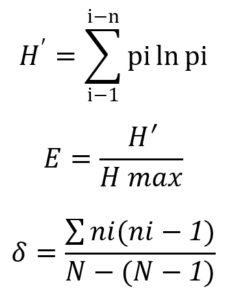ISSN: 0973-7510
E-ISSN: 2581-690X
The growth and quality of Cilembu Sweet Potato is influenced by the colonization of plant growth promoting rhizobacteria (PGPR). In this study, cultivation medium (soil) were sampled on different period of growth time (0 – 4 months) in two different locations, Cilembu (LCI) and Jatinangor (LJA). Isolation were done based on several selective ability of PGPR, followed by sequencing using 16S rRNA. Based on soil properties, LJA contains more clay and slightly more acidic than the LCI, where the C/N ratio at LCI is higher than LJA (13:10). 17 isolates of bacteria and two isolates of Actinomycetes were found in LCI samples, while LJA samples had 17 isolates of bacteria and one isolate of Actinomycetes. The highest abundance of rhizosphere bacteria at LCI and LJA belongs to Firmicutes division, with 58% and 83% of presence, respectively. PGPR diversity increases in LCI site, while it drops after the third month in LJA site. This result indicates that PGPR diversity in LCI site may contribute in taste establishment of Cilembu Sweet Potato.
Cilembu sweet potato, PGPR, Rhizosphere, Ipomoea batatas
Sweet potato (Ipomoea batatas L) is one of the most potential food commodity to complement or even subtitute other main food commodities. Sweet potato has a very high content of carbonhydrate compared to rice, maize, and cassava. In addition, sweet potato also has high yielding potential and atdaptability under wide range on environmental conditions1. Other than the usage of sweet potato as food material, many developed countries (including Indonesia), has used sweet potato as raw material for fermentation, textile, glue, cosmetic, pharmaceutical, and beverage industries1.
Indonesia is one of the five main producers of sweet potato during 1990 to 2009. Productivity of sweet potato in Indonesia is approximately 13.93 tons/ha, which exceeds the average productivity of sweet potato in the world. These sweet potatoes are mainly harvested in Cilembu, West Java, Indonesia. Rancing sweet potato is a sweet potato cultivar from Cilembu that is well-known for its honey-like sweet taste, which is much sweeter than other types of sweet potato. This cultivar is already popular since 1990s, not only in local communities, but also in global scale. Besides Rancing, other local varieties of sweet potato in Cilembu, such as Neerkom and Eno, were also known to be existed but eventually extinct. These varieties needed around eight months harvest time, a longer period than Rancing2.
Sweet potato varieties are now cultivated in other sites beside Cilembu, but unfortunately these sites are not able to produce similar taste as the sweet potatoes from Cilembu. In artificial and natural ecosystems, the existence of relationships between bacteria and plants plays an important role in the growth and health of plants, including the establishment of many characteristics of plants. Moreover, some factors related to spatial variation are also known to take effect in microbial community, such as pH, salinity, soil fertility, type of soil, and some other biotic factors (nematodes, vegetation type, etc.) 3,4. These findings may indicate that rhizosphere bacteria, specifically in Cilembu, may become an important factor that contribute in the taste establishment and optimum growth of Rancing sweet potato.
Eventually, several studies about sweet potato was done related to the isolation and identification of PGPR bacteria, as well as the use of the bacteria as biological control agents, anti-pathogens, and growth promoter during the sweet potato’s cultivation period5. However, information about the PGPR bacterial diversity and community variations during the sweet potato growth phase is very limited. This study aims to determine PGPR communities during the growth of sweet potatoes, and also examine the effect of spatial variation (different site of cultivation) to the PGPR communities. This study also serve as a preliminary study of further identification and screening of PGPR communities which plays important roles on the taste establishment of Cilembu sweet potato.
Research sites
The soil samples were collected from two different sites, Cilembu (LCI) and Jatinangor (LJA). These sites are located in West Java, Indonesia. Rhizosphere soil sampling from each site were done five times with the span of one month (0-4 months).
Isolation and identification of rhizosphere bacteria from sweet potato roots
One gram of Rhizosphere soil was added into nine ml physiologist solutions (0.85%). Serial dilution were done for six times, and 0.1 ml of the final solution was inoculated using spread method to the petri dish filled with NA medium6. After 24 – 48 hours of incubation, colonies of bacteria analyzed by macroscopic and microscopic test.
Selection of PGPR isolates
Nitrogen fixation ability
Rhizosphere bacteria isolates were inoculated to Nitrogen Free Broth (NFB) with spot methods and incubated for three days, where bacteria with nitrogen fixation ability may changes the media color to light blue or dark blue. Ammonification test were also done by inoculating the isolates to peptone broth medium. The level of ammonia formed can be measured by observing the yellow color founded after the addition of Nessler reagent7.
Phosphate solubilization ability
All isolates were screened on Pikovskayas medium for phospate solubilization test. Tri-calcium phosphate (TCP) as a source of phosphate were added to the medium. After 48 hours of incubation in 30oC, presence of clear halo around the growth indicates phospate solubilization7.
Ability to produce phytohormone (IAA)
IAA-producing bacteria were tested using both nutrient broth and Salkowski reagent. Isolates were cultured on King’s B broth with and without 0.1 g / l L- tryptophan and incubated at 28 ± 20oC for 24 hours on a shaker. After the culture was incubated 24 hours, the culture was centrifugated at 4000 rpm for 20 minutes. One milliliter of supernatant was taken and added to 1 ml of Salkowski reagent (12 g/L FeCl3 at 429 ml/L H2SO4) and incubated in the dark for 22 – 25 minutes at room temperature. The intensity was read at 535 nm using a UV spectrophotometer5.
Amylolytic & Cellulolytic activities
Identification of amylolytic & cellulolytic bacteria were done by inoculating 5 mL of the isolates to agar plates with specific contents. For amylase production test, using of medium Starch Agar (Agar: 12g/L, starch: 10g / L , and Beef extract : 3g/L)8. On the other hand, cellulolytic activity test used medium which contains CMC (Carboxymethylcellulose 0.5 g/L, K2HPO4 0.1 g/L, KCl 0.1 g/L, MgSO4 0.5 g/L, Yeast extract 0.5 g/L, Glucose 0.1 g/L and order 1.7%)9. Gram’s iodine was added within 0.5 ìm round papers, which were placed on the agar before 48 hours incubation period (Cappucino and Sherman 2008). Positive cellulolytic isolates will form clear zone around the papers, and larger diameter of the clear zone may represents a higher level of cellulose or amylase production.
Measurement of diversity, evenness, and dominance index
Diversity of bacterial rhizosphere on Rancing sweet potato was calculated with the Shannon-Wiener Index (H’), where the index value are classified into three different levels: low, average, and high. Calculation of evenness index (E) was done to explain the distribution patterns of individuals of each species using:
Simpson Index (δ) were calculated to find the dominating species in the population.
Identification of bacteria using 16S rRNA sequence
Isolates were grown on NB medium and incubated at 120 rpm shaker (28°C) for 24 hours. DNA from isolates of PGPR efficiently extracted. 16S rRNA genes were amplified in thermocyler (Eppendorf thermocycler) using universal primers under the following conditions: initial denaturation at 95°C (5 minutes), denaturation at 95°C (30 seconds), annealing at 52°C (30 seconds), and primer extension at 72°C (2 minutes) followed by the final elongation (10 minutes). Products reinforced were checked for purity by electrophoresis on a 1% agarose gel. DNA extracted was calculated (100 ng/ml) and was being sequenced with the help of an automated sequencer. The DNA sequences obtained were analyzed with basic sequence alignment BLAST program and were run against the database from NCBI (National Center for Biotechnology Information Blast).
Physics-Chemical Condition and Climate Profile
Based on the climate profile analysis, LCI and LJA were different in many aspects (Table 1). Althrough it is not significantly different in terms of soil temperature and soil humidity, other properties such as air temperature, air humidity, and pH in both site are considerably different. Soil pH at LJA were higher that at LCI.
Table (1):
Climate Profile of Cilembu (LCI) Site and Jatinangor (LJA) Site
| Climatic Properties | Site | |
|---|---|---|
| Cilembu | Jatinangor | |
| Location | S06°54’21.1 | S06°55’06.00 |
| E107°50’40.1 | E107°46’56.3 | |
| Altitude (masl) | 974 | 757 |
| Air Temperature (°C) | 24 – 26 | 26 – 28 |
| Air Humidity (%) | 76 – 97 | 70 – 90 |
| Light Intensity (Lux) | 3970 | 2790 |
| Soil Temperature (°C) | 22 – 26 | 22 – 27 |
| Soil Moisture (%) | 80 – 86 | 70 – 80 |
| Soil pH | 6.9 – 7.0 | 5.9 – 6.9 |
The ratio between C and N cotents in soil (C:N Ratio) strongly related to the amount of mobile N for plants and the decomposition rate of materials beneath the soil. Higher value of C:N ratio will lead to the increase of microbial activity to produce CO2, which also cause the depression of soil nitrates or N immobilization10. As the value of C:N ratio goes up, the amount of immobilized N will rise consequently and cause N defficiency. Based on the result in Table 2, soil in Cilembu site has higher C:N Ratio (13) compared to soil from Jatinangor site (10). Generally, these values are considered to be low. Significant N immobilization is known to cause N deficiency when C:N Ratio is more than 3010. Therefore, soils in Cilembu and Jatinangor may still support crop growth, specifically by supplying enough N.
Cation Exchange Capacity (CEC) of soil from Cilembu is lower than soil from Jatinangor (Table 2). The value of CEC represents the capability of the soil to hold and exchange cations11. This process is very important to assure that the soil can support nutrient exchange and distribution to plants. Many factors influence CEC value, including soil proportions. Hazelton and Murphy11 suggested that higher CEC can be found in soil with high proportion of clay and organic matter, as seen in this study. Nevertheless, CEC value of both sites can still be classified in the same category, “high CEC”, which range around 15-40 cmol(+) Kg-1. High value of CEC indicates that both sites may hold enough macronutrients to be accessed by plants.
Table (2):
Physics-Chemical Conditions of Soil from Cilembu (LCI) Site and Jatinangor (LJA) Site
| Soil Properties | Site | |
|---|---|---|
| Cilembu | Jatinangor | |
| Texture | Clay | Clay |
| Sand Proportion (%) | 21.00 | 8.00 |
| Dust Proportion (%) | 33.00 | 28.00 |
| Clay Proportion (%) | 46.00 | 64.00 |
| pH in H2O | 6.50 | 5.70 |
| pH in KCl | 5.20 | 4.80 |
| C Organic (%) | 3.12 | 2.16 |
| N Organic (%) | 0.23 | 0.22 |
| C/N Ratio | 13.00 | 10.00 |
| P2O5 (ppm) | 15.70 | 86.70 |
| P2O5 (mg/100g) | 60.92 | 149.48 |
| K2O | 57.31 | 18.25 |
| CEC (cmol(+)/kg) | 21.15 | 24.69 |
| Base Saturation (%) | 65.00 | 41.00 |
| K Total (cmol(+)/kg) | 1.73 | 0.55 |
| Ca Total (cmol(+)/kg) | 9.79 | 8.01 |
| Mg Total (cmol(+)/kg) | 1.91 | 1.38 |
| Na Total (cmol(+)/kg) | 0.39 | 0.13 |
| S Total (ppm) | 30.60 | 11.90 |
| Fe Total (ppm) | 4.00 | 7.80 |
| Al Total (ppm) | 285.70 | 32.10 |
| Cu Total (ppm) | 0.60 | 0.50 |
| Zn Total (ppm) | 1.00 | 0.70 |
| Mn Total (ppm) | 10.90 | 36.10 |
Generally, soils in Cilembu and Jatinangor site are still in good conditions and may support the growth of rancing sweet potato. Jatinangor site has slightly better value of CEC and C/N Ratio, which represents its ability to support important proccesses in plants nutrient uptake. However, higher pH value in Cilembu site is much favorable for macronutrients, and boost the macronutrients availibility in the soils. Macronutrients are critical for plants as main components of organic compounds that build plants structure. Therefore, soil in Cilembu site is more suitable and fertile for rancing sweet potato cultivation compared to soil in Jatinangor site.
Isolation and grouping of Rhizosphere Bacteria
Based on the results from isolation and identification, different number of isolates were obtained from the two locations (LCI and LJA) during the growth phase. Five samples were obtained during 4 months of growth, where 7, 13, 16, 14, 17 isolates were found in LCI site, and 9, 12, 13, 11, 17 isolates in LJA site (Fig. 1.a.) Microscopic and macroscopic identification showed that rhizosphere bacteria in Cilembu sweet potato can be classified into three different groups, namely Firmicutes, Proteobacteria, and Actinobacteria (Fig. 1.b). Microbial composition in rhizosphere are reported to be different with microbial composition in bulk soil by several studies12. The study showed that at the level of phylum, microbial community in rhizosphere and bulk soil are different, and the phylas founded had distinctive growth dynamics during the growth of soybean in the field. Phylas founded in this study are known to be found in rhizosphere, roots, stems, and leaves tissue of many plants13.
Firmicutes bacteria groups in LCI isolates were founded to have the highest abundance, with the percentage of 58%. This trend can also be found in LJA isolates, where 83% of the whole bacteria belongs to Firmicutes division (Fig. 1.b). These numbers may indicate the domination of this division in the bacteria community of Cilembu sweet potato, as the percentage range above 50%. Firmicutes can repress the other bacteria as it produce antibiotics and ubiquitous in the soil (eq. Bacillus)14.
Meanwhile, the abundance of Proteobacteria and Actinobacteria divisions on LCI isolates were recorded to be as much as 32 % Proteobacteria and Actinobacteria 11%, respectively. Similar order were also founded on LJA isolates, where Proteobacteria and Actinobacteria were founded for as much as 11% and 6%, respectively (Fig. 1).
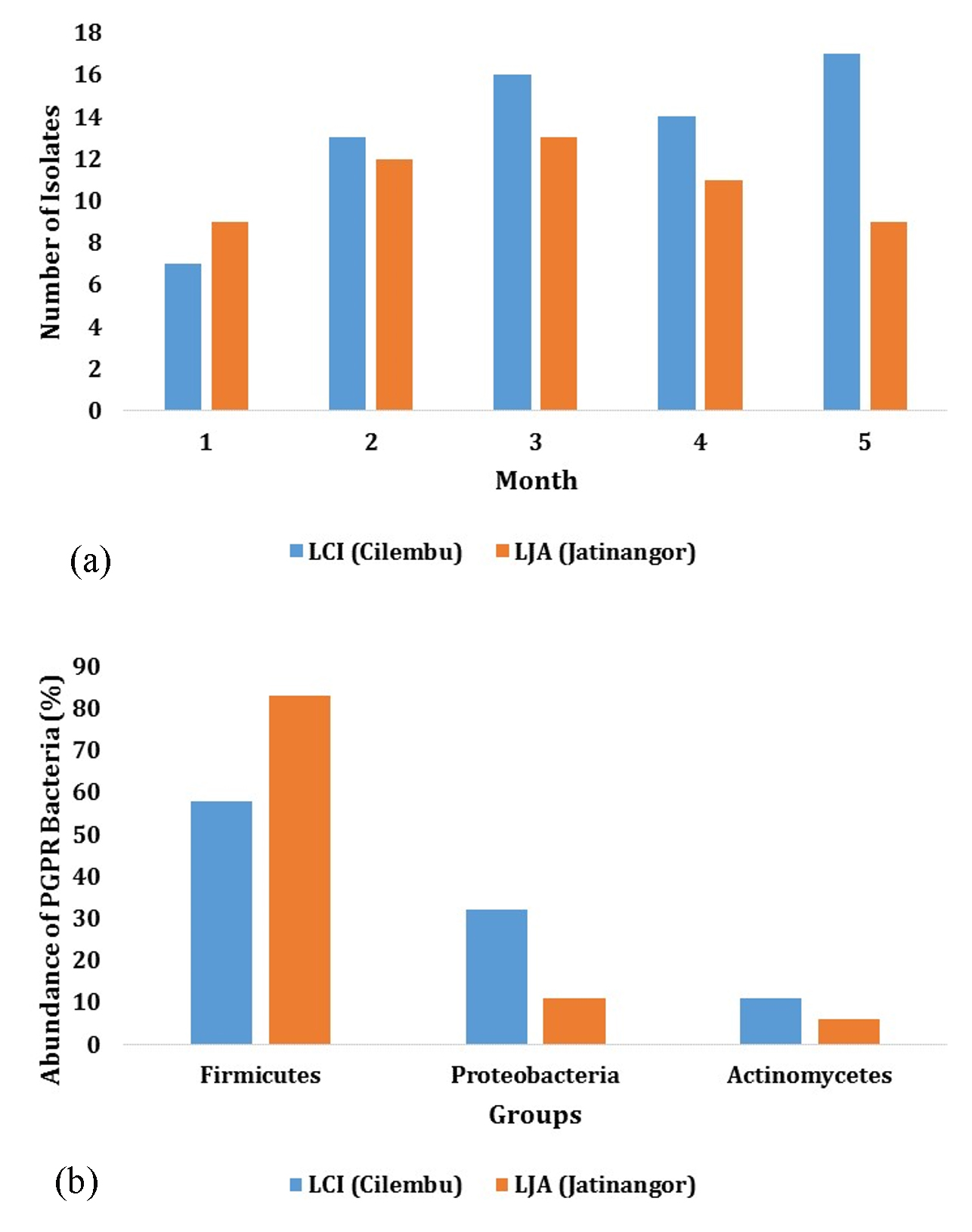
Fig.1. (a) The Number of Isolates and (b) Abundance per Group of PGPR bacteria in the rhizo-sphere at Cilembu (LCI) and Jatinangor (LJA) Site
The dynamics of PGPR during Cilembu sweet potato’s growth phases
Bacteria founded were also grouped based on its ability as PGPR. During the growth of Rancing sweet potato, the colony number has increased each month in LCI site for all groups of PGPR (Fig. 2.a). Different trend were found in LJA site. Despite of the increase of bacteria species number founded during the growth, the abundance of bacteria was decreased over the time period (Fig. 2.b). Most of PGPR bacteria only increased during the first two month, and later decline significantly. This phenomenon may relies on interaction between the soil, plants and microorganisms15. As mentioned before, micro- and macro- nutrients are known to be higher in LCI site compared to LJA. This condition may limit the lifespan of each bacteria and cause the decreasing number of bacteria colony in LJA site.
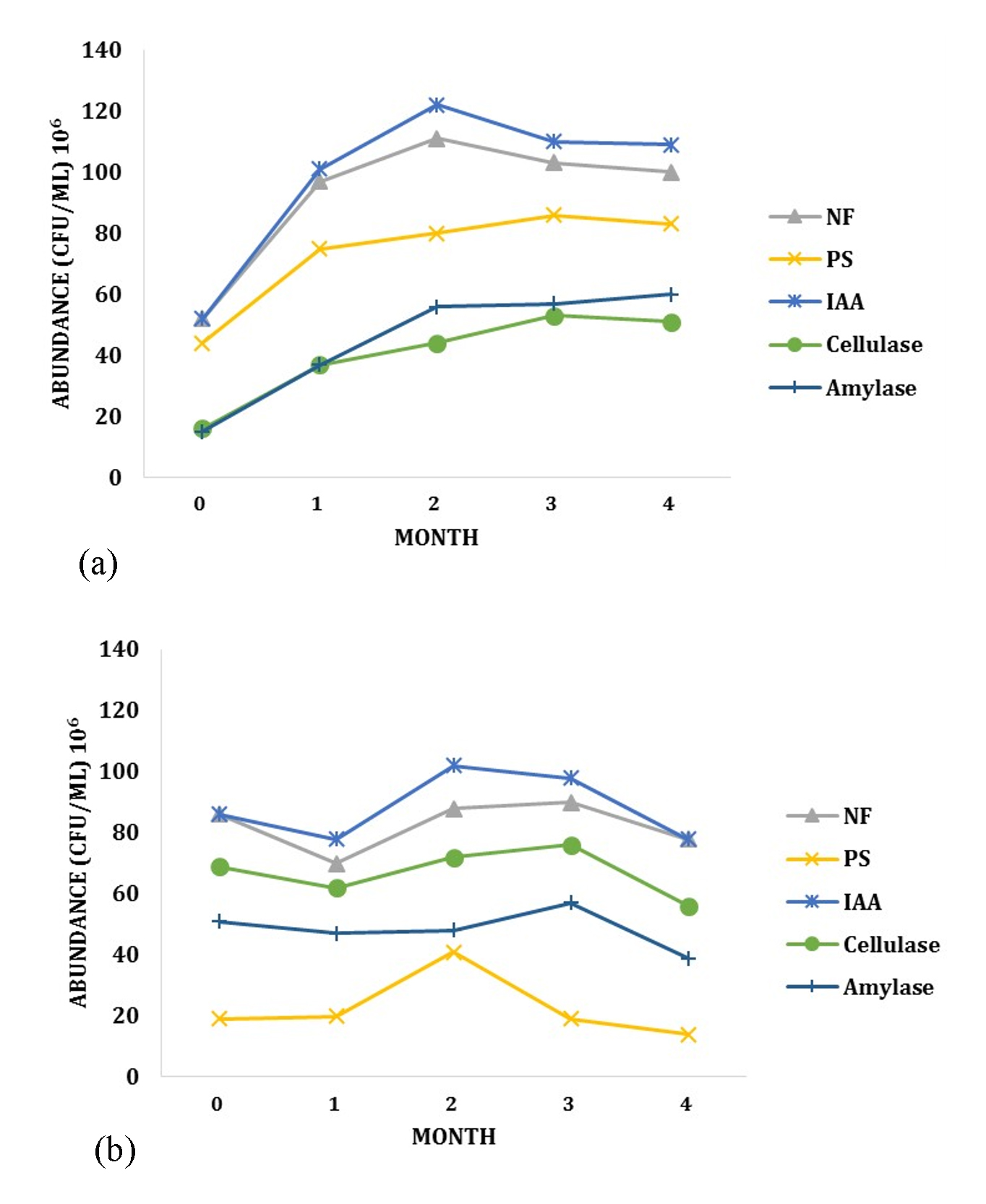
Fig. 2. The Dynamics of PGPR bacteria groups during the growth of sweet potato in (a) LCI site and (b) LJA Site
The other factors that may relate to the differences is metabolic enzymes. The metabolic enzymes from PGPR play important roles during plants growth. The presence and activity of these enzymes depend on many aspects, especially the availability of C organic. LCI soil contained higher amount of C organic, and this may lead to a more suitable environment for PGPR bacteria to grow.
The dynamics of PGPR community during the growth of Cilembu sweet potato can also be influenced by the metabolites from host plants. Higher growth rate may cause the increase of metabolites production, and this phenomenon may leads to the increase of PGPR diversity and abundance
Dynamics of abundance, and diversity of PGPR at different sites
The abundance and the diversity index of Rancing sweet potato rhizosphere bacteria from LCI and LJA site was analyzed based on the number of bacterial cells and the frequency of species appearance during growth phases. In this study, the growth of Rancing sweet potato were divided into three phases; early phase, intermediate phase, and final phase. Early phase refers to the first month of growth, while the intermediate phase refers to the second month. Final phase range from third to fourth month of growth.
Dynamics and abundance of the rhizosphere bacteria from both sites has fluctuated during growth. The number of species from LCI isolates has increased from 6 PGPR species on the early phase to 11 species on intermediate phase. On these phases, the abundance of PGPR also increased from 6,3 x 107 to 1,33 x 108 CFU (Fig. 3). For LJA isolates, the abundance of bacteria from phase one to phase two was also changed (8,6 x 107 to 1,08 x 108 CFU) and the type of bacteria increased from 9 to 13 bacterial isolates (Fig. 4).
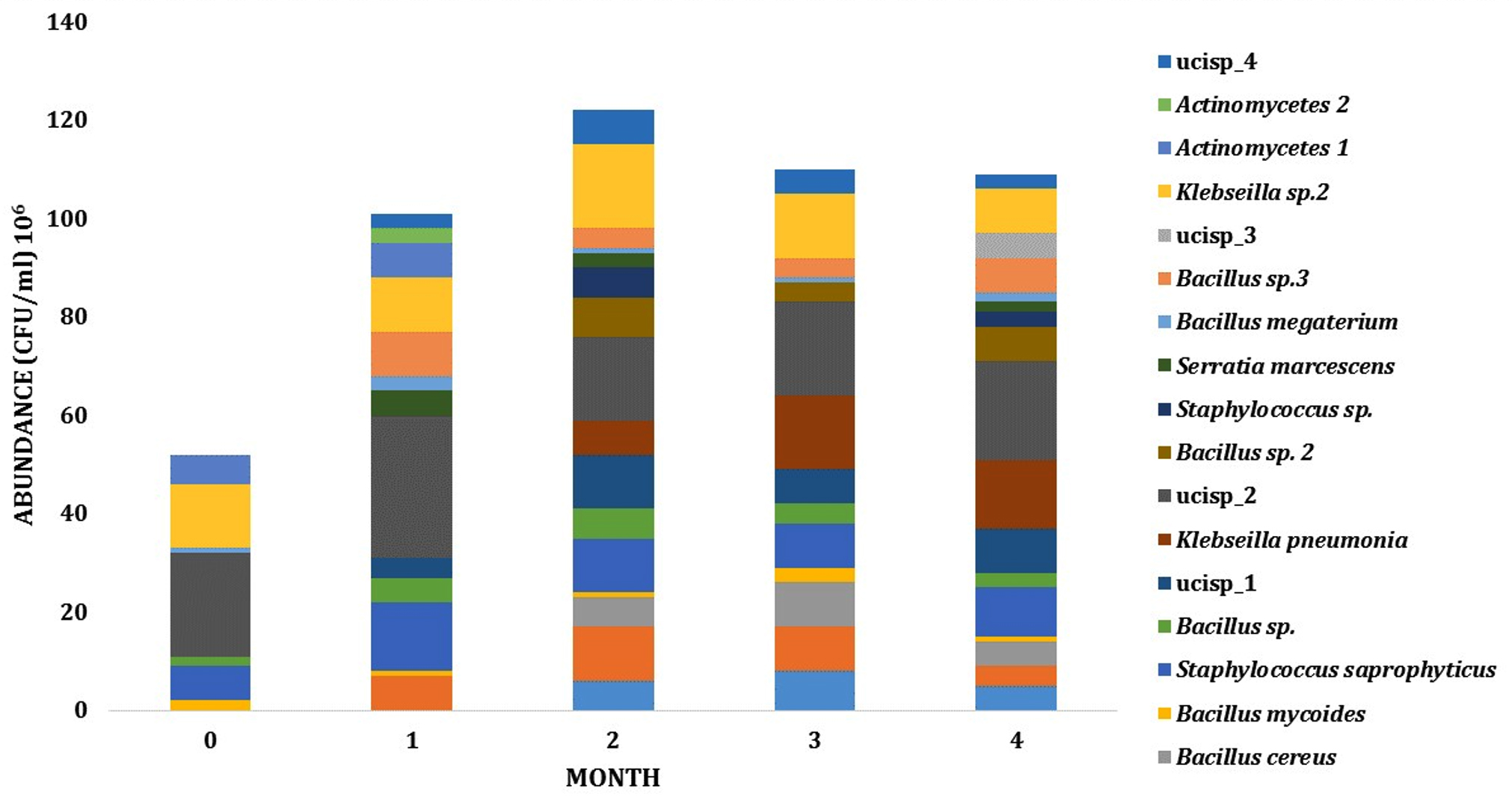
Fig. 3. Dynamics of PGPR abundance in LCI site during growth
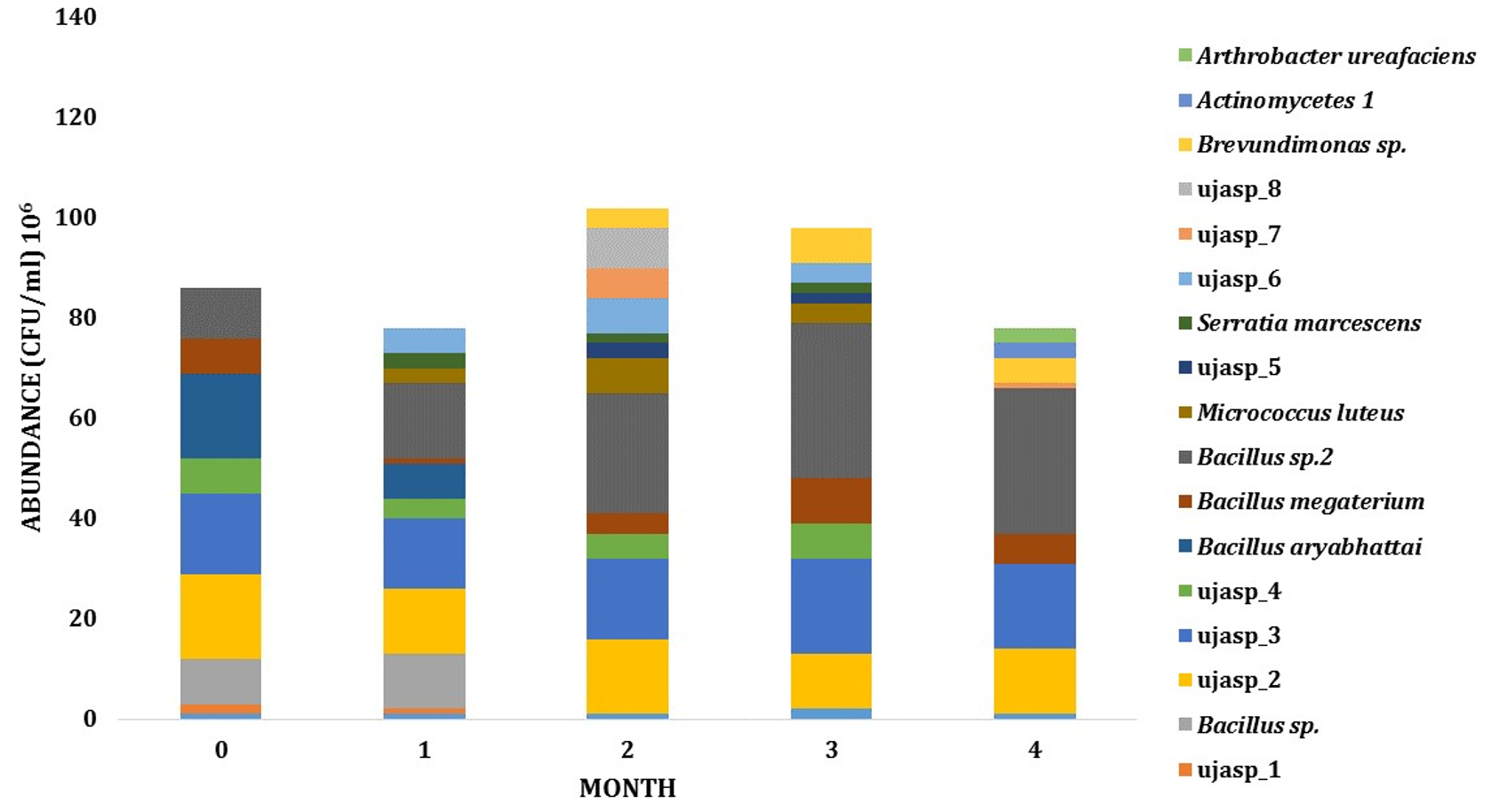
Fig. 4. Dynamics of PGPR in LJA site during growth
Diversity index dynamics of rhizosphere bacteria from LCI and LJA showed significant differences (Fig. 5). The diversity index used was Shannon-Wiener (H ‘), which may determine the diversity level of the PGPR community. The results indicate that the diversity of each phase of growth for both sites are in a sufficient productivity level, where ecological pressures is moderate, and ecosystem conditions is in balance. The level of microorganisms diversity is known to be influenced by several factors, including the interaction between plants, soil fertility, physical environment, and other microorganism’s pressure16.
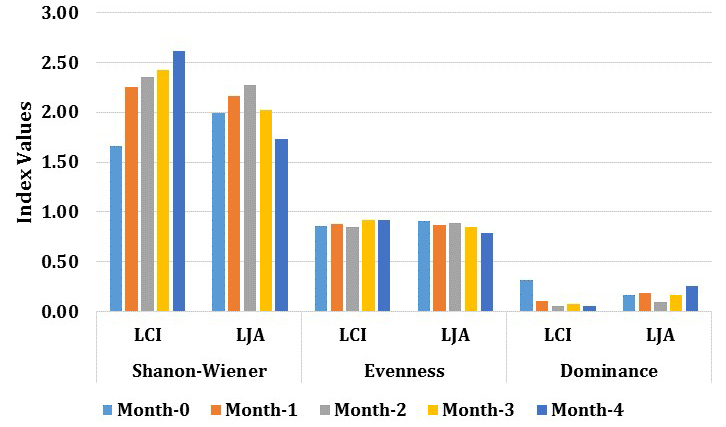
Fig. 5. Average of bacterial diversity index: Shannon-Wiener diversity (H’), Evenness (E), and dominance (δ) for each location during the growth phase.
The results indicate the existence of three groups of PGPR in the rhizosphere bacterial community of Cilembu sweet potato from both sites. Different abundance level were found for Firmicutes, Proteobacteria, and Actinobacteria division (LCI: 58, 32 and 11% while the LJA: 83, 11, and 6%). The number of PGPR species (Firmicutes & Proteobacteria) at LCI increase during the growth, while PGPR species in LJA decrease. Actinobacteria only present at 0 months and 1st month at LCI, while at LJA present at the final phase (4th month). The rhizosphere bacterial diversity in LCI increased during the growth phase with sequential values (H’ are 1.66; 2.25; 2.34; 2.42; 2.61) while the LJA decline after the initial phase of tuber formation (H’ are 1.98; 2.16; 2.27; 2.02; 1.73). This results indicate that the microbial communities and dynamis in LCI site is different with LJA site and therefore may contribute in the taste establishment of produced sweet potato.
Understanding the composition, diversity, and important role of rhizosphere bacteria will be beneficial in agriculture to reduce dependence on chemical fertilizers that are clearly known to have negative effects on environment and human health. By understanding the diversity of PGPR as a component of the rhizosphere ecology, the quality of agricultural products and the cultivation of sweet potato in other areas can be improved. Further, environmental engineering may become possible to design the environmental conditions and cultivation processes resulting in the same taste and quality of sweet potato as the one in Cilembu (LCI site).
ACKNOWLEDGMENTS
The author would like to thank School of Life Sciences and Technology, Institut Teknologi Bandung, and DIKTI for all the supports and facilities provided.
- Wang, Z., Li, J., Luo, Z., Huang, L., Chen, X., Fang, B., Li, Y., Chen, J., Zhang, X. Characterization and development of EST-derived SSR markers in cultivated sweetpotato (Ipomoea batatas). BMC Plant Biology, 2011; doi:10.1186/1471-2229-11-139
- Maulana, H.B., Waluyo, Karuniawan, A. Status of sweet potato cultivation of Neerkom and Eno varieties in sweet potato production centers Cilembu Sumedang District. Paper presented at the National Seminar Peripi Komda Breeding Banyumas Based Local Wisdom Facing Potential and Challenges of Globalization 8-9 July 2011 in UnSoed Purwokerto, Central Java.
- Fierer, N., Jackson, R.B. The diversity anf biogeography of soil bacterial community. Proc. Natl. Acad. Sci. U S A, 2005; doi:10.1073/pnas.0507535103
- Lozupone, C.A., Knight, R. Global patterns in bacterial diversity. Proc. Natl. Acad. Sci. U S A, 2007; doi:10.1073/pnas.0611525104
- Farzana, Y., Radziah, O., Kamaruzaman, S., Mohd Said, S. Characterization of beneficial properties of plant growth-promoting rhizobacteria isolated from sweet potato rhizosfer. African Journal of Microbiology Research, 2009; 3(11):815-821.
- Benson, H.J., Brown, A.E. Microbiological application: laboratory manual in general microbiology, 4th edn. New York: 2010
- Cappuccino, J.G., Sherman, N. Microbiology: a laboratory manual eighth edition. San Fransisco: 2008
- Atlas, R.M. Handbook of microbiological media, 4th edn. Washington D.C.: 2010
- Kasana, R.C., Richa, S., Hena, D., Som, D., Arvind, G. A rapid and easy method for the detection of microbial cellulases on agar plates using Gram’s iodine. Curr. Microbiol., 2008; 57(5):503-507
- Power, J.F., and R. Prasad. 1997. Soil Fertility Management for Sustainable Agriculture. Florida: CRC Press
- Hazelton, P., and B. Murphy. 2016. Interpreting Soil Test Results: What Do All the Numbers Mean?. Collingwood: CSIRO Publishing Australia
- Sugiyama, A., Ueda, Y., Zushi, T., Takase, H., Yazaki, K. Changes in the bacterial community of soybean rhizospheres during growth in the field. PLOS ONE, 2014; doi: 10.1371/journal.pone.0100709
- Bodenhausen, N., Harton, M.W., Bergelson, J. Bacterial communities associated with the leaves and the roots of Arabidopsis thaliana. PLOS ONE, 2013; 8(2):e56329.
- Dunne, C., Delanny, I., Fenton, A., O’Gara, F. Mechanisms involved in biocontrol by microbial inoculants, Agronomie, 1996; 16:721-729.
- Gianfreda. Enzymes of importance to rhizosphere processes. Journal of soil Science and Plat Nutrition, 2015; 15(2):283-306.
- Subba Rao, N.S. Soil microorganism and plant growth. Florida: 1995.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.