect of Chloroformic Extract of Tanacetum parthenium in the treatment of Giardia lamblia infection in Balb/c Mice. J Mazand Univ Med Sci. (2014) 24(Supple 1): 157-165(Persian).
- Akhlaghi L, Mafi M, Oormazdi H, Meamar A. R ,Shirbazou S and Tabatabaie F . Frequency of intestinal parasitic infections and related factors among primary school children in Abyek township of Qazvin province (2011 -2012) Annals of Biological Research, 2013; 4(8):22-26
- Karaby O, Tamer A. Treatment of Gardiasis. World J Gastroenterol; 2004; 15(8): 1215-1217.
- Einarsson E, Ma’ayeh S, Svärd SG An up-date on Giardia and giardiasis, Curr Opin Microbiol., 2016; 5; 34:47-52. doi: 10.1016/j.mib.(2016) 07.019. [Epub ahead of print]
- Ian H. McHardy, Max Wu, Robyn Shimizu-Cohen, Marc Roger Couturier, and Romney M. Humphries, Detection of Intestinal Protozoa in the Clinical Laboratory, J Clin Microbiol.; 2014; 52(3): 712–720
- Stallmach A, Hagel S, Lohse AW. Diagnostic workup and therapy of infectious diarrhea. Current standards. Internist (Berl), 2015; 56(12):1353-60. doi: 10.1007/s00108-015-3756-2.
- Jahan N , Khatoon R , and Ahmad S Comparison of Microscopy and Enzyme Linked Immunosorbent Assay for Diagnosis of Giardia lamblia in Human Faecal Specimens, J Clin Diagn Res. 2014; 8(11): DC04–DC06.
- Fakhar M. Text book of common parasitic diseases in north of Iran.1th ed. Mazandaran: Shelfin., 2011: p: 145-152.
- Garcia LS. Diagnostic medical Parasitology. 4th ed. Washington DC, ASM press, 2001.
- Flores A, Esteban JG, Angles R, Mas-Coma S. Soil transmitted helminth infections at very high altitude in Bolivia. Trans roysoc trop Med Hyg. 2001; 95: 272-277.
- Parija SC, Bhattacharya S, Padhan P, Shivaprakash MR. Evaluation of formalin-acetone sedimentation in the concentration of stool for intestinal parasites. Trop Doct, 2003; 33: 163-164.
- Cringoli G , Coprological diagnosis: what’s new? Parassitologia.; 2004; 46(1-2):137-9.
- Shaddel M, Nazari p.M, Abadi AR. Comparative sensitivity of formol- detergent technique with formalin- ether and direct technique for detection of intestinal parasite. J AUMS. 2006; 4(3): 895-899.
- Yimer M, Hailu T, Mulu W, Abera B, Evaluation performance of diagnostic methods of intestinal parasitosis in school age children in Ethiopia. BMC Res Notes. 2015; 26;8:820. doi: 10.1186/s13104-015-1822-4.
- Ahmadi N, Gachkar L, Pakdad K, Ahmadi O. Ability of methods Formalin-ether, Wet mount and Formalin-acetone in the diagnosis of intestinal parasitic infections. Iranian J infect Dis Trop med. 2007; 12(38): 43-47.
- Cringoli G, Rinaldi L, Veneziano V, Capelli G, Scala A The influence of flotation solution, sample dilution and the choice of McMaster slide area (volume) on the reliability of the McMaster technique in estimating the faecal egg counts of gastrointestinal strongyles and Dicrocoelium dendriticum in sheep. Vet Parasitol. 2004; 13; 123(1-2):121-31.
- Hassan Mergani M, Al-Shebani Mohammed M, Khan N, Bano M, Hafeez Khan A, Detection of intestinal protozoa by using different methods, 2014; 2(2),28-32
- Amiri M, Nazemi S, Raei M, Chaman R, Norouzi P. A Comparison of Direct Technique and Formalin-Ether Method in Determining Parasitic Infection among Health-Card Applicants in Shahroud City. M L J , 2013; 7 (3):69-74.
- Fritsche TR, Selvarangan R. Medical parasitology. In: McPherson RA, Pincus MR, eds. Henry’s Clinical Diagnosis and Management by Laboratory Methods. 22td ed. Philadelphia. Saunders Elsevier, 2011.
- Intapan P.M, Maleewong W, Wongsaroj T, Singthong S, Morakote N. Comparison of the Quantitative Formalin Ethyl Acetate Concentration Technique and Agar Plate Culture for Diagnosis of Human Strongyloidiasis. J Clin Microbiol. 2005; 43(4): 1932–1933.
- Rahimi-Esboei B., Gholami Sh., Azadbakht M., ziaei H. Effect of Hydroalcholic extract of Artemisia annua on cysts of Giardia lamblia in invitro. Mazand Univ Med Sci., 2012; 22(90): 72-80 (Persian).
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


