ISSN: 0973-7510
E-ISSN: 2581-690X
One of the most important problems faced by microbiologists is to preserve bacterial isolates in the best state to study and further diagnosis. The current study aims to provide a summary of experimental results to maintain two species of bacteria alive after being stored by using some additives. This study found that the best temperature to preserve Staphylococcus aureus was -20°C for a year, while for Escherichia coli it was the same temperature except in using Glycerol (G) 100% and Food oil (FO) methods. The optimum method to preserve S. aureus was by using Normal Saline (NS), while Distilled Water (DW) was the optimum method to preserve E. coli at temperatures (4, 25 and -20)°C for a year, the phenotypic patterns for examining bacteria were maintained except in NS at 4°C for S. aureus after a year ago. Glycerol was used alone at concentrations (100, 50, 30 and 15)%, and another group used G+NS in the same volumes, good results were achieved when it used alone or with NS to preserve bacteria for six months at 4°C except for methods of G100% and (G100% + NS) for examining bacteria. FO has never been used as preservation liquid, it is successful to survive S. aureus at -20°C for a year, and when it was added to NS, E. coli survived for a year at three temperatures (4, 25 and -20)°C, while S. aureus didn’t survive for a year when FO+NS method used at room temperature. The precipitation method was used for bacterial suspension, then added the preserving liquid, but the results were not effective compared to the First method.
Preservation of bacteria, Staphylococcus aureus, Escherichia coli, Distilled water, Normal saline, Food oil, Glycerol
Preservation of bacteria is very important in the bacteriological laboratory. After obtaining bacterial isolates, they should be stored to protect them from contamination, so that they can be studied, diagnosed and tested later. It is difficult to keep microorganisms alive and pure, without any changes in physiological or genetic status when they are cultured on solid media and the accumulation of substances resulting from microbial activity in culture media. Over time, nutrient cultured media lose the proper moisture for growth, their basic substances run out, and the activity of bacteria decreases1,2. In general, mutations occur when bacteria are re-culturing on solid media3. Preservation methods are based on the nature of the bacteria, the duration of the storage, and the facilities in the laboratory4. Numerous methods have been used to preserve bacteria over the years5, and the most common and successful cryopreservation and lyophilization3. Cryoprotectants such as glycerol and dimethyl sulfoxide (DMSO) are currently used as additives in preserving microbes. These protective materials adsorbed at the surface of microorganisms and envelop the cells, thus providing a cover for the formation of ice crystals over long-term preservation. Glycerol work as membrane permeability and aids glazing by exchanging water in cells and making hydrogen bonds with water molecules for a protective effect6. The optimal method to preserve microorganisms has not been clearly defined and it differs from one species to another. The preservation liquid which used includes distilled water, Normal saline, tryptic soy broth, and nutrient broth, and it can all be used with or without cryopreservants7,8. We need to develop new strategies aimed at ensuring greater stability and storage of the functions of microorganisms9, at the same time low cost. The study aimed to find an effective and economical for preserving bacteria by using different temperatures and minimizing space at the time of preservation.
This study lasted from April / 2018 to June / 2019.
Bacterial isolates
Two pathogenic strains provided from Lab. of Microbiology / College of Veterinary Medicine / University of Mosul were tested, one of them is S. aureus as an example of gram-positive bacteria, and the other is E. coli as an example of gram-negative bacteria.
Culture method
5 – 7 colonies from each of the two species of bacteria which is tested and grown on Brain Heart Infusion Agar (BHIA) (Lab M. a Neogen company, UK) transferred to a micro-centrifuge tube (Mct.) (Vol. 1.5 ml., Dia. 10.6×39.5 mm. Citotest®, Barcopharma company) containing 1 ml. of preservation liquid1,8.
Preservation methods
Preservation liquid consists of one of these Substances: Distilled water, Normal saline, and Glycerol and Food oil, Table (1).
Table (1):
The Substances used as preservation liquid.
Substance |
Abbreviation |
Conc. % |
Sterilization |
Manufacture company |
|---|---|---|---|---|
Distilled water |
DW |
—— |
Autoclave |
—— |
Normal saline |
NS |
0.9 (Sodium Chloride) |
Autoclave |
POLIFARMA, Ergone, Tokirdag, Turkey |
Glycerol |
G |
100 50 30 15 |
Filtration |
Scharlau, Scharlab S.L., Gato, Pereze, Spain |
Glycerol with Normal saline |
G+NS |
Same volumes of them (0.5 ml.) G100+NS G50+NS G30+NS G15+NS |
Filtration |
—— |
Food oil |
FO |
—– |
Filtration |
Aldar oil, Sun Flower oil, Etihad food industries company, Babylon, Iraq |
Food oil with Normal saline |
FO+NS |
Same volumes of them (0.5 ml.) |
Filtration |
—— |
The Mct. tubes which contain the preservation liquid were maintained at temperatures of 25°C, 4°C (Refrigerator, Arcelik company, Turkey), and -20°C (Freezer, Arcelik company, Turkey). Room temperature ranges from 20 – 30 at rate 25°C.
The preservation methods were also tested by Precipitation, in this method bacteria were cultured in McCartney bottles containing nutrient broth (LAB M. a Neogen company, UK), then distributed into Mct. tubes and precipitated by the centrifuge (NF048, Nuve, Turkey) at 3000 ppm. for 3 minutes, then the glycerol and food oil (Preservation liquid) were added after discarding the supernatant, by using the concentration and volume of glycerol and food oil as in Table (1). Mct. tubes stored at three temperatures (25, 4 and -20)°C.
Viability test
To determine the viability of bacteria, 1 Mct. of preserved bacteria was examined after 1, 2 weeks, 1, 3, 6 and 12 months respectively at three temperatures. Bacteria in a preserving liquid were re-cultured on the BHIA and incubated at 37°C for 24 hours. For testing of phenotypic properties of the preserved bacteria, they were inoculated on Milk agar (MA), which was prepared as nutrient agar and 1.5% skim milk, to detect golden endo-pigmentation produced by S. aureus, E. coli was cultured on Eosin Methylene Blue (EMB) (Lab M. a Neogen company, UK) to detect the Metallic sheen phenomenon8,10.
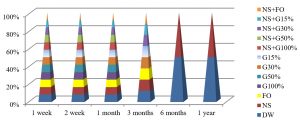
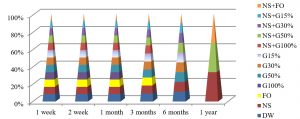
Fig. 4, shows that the best method to maintain the viability of S. aureus at 25°C were by using DW and NS after one year of preservation, while all preservation methods except G100%, G50%, NS+G100% or NS+G50%, maintained these bacteria alive for 3 months at this temperature.
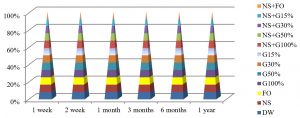
Fig. 5, shows that the best method to maintain the viability of S. aureus for a year at 4°C was by using NS+G50% or NS+FO methods, while all preservation methods except FO, G100% and NS+G100%, maintained S. aureus alive for 6 months.
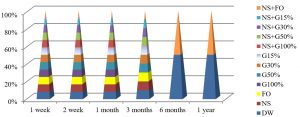
The best temperature for maintaining S. aureus alive was -20°C for one year, in all preservation methods used in the study, as shown in Fig. 6.
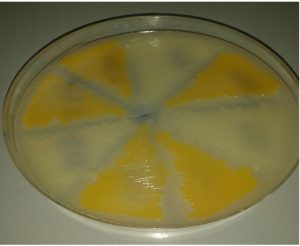
These methods maintained the phenotypic pattern of the endo-pigmentation production (golden pigmentation) and observed after S. aureus cultivation in milk agar at 37°C for 24 hours, except by using normal saline alone at 4°C which bacteria survived for a year, but golden pigmentation disappeared in this medium at the end of preservation period, Fig. 2.
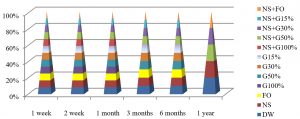
Fig. 7, shows that the best method to maintain the viability of E. coli at 25°C was by using DW and NS+FO for one year of preservation, while by using all preservation methods except G100% and NS+G100%, maintained these bacteria alive for 3 months at this temperature.
Fig. 8, shows that the best method to maintain the viability of E. coli for a year at 4°C by using DW, NS, NS+G50%, NS+G30% or NS+FO methods, while all preservation methods except G100% and NS+G100%, maintained E. coli alive for 6 months.
The best temperature for maintaining E. coli alive was -20°C in one year, in all preservation methods used in the study except for the FO and G100% methods, bacteria can’t grow after one year of preservation at this temperature, as shown in Fig. 9.
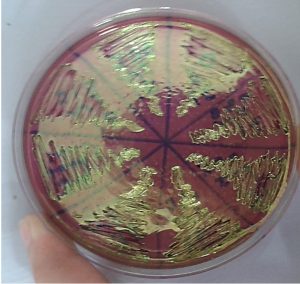
All methods used in this study maintained the phenotypic pattern of the metallic sheen production and observed after E. coli cultivation in EMB medium, at 37°C for 24 hours, Fig. 3.
In the Precipitation method, S. aureus couldn’t survive for one year at 25°C in all preservation liquid, used FO alone maintained these bacteria alive for 6 months at this temperature. At 4°C S. aureus survived for a year by using FO while using G50% or G15% maintained S. aureus alive for 3 months. The optimum preservation method is -20°C by using G30% or G15% or FO methods, the bacteria survived for a year, while by using G100% or G50% maintained S. aureus alive for 6 months.
In all preservation methods used, E. coli was maintained alive for a month at 25°C. At 4°C E. coli survived for a year by using G30% or G15%, while using FO maintained E. coli alive for 3 months. The best temperature for preservation of E. coli by using Precipitation method is -20°C, the bacteria survived for a year in G30% or G15% or FO, while by using G100% or G50% maintained E. coli alive for 6 months.
It is important to develop an easy and convenient technique for maintaining the viability, function, morphology and genetic traits of pure isolates to allow maximum restoration after storage11. The present study found that the best temperature of preservation for S. aureus and E. coli was -20°C for a year, in all preservation methods used in our study except G100% and FO methods in E. coli, Fig. 6 and 9. To date, cryopreservation is considered the best method for storing bacteria, and the best survival rates are recorded using this method1. In the cryopreservation method, bacteria are stored at a frozen temperature to minimize or prevent metabolism and physical change12.
The current study found that S. aureus bacteria survived for one year in normal saline, while E. coli survived in sterile DW in the same period at (4, 25 and -20)°C. This is confirmed by researchers from Wyndmoor, PA, USA8, they were observed that bacteria could remain alive in water and PBS for 2 years at room temperature range between (20-25)°C, and showed that G+ bacteria can be maintained them in PBS better than DW, while the study4 conducted at University of California, USA too, confirmed that the bacteria they studied could survive in DW more than 20 years. The present study does not agree with researchers8, that S. aureus was unable to give golden endo-pigmentation at 4°C by NS preserving method. This may be due to a lack of continuous supply or fluctuations in electricity during the period under study, due to the city’s conditions after liberation. Researchers from the USA (Liao et al.) and Australia (Banning et al.)8,13, reported that E. coli survived in sterile water and groundwater for several months respectively. This is the conclusion from the current study using DW as a preservative, the tested bacteria survived until the end of the study period. In another study by Ji and Jin of China14, found that the best option for bacterial preservation, is to use DW alone without any addition at 4°C, which agrees with the present study on E. coli, but it is not comparable to our study of S. aureus, where it could not live for a year in DW at 4°C, Fig. 5 and 8. Current findings contrast with those of researchers in the last century, where they pointed out that most microorganisms die if suspended with DW, except Pseudomonas solanacearum, they remained alive for 10 years in the water at room temperature while dying when stored in the refrigerator15. Despite the simplicity of using DW as a preservation liquid, it has not been widely used by microbiologists, due to insufficient data and studies related to it to verify the feasibility of this method or not8.
The present study is the first to use food oil alone or with normal saline as a preservation liquid for the conservation of bacteria, where no other studies have preceded. The FO+NS proved effectiveness in the preservation of E. coli at three temperatures used, Fig. 7-9, but S. aureus could not survive at 25°C Fig. (4), while using FO alone preserve S. aureus for a year at -20°C, Fig. 6, this may be due to the different composition of the cell wall in G+ve from G-ve bacteria. The use of FO alone or with NS maintained the phenotypic patterns of preserved bacteria. Many studies successfully used liquid paraffin or mineral oil, which prolongs the microbial growth life, prevents evaporation and reduces the rate of metabolism of the medium by reducing oxygen. This is a simple and economical method for maintaining pure cultures of bacteria. In this method, the oil or paraffin is poured over the slant of culture and stored at room temperature. This condition helps pure culture to remain in a dormant state and the culture can be preserved from months to years16.
There was a recorded report that Pseudomonas, Bacillus, and E. coli remained alive below the oil for 3 years and that their phenotypic characteristics did not change14. In a study conducted by Yamasato et al., from Japan17, they observed that 75-85% of the strains tested, remained viable under liquid paraffin at 10°C for 2 years and at -53°C for 3.5 years.
The researcher classified the Cryoprotectants as penetrating or non-penetrating the cell, Glycerol belongs to the latter one3. It is one of the most important types of cell-conserving substances18, that act to surround the cells, delay the freezing of intracellular substances in these cells and reduce the solution effects1. It prevents ice crystallization and as a result, bacteria remain alive longer3, it is a strong substance that prevents water from entering the cell19. Frozen culture is the most commonly used preservation method20. We can maintain Microorganisms by freezing broth cultures at -5 to -20°C for 1-2 years in suitable containers21. Chen and Jin’s study from China22 indicated that cryopreservation is easier and cheaper compared to lyophilization methods, and if freezing processes are successful, the bacterial mass survives for up to 35 years.
In this study, glycerol was used in many concentrations alone or with NS and found that these concentrations were good for preserving bacteria at -20°C, this does not correlate with the researchers’ study from India3, they may be due to the concentration of glycerol used in their study by 10-15% or without normal saline. An expanded study17 indicated that the use of a slanted agar method then covered aerobic bacteria growth by 10% glycerol, maintained the bacteria at -53°C for 16 months. In another study12, the researchers reported that the bacteria kept in 20% glycerol including Staphylococcus that managed to survive for 3 months and there are no changes in biochemical properties, this is the result of the current study when using glycerol by 15% and 30% for the same period at 25°C.
Our study contradicts with Laurin et al., from Canada23, where they found that the use of 15% glycerol at -20°C was sufficient to keep the bacteria for more than a week, while in this study, glycerol managed to keep the bacteria alive for a year at this temperature except for G100% method for preserving E. coli, Fig. 6 and 9.
In this study the Precipitation method was also used, the optimum temperature of preservation in this method is -20°C as well, but at a concentration of G50% and G100% in both S. aureus and E. coli, the bacteria couldn’t survive for a year, the preservation using FO at 4°C gave a result of S. aureus and remained alive for a year, while using G30% and G15%, E. coli also survived for a year at 4°C.
The difference in the result between the previous method and the Precipitation method in the use of FO or glycerol as a preservative, maybe due to the mechanical deposition of the suspended bacteria and then the oil is added, while in the first method the bacterial inoculation was added to the preservation liquid, so the FO or glycerol surrounding from all direction and maintaining it.
According to the data collected indicate that the preservation liquid consisting of one or both of these substances DW, NS, G, and FO, will provide protection against loss of viability and may increase the long-term preservation when maintained at -20°C.
ACKNOWLEDGMENTS
We thank the Department of Microbiology of Veterinary Medicine, University of Mosul, Iraq for their support to complete this study.
FUNDING
None.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by the author.
AVAILABILITY OF DATA
All datasets created or investigated during this study are involved in the manuscript and/or the Supplementary Files.
- Tedeschi R and De Paoli P. Collection and Preservation of Frozen Microorganisms. Methods Biobanking, 2010; 313-326.
Crossref - Liu H, Fung DYC, and Hoffmans C. Chalk culture method for preserving bacterial cultures and its applications. J. Rapid Methods Auto. Microbiol., 2003; 11: 163-224.
Crossref - Prakash O, Nimonkar Y, and Shouche YS. Practice and prospects of microbial preservation. FEMS Microbiol Lett., 2012; 339(1): 1-9.
Crossref - Iacobellis NS and DeVay JE. Long-term storage of plant pathogenic bacteria in sterile distilled water. Appl. Environ. Microbiol., 1986; 52: 388-389.
Crossref - Chitte RR and KG. Preservation of Thermophilic Bacterial Spores Using Filter Paper Disc Techniques. J. Bioprocess. Biotech., 2015; 5(4).
Crossref - Martin-Dejardin F, Ebel B, Lemetais G, Minh HNT, Gervais P, and Cachon R. A way to follow the viability of encapsulated Bifidobacterium bifidum subjected to a freeze-drying process in order to target the colon: Interest of flow cytometry. European Journal of Pharmaceutical Sciences, 2013; 49: 166-174.
Crossref - She RC and Petti CA. Procedures for the Storage of Microorganisms. Chapter 11 in Manual of Clinical Microbiology, 2015; 1:161-168. ASM press, Washington, DC,
Crossref - Liao CH and Shollenberger LM. Survivability and long-term preservation of bacteria in water and in phosphate-buffered saline. Lett. Appl. Microbiol., 2003; 37: 45-50.
Crossref - Alonso S. Novel Preservation Techniques for Microbial Cultures, Chapter 2 in: Novel Food Fermentation Technologies, Food Engineering Series, Springer International Publishing Switzerland, 2016; 7-33.
Crossref - Mahmmoud EN. Study of Coagulase-Negative Staphylococci (CNS) in Bovine Subclinical Mastitis. 2006; Thesis [Master Science]. College of Veterinary Medicine / University of Mosul.
Crossref - Philip N, Garba B, and Neela VK. Long-term preservation of Leptospira spp.: challenges and prospects. Appl. Microbiol. Biotech., 2018; 102(13): 5427-5435.
Crossref - Angshumanjana, Anirbanjana, Dipjitdey, Arijitmajumdar, Jayantabikashdey, and Tudu N. Selection of Storage Methods for Maintenance of Different Stock Cultures. Int. J. Curr. Microbiol. App. Sci., 2016; 5(10): 1097-1104.
Crossref - Banning N, Toze S, and Mee BJ. Escherichia coli survival in groundwater and effluent measured using a combination of propidium iodide and the green fluorescent protein. J. App. Microbiol., 2002; 93: 69-76.
Crossref - Ji YX and Jin RC. Effect of different preservation conditions on the reactivation
performance of anammox sludge. Sep Puri Technol., 2014; 133: 32-39.
Crossref - Heckly RJ. Preservation of microorganisms. Adv. Appl. Microbiol., 1978; 24: 1-53.
Crossref - Hartsell SE. The Preservation of Bacterial Cultures under Paraffin Oil. Appl. Microbiol., 1953; 1(1): 36-41.
Crossref - Yamasato K, Okuno D, and Ohtomo T. Preservation of bacteria by freezing at moderately low temperatures. Cryobiol., 1973; 10: 453-463.
Crossref - Samir A and Wasfy MO. A simple technique for long-term preservation of leptospires. J. B. Microbiol., 2012; 53(3): 299-301.
Crossref - Weng L, Chen C, Zuo J, and Li W. Molecular dynamics study of effects of temperature and concentration on hydrogen-bond abilities of ethylene glycol and glycerol: implications for cryopreservation. J. Phys. Chem, 2011; 115(18): 4729-4737.
Crossref - Cody WL, Wilson JW, Hendrixson DR, McIver KS, Hagman KE, Ott CM, and Schurr MJ. Skim milk enhances the preservation of thawed −80°C bacterial stocks. J. Microbiol. Methods., 2008; 75(1): 135-138.
Crossref - Ali M, Oshiki M and Okabe S. Simple, rapid and effective preservation and reactivation of anaerobic ammonium oxidizing bacterium “ Candidatus Brocadia sinica.”. Water Res., 2014; 57: 215-222.
Crossref - Chen H and Jin RC. Summary of the preservation techniques and the evolution of the anammox bacteria characteristics during preservation. Appl. Microbiol. Biotechnol., 2017; 101(11): 4349-4362.
Crossref - Laurin V, Labbe V, Juteau P, Parent S and Villemur R. Long-term storage conditions
for carriers with denitrifying biomass of the fluidized, methanol-fed denitrification reactor of the Montreal Biodome, and the impact on denitrifying activity and bacterial population. Water Res., 2006; 40(9): 1836-1840.
Crossref
© The Author(s) 2020. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.