ISSN: 0973-7510
E-ISSN: 2581-690X
Among the main global health concern is the rampant rise in antibiotic resistant bacteria. One of the appealing and promising strategies to combat this menace is to target the adaptive mechanism called quorum sensing (QS) used by bacteria to survive. Exploratory research on anti-QS compounds derived from natural products has been a promising area. The present study investigated methanolic extracts from 26 plants to compare their anti-QS activity using the QS biosensor strain Chromobacterium violaceum American Type Culture Collection (ATCC 12472) (Microbial Type Culture Collection MTCC2656). QS-mediated violacein pigment inhibition was carried out using agar well diffusion method with concentrations ranging from 10 mg/ml to 100 mg/ml. Leaf extracts of Mangifera indica and Pimenta dioica and peel extract of Punica granatum were the only three plants found to exhibit violacein inhibitory potential till 10 mg/ml. The result of the minimum inhibitory concentration (MIC) showed 1.6 mg/ml for M. indica and P. dioica and 6.25 mg/ml for P. granatum. Further, violacein inhibitory properties of these extracts at and below MIC were evaluated by well diffusion assay (qualitative) and by flask incubation assay (quantitative). The zone of inhibition (well diffusion assay) was found to be 14.51 ± 0.63 mm to 10.37 ± 0.68mm for M. indica, 15.23 ± 0.57 mm to 9.62 ± 1.29 mm for P. granatum and 17.01 ± 0.1 mm to 13.14 ± 0.18 mm for P. dioica. The inhibitory effect of the plant extracts via quantitative assay on violacein ranged from 83-49%, 89-81%, and 89-49% for M. indica, P. granatum, and P. dioica respectively. Our findings suggested the potential of M. indica, P. granatum, and P. dioica methanolic extracts as a source of effective inhibitors of QS-mediated violacein production.
Antibiotic Resistance, Chromobacterium violaceum, Plant Extracts, Quorum Sensing, Violacein, Virulence Factors
The increased prevalence of multidrug-resistant bacteria is taking the world back to the pre-antibiotic era1 and has encouraged researchers to focus on alternative approaches.2 Antibacterial resistance exhibited by many bacteria is reported to be associated with QS-regulated behaviour. QS is the density-dependent process that allows bacterial interaction for survival and subsequent enhancement in their pathogenicity.3 One of the promising options to deal with pathogenic bacteria is to interfere with the mechanism leading to their virulence factors production. Researchers are trying to fight infections due to pathogenic bacteria by identifying methods/compounds that target particular modulators of QS-mediated virulence factors.4 Current studies suggest that various virulence factors produced by bacteria (both Gram-negative and Gram-positive) are related to QS which are facilitated by auto inducers. Auto-inducing peptides (AIP) produced by Gram-positive and acyl homoserine lactones (AHL) by Gram-negative bacteria are not just known to contribute to inter-microbial communication but also directly or indirectly cross-communicate with their host.1,5 Novel ways that block or manipulate the QS pathways are evolving as promising antipathogenic or antivirulence therapeutic strategies to combat bacterial colonization and infection.6
Despite the multitude of antibacterial agents available, resistance to them is increasing primarily due to their misuse or overuse coupled with poor infection prevention and control. Plant extracts with diverse array of phytoconstituents like alkaloids, flavonoids, phenols, saponins, coumarins and terpenes have been considered effective in inhibiting the multidrug resistance phenotype of pathogens via their counter-action towards diverse factors that promote the emergence of drug resistance.7 The antibacterial potential of various phytochemicals as well as their effect on virulence factors production such as swarming motility, pyocyanin and biofilm production have been reported. Inhibitory properties of quercetin, a common phytoconstituent, on virulence factors and biofilm development in Pseudomonas aeruginosa PAO1, have been reported.8 A study that investigated the effect of selected phenolic secondary metabolites on QS-related virulence factor production of PAO1, demonstrated the reduction in the pyocyanin and biofilm formation by caffeic and ferulic acids.9
Attempts to develop easy and rapid techniques utilizing model organisms to identify new and potent QS inhibitors (QSI) have resulted in an increased interest towards natural products from both microbes and plants.1 C. violaceum has been frequently used for QS investigations as a model organism because of its indicator ability associated with QS-regulated violacein biosynthesis, and its suitability in understanding how molecules interact and affect the QS mechanism of microorganisms. This property of C. violaceum has been explored for understanding how disprupting bacterial communication can affect their pathogenicity in qualitative and quantitative terms.
Detailed understanding on QS regulated virulence factor production has helped scientists identify different mechanisms of bacterial pathogenicity. Therefore it is important for scientists to focus on novel ways and products particularly from plants for interrupting QS.10 The plant extracts which exhibited quorum sensing mediated pigment inhibition in C. violaceium appear promising for use as sources of phytochemicals that will reduce bacterial resistance via inhibition of diverse virulence factor production. In this context, the present study was designed to investigate 26 medicinal plant extracts to validate their quorum sensing inhibition potential and relative quorum modulatory efficiency.
Plant material collection and authentication
Twenty-six plant materials were sourced from Ernakulam city, Kerala, India, Indian Institute of Horticultural Research Bangalore (IIHR) and online ayurvedic product dealers. Out of this, 23 plants were authenticated by Dr. P.E. Rajasekharan, Principal Scientist, Division of Flower and Medicinal Crops, IIHR, Bangalore. The remaining three plants were authenticated by Dr. V. Rama Rao, Research Officer (Botany) at Central Ayurveda Research Institute, Uttarahalli, Bengaluru-560109, with voucher numbers RRCBI-13286 (Punica granatum), RRCB1-MUS110 (Mangifera indica) and RRCBI-mus411 (Pimenta dioica). The list of plants screened for their quorum sensing inhibitory activity is given in Table 1.
Table (1):
List of Indian medicinal plants screened for antiquorum sensing activity against C. violaceum MTCC 2656
No |
Plants |
Family |
Common name |
Parts used |
Ref. |
|---|---|---|---|---|---|
1. |
Aegle marmelos |
Rutaceae |
Indian bael |
leaves |
11,12 |
2. |
Aloe barbadensis miller. |
Asphodelaceae (Liliaceae) |
aloe |
leaves |
13 |
3. |
Asparagus recemosus |
Asparagaceae |
shatawari |
root |
14,15 |
4 |
Azadirachta indica |
Meliaceae |
neem |
leaves |
16 |
5 |
Bacopa monnieri |
Plantaginaceae |
brahmi |
leaves |
17,18 |
6 |
Baliospermum montanum |
Euphorbiaceae |
danti,wild castor |
leaves |
19 |
7 |
Bryophyllum pinnatum |
Crassulaceae |
miracle leaf |
leaves |
20 |
8 |
Calotropis gigantea L. |
Apocynaceae |
giant milk weed |
leaves |
21 |
9 |
Cassia fistula |
Fabaceae |
golden shower |
leaves |
22 |
10 |
Chromolaena odorata |
Asteraceae |
siam weed, christmas bush |
leaves |
23,24 |
11 |
Cissus quadrangularis |
Vitaceae |
veldt grape, asthisamharaka |
stem |
25 |
12 |
Citrus reticulata L.var |
Rutaceae |
mandarin orange |
Pericarp(of fruit)) |
26 |
13 |
Gmelina arborea |
Lamiaceae |
gamhar, white teak |
leaves |
27,28 |
14 |
Glycyrrhiza glabra |
Fabaceae |
licorice |
root |
29 |
15 |
Gymnema sylvestre |
Apocynaceae |
gurmar, Australian cowplant |
leaves |
30 |
16 |
Hemidesmus indicus |
Apocynaceae |
anantmool |
roots |
31 |
17 |
Hemigraphis colorata |
Acanthaceae |
red ivy |
leaves |
32 |
18 |
Mangifera indica |
Anacardiaceae |
mango tree |
leaves |
33 |
19 |
Moringa oleifera |
Moringaceae |
drumstick tree |
leaves |
34 |
20 |
Pimenta dioica |
Myrtaceae |
Jamaica pepper |
leaves |
35 |
21 |
Piper betle |
Piperaceae |
betel |
leaves |
36 |
22 |
Piper nigrum |
Piperaceae |
black pepper |
Leaves |
37 |
23 |
Psidium guajava |
Myrtaceae |
common guava |
leaves |
38 |
24 |
Punica granatum |
Lythraceae |
pomegranate |
peel and membrane |
39 |
25 |
Syzygium cumini |
Myrtaceae |
Jamun |
leaves |
4 |
26 |
Vitex negundo |
Lamiaceae |
Chinese chaste tree |
leaves |
40 |
Preparation of plant extracts
Plant materials consisting of leaves, roots and peels were washed in tap water, shade dried and ground to powder. Two hundred milliliters of absolute methanol was added to 20 grams of the powdered plant materials and kept in the orbital shaker incubator at room temperature. After 48 hours of extraction, filtration was done using filter paper (Whatman No. 1) and was dried at 45°C. 31 The dried crude extracts were stored at -20°C until further analysis. Stock concentration of 50 mg/ml was made in 1% DMSO from which further assays were performed.41
Bacterial strain and culture conditions
Lyophilized culture of C. violaceum (MTCC 2656) was obtained from MTCC, IMTECH, Chandigarh, India. This bacteria is used as biosensor strain because it produces a purple pigment called violacein that is QS regulated. The strain was routinely cultured in Luria-Bertani (LB) broth (1% NaCl, 1% tryptone, 0.5% yeast extract) between 30-37°C.1 Glycerol stock cultures of the organism were made and stored at -80°C for further use.
Media and chemicals
LB agar (Miller), Muller Hinton Broth (MHB), LB broth (Miller), and glycerol were purchased from Sisco Research Laboratories (SRL) Pvt. Ltd, Mumbai. p-iodonitrotetrazolium violet (INT) was supplied by Himedia Laboratories, Mumbai. Cinnamon oil was purchased from essancia (https://essanciaonline.com). All the chemicals used for the study were of analytical grade.
Assays for anti-QS activity
Screening for violacein inhibition (Qualitative assay)
The agar well diffusion assay was performed to screen the violacein pigment inhibitory activity of 26 selected medicinal plants on C. violaceum MTCC 2656 strain. Overnight bacterial cultures in LB broth were standardized to 0.5 McFarland and 0.1 ml of the inoculum was spread onto LB agar plates.42 Wells of 8 mm diameter were made on the agar plates, and 100, 75, 50, 25, and 10 mg/ml of the plant extracts were added to the wells and incubated for 24 hrs at 30°C. Cinnamon oil (2 µl /ml) and 1% DMSO served as positive and negative controls, respectively. The quorum inhibitory effect was determined by measuring the diameter ( mm) of the zone of violacein inhibition around the wells.1
Determination of MIC
MIC of the plant extracts, which exhibited violacein inhibition up to 10 mg/ml was performed. A two-fold serial dilution method in 96 well microtiter plate was used for MIC determination. An aliquot of 50 µl MHB was added to the wells of microtiter plate (1-10th well). Methanolic extract (50 mg/ml) was made in 1% DMSO. From the 50 mg/ml stock prepared, 100 µl was added to the first well. A serial dilution of the extracts was made by mixing and taking 50 µl to the subsequent well up to the 10th well. The last 50 µl was discarded from the 10th well. Bacteria, MHB broth and 10 µg/ml of gentamycin (positive control) were added to the 11th well. The media (MHB broth) and bacteria (12th well) was considered as negative control.43
Bacterial cultures were grown overnight in MHB and were adjusted to a turbidity of 0.5 Mc Farland equivalent to 1.5 × 108 cfu/ml. The inoculum was diluted 100 times with MHB, and 50 µl of this was added into each well (1-12) to arrive at bacterial suspension with a density of 5 × 105 CFU/ml.44 The bacteria were exposed to extract concentrations ranging from 50 to 0.1 mg/ml and were incubated for 24 hrs at 37°C. 40 µl of 0.2 mg/ml of INT was added to each well and further incubated for 1 hr. The change in the colour of dye from colourless to pink was due to the live and active bacteria. MIC of the plant extracts were performed in 3 independent trials in triplicates.45
Qualitative violacein inhibition assay
The agar-well diffusion assay was performed on the selected plant extracts which exhibited violacein inhibition at and sub-MIC concentration following the method described in the screening for violacein inhibition above.1
Quantification of violacein pigment (flask incubation assay)
The quantification of violacein pigment was performed using the method described by Choo et al.46 with slight modifications. C. violaceum MTCC 2656 was incubated in LB broth and kept at 30°C for 24 hrs. The bacterial turbidity was later adjusted to 0.5 McFarland using UV–visible spectrophotometer which is equivalent to 1.5 × 108 cfu/ml. One ml of this bacterial suspension was added to conical flask containing LB broth and plant extracts to achieve the following concentrations (6, 5, 4, 3, 2, 1, 0.5 mg/ml) for P. granatum peel and (1.6, 1.4, 1.2, 1.0, 0.8, 0.6 mg/ml) for M. indica and P. dioica leaves. Bacteria and broth only served as negative control, while 2 µl/ ml cinnamon oil as positive control. After overnight incubation, 1 ml from each flask was transferred to Eppendorf tubes and centrifuged at 13000 rpm for 10 minutes to precipitate violacein. The pellets obtained were resuspended in 1 ml DMSO. After rigorous mixing for 30 s to solubilize violacein pigment, samples were centrifuged at 13000 rpm for 10 minutes to remove the cells. Two hundred microliters of the suspension were transferred to 96 well microtiter plate and the absorbance was taken at 585 nm spectrophotometrically (VarioskanTM lux multimode plate reader, Thermo Scientific United States). The experiments were carried out in three independent trials in triplicates. The mean absorbance of the samples were determined and the violacein inhibition was calculated using the equation below:
% violacein Inhibition = OD(control) – OD(test) * 100 / OD (control)
Where OD(control) is the average OD of negative control (bacteria and broth). OD (test) is the average OD of the test for each concentration of the extract.47
Statistical analysis
The experiments were carried out in triplicate of 3 replicate analysis and the data were presented as mean ± standard error of the mean (SEM). One way analysis of variance (ANOVA) followed by Tukey’s mutiple comparison to understand statistical significance between the control and the various concentrations of treatments was used. GraphPad Prism (Version 8.0.1) was used for statistical analysis and p value ≤ 0.05 was considered statistically significant.
Screening of plants for their antiquorum sensing activity
Results of the twenty-six (26) plant extracts screened for their quorum sensing mediated violacein inhibition in C. violaceum MTCC 2656 are presented in Table 2. For initial screening, the plant extracts were tested for the violacein inhibition using concentrations ranging 10-100 mg/ml. Among the plant extracts that exhibited violacein inhibition, P. betle (leaf), P. nigrum (leaf), P. guajava (leaf) and S. cumini (leaf) showed inhibition up to 25 mg/ml while M. indica (leaf), P. dioica (leaf) and P. granatum (rind) showed violacein inhibition up to 10 mg/ml. The three plants (M. indica, P. granatum and P. dioica), which gave violacein inhibition at 10 mg/ml and below, were shortlisted for this study.
Table (2):
Screening results of plant extracts for their violacein inhibition
No. |
Plants |
Inhibition of violacein production |
|---|---|---|
1. |
Aegle marmelos |
negative |
2. |
Aloe barbadensis miller |
negative |
3. |
Asparagus recemosus |
negative |
4. |
Azadirachta indica |
negative |
5. |
Bacopa monnieri |
negative |
6. |
Baliospermum montanum |
negative |
7. |
Bryophyllum pinnatum |
negative |
8. |
Calotropis gigantea L. |
negative |
9. |
Cassia fistula |
negative |
10. |
Chromolaena odorata |
negative |
11. |
Cissus quadrangularis |
negative |
12. |
Citrus reticulata L.var |
negative |
13. |
Gmelina arborea |
negative |
14. |
Glycyrrhiza glabra |
negative |
15. |
Gymnema sylvestre |
negative |
16. |
Hemidesmus indicus |
negative |
17. |
Hemigraphis colorata |
negative |
18. |
Moringa oleifera |
negative |
19. |
Vitex negundo |
negative |
20. |
Mangifera indica |
positive |
21. |
Pimenta dioica |
positive |
22. |
Piper betle |
positive |
23. |
Piper nigrum |
positive |
24. |
Psidium guajava |
positive |
25. |
Punica granatum |
positive |
26. |
Syzygium cumini |
positive |
Positive = showing violacein inhibition, Neagative = not showing violacein inhibition.
Minimum inhibitory concentration (MIC)
Three plants (M. indica, P. granatum and P. dioica) that had violacein pigment inhibition potential till 10 mg/ml were selected to check their minimum inhibitory concentration against C. violaceum MTCC 2656. Table 3 presents the results from this experiment.
Table (3):
MIC of top 3 violacein inhibiting plant candidates
Plant extracts |
MIC against C.violaceum 2656 |
|---|---|
M. indica |
1.6 mg/ml |
P. granatum |
6.25 mg/ml |
P. dioica |
1.6 mg/ml |
Qualitative violacein inhibition assay
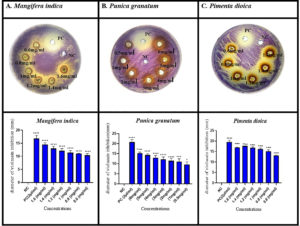
Agar well diffusion assay was performed for the selected plant extracts using MIC and sub-MIC concentrations (Table 4). Loss of purple colour around the wells in which plant extracts were placed is indicative of their quorum inhibitory activity (Figure 1).
Table (4):
Plant extract Concentrations used for violacein inhibition assays
Plant extracts |
Concen. used (mg/ml) |
|---|---|
M. indica |
1.6, 1.4, 1.2, 1.0, 0.8, 0.6 |
P. granatum |
6, 5, 4, 3, 2, 1, 0.5 |
P. dioica |
1.6, 1.4, 1.2, 1.0, 0.8, 0.6 |
Figure 1. Violacein inhibition by well diffusion assay
Data were considered as mean ± SEM of three independent biological replicates. p values were calculated using one-way ANOVA. Tukey’s post hoc was used for multiple comparisons. p ˂ 0.05 is considered significant. (*p < 0.05, ***p < 0.001, ****p < 0.0001). PC = positive control (cinnamon oil), NC=negative control (1% DMSO). All the treatments were compared with the negative control”
From the above results (Figure 1), it was observed that the quorum inhibitory activity was concentration-dependent. With the decrease in the concentration of extract, the diameter of the clear zone of pigment inhibition around the wells also decreased.
Violacein quantification
Violacein pigment was quantitatively determined in response to the above-mentioned concentrations (Figure 2) of the shortlisted plant extracts. Concentration-dependent pigment inhibitory effect was observed. These inhibitory effect of the plant extracts on violacein ranged from 83% – 49%, 89%, -81%, and 89%-49% for M. indica, P. grantum and P. dioica, respectively.
Figure 2. Quantification of violacein pigment in response to treatment
Data were considered as mean ± SEM of three independent biological replicates. p values were calculated using one-way ANOVA. Tukey’s post hoc was used for multiple comparisons. p < 0.0001 is considered significant (****p < 0.0001). PC = positive control (2 µl/ml of cinnamon oil), NC=negative control (LB broth +bacteria only). All treatments were compared with the negative control. Different Concentrations of the 3 plant extracts were expressed in mg/ml
Antipathogenic strategies to treat bacterial infections by interrupting quorum sensing regulated virulence factors production have recently drawn interest due to the continuing emergence and spread of multidrug-resistant bacteria.48 The dependence on medicinal plants for managing bacterial infections has been in practice for ages despite the availability of modern medicines. The richness of phytochemicals in them that target diverse pathogenicity-associated pathways contribute to either killing or interfering with the growth of the pathogens, offering a range of microbial infection management agents. For this purpose, in the present study, C. violaceum (MTCC 2656), a bio-reporter strain known for the production of a purple-coloured pigment violacein which is a quorum sensing system-regulated phenotype10 was utilised to screen 26 methanolic plant extracts for their quorum-sensing inhibition potential. Seven of the screened plant extracts ‘P. betle (leaf), P. nigrum (leaf), S. cumini (leaf), P. guajava (leaf), M. indica (leaf), P. dioica (leaf) and P. granatum (rind) have shown anti QS activity at a higher concentration of 25-100 mg/ml. Among these, M. indica (leaf), P. dioica (leaf) and P. granatum (rind) were chosen further as they exhibited pigment inhibitory activity at concentrations of 10 mg/ml and below. The MIC of these three selected plant extracts were performed, and the pigment inhibitory potential at and sub-MIC levels showed significant pigment inhibition (P < 0.0001) compared to the negative control.
In our study, P. granatum peel gave an opaque zone of violacein inhibition of 15.23 ± 0.57 mm to 9.67 ± 1.3 mm at concentrations ranging from 6 mg/ml to 0.5 mg/ml, respectively. Similar results were reported by Mehta et al.49 at lower concentrations of 1.5 mg/ml to 0.075 mg/ml, exhibiting pigment inhibitory effect of 17 mm to 8 mm, respectively. The difference in violacein inhibition in the above report could be due to the different extraction procedures used. In another study by Oh et al.,50 the quorum inhibitory activity of pomegranate peel extract (PPE) using 75% aqueous methanol at 10 mg/ml was 26 mm. The absolute methanol extract of P. granatum in our study showed violacein inhibition of 22 mm at a similar concentration. This difference could also be due to differences in the method of extraction.
Husain et al.33 reported that the methanolic subfraction of M. indica inhibited violacein by 19 mm and 17 mm at concentrations 800 and 1600 µg/ml. Crude methanolic extracts used in our study showed pigment inhibition of 14.51 ± 0.63 mm and 11.08 ± 0.08 mm, respectively, at concentrations 1600 and 800 µg/ml. Despite the differences in the method of extraction, violacein inhibition at 1600 µg/ml is comparable to the result of Husain et al. above.
Vasvi et al.35 used 90% ethanol for the extraction of P. dioica leaves, and they have reported that the crude plant extract showed weak violacein pigment inhibition at a concentration ranging from 0.5-1 mg/ml. The results obtained from our study using crude extracts showed inhibition at concentrations of 1 mg/ml(16.22 ± 0.41 mm) and 0.6 mg/ml(13.14 ± 0.18 mm).
QS inhibitory property of the three plants were further quantitatively determined using the flask assay.49 In our study, P. granatum showed a substantial decrease in the percentage of violacein (80.76 ± 0.58%) at a concentration of 0.5 mg/ml, which is higher than 41.92% at a concentration of 0.6 mg/ml, as reported by Mehta et al.49 Similarly, a study by Oh et al.50 showed percentage violacein inhibition of 78.5%, 70%, 45% and 23% by P. granatum at concentrations of 2, 1.5, 1 and 0.5 mg/ml, respectively. Compared to these results, our P. granatum extracts showed a higher percentage of violacein inhibition of 86.92 ± 1.02%, 84.84 ± 1.70 and 80.76 ± 0.58% at similar concentrations of 2, 1 and 0.5 mg/ml, respectively.
The study conducted by Yang et al.51 revealed that the tannin-rich fraction from pomegranate rind (TFPR) effectively inhibited violacein production by 49.65% and 29.88% at concentrations of 0.312 and 0.625 mg/ml, respectively, in C. violaceum ATCC 12472. Our methanolic extract of P. granatum showed a better violacein inhibition of 80.76% at 0.5 mg/ml.
Yet in another study by Husain et al., the methanolic sub-fraction of the petroleum ether extract of M. indica decreased violacein production by 83.6%, 55.6% and 32.1% at concentrations of 0.8, 0.4 and 0.2 mg/ml, respectively.33 The violacein inhibitory effect achieved in our study by crude methanolic mango leaf extract at concentrations of 1 mg/ml (67.35%), 0.8 mg/ml (53.33%), and 0.6 mg/ml (48.5%) did not agree with the above study possibly due to differences in the method of extraction used. Quantitative violacein inhibitory assay using methanolic leaf extract of P. dioica in our study showed a percentage inhibition of 78.67 ± 10.79% at a concentration of 1 mg/ml. These results agree with the finding of Vasavi et al.,35 who conducted the quantification assay using the purified ethyl acetate fraction (EAF), and the percentage of violacein inhibition was more than 80% at concentrations of 0.5-1 mg/ml. ALrashidi et al.52 reported that the P. dioica essential oil showed percentage pigment inhibition of (38.25 ± 1.8%) and (32.34 ± 1.3%) at concentrations of 1.25 mg/ml and 0.625 mg/ml, respectively, in CV 12472. Our results using the P. dioica leaf extracts showed better percentage pigment inhibition of (87.81 ± 2.81%) and (49.78 ± 1.75%) at similar concentrations of 1.2 mg/ml and 0.6 mg/ml, respectively.
Al-Hussaini and Mahasneh,53 in their work on QS-inhibiting plant extracts, presented anti-QS activity as mild (8 to 10.5 mm), moderate (13 mm), and strong (18 mm) based on the diameter of the zone of violacein inhibition. The present study also attempted to compare the performance of QS inhibitory properties of the three selected plants via well diffusion assay (Qualitative) and flask assay (quantitative). At 1 mg/ml extract concentration, P. dioica appears to have significant (p < 0.05) QS inhibition in qualitative assay compared to M. indica and P.granatum.
Quantitative assay performance of this corresponding concentration (1 mg/ml) did not show significant QS inhibition. But P. granatum (0.5 mg/ml) has shown a significant (p < 0.0001) QS inhibition compared to the closer concentration (0.6 mg/ml) of M. indica and P. dioica.
Findings from the present study suggest the effectiveness of the quantitative violacein inhibition assay for a better comprehension of QSI properties by plant extracts. The observed difference could be attributed to the behaviour of the bacteria in the different media used in culture.47
The observed differences in the QSI of plant extracts could be due to the different types of phytochemicals present in them.54 Prior research has stated that P. dioica leaf extracts are rich in glycosides, alkaloids, carbohydrates, flavonoids, tannins, and proteins contributing to its diverse pharmacological properties. The major constituents of the essential oil extracted from this plant include methyl eugenol, eugenol, myrcene, b-caryophyllene, cineole, and limonene. A significant quantity of eugenol, a phenolic compound present in this essential oil, is known for its wide-ranging antibacterial and anti-quorum sensing activities.52,55 Accumulated research demonstrates the components of P. granatum exhibiting anti-inflammatory, antibacterial, antiviral, and antifungal properties.39 Research validates that the antibacterial effect of P. granatum is attributed to its abundant phenolic components, hydrolysable tannins, and anthocyanins.56 Compounds like punicalagin, chlorogenic acid, rutin, epicatechin, gallic acid, caffeic acid, quercetin, catechin, and cinnamic acid are the primary phytochemical compounds that exhibit significant quorum sensing inhibitory potential.39,57 M. indica leaves have been reported to contain mangiferin, phenolic acids, benzophenones flavonoids, ascorbic acid, carotenoids, xanthones, tannins, terpenoids and tocopherols, which have antioxidants and antibacterial activities.58,59 The high concentration of gallic acid in the methanolic leaf extract of M. indica was suggested as a reason for its higher anti-QS activity.33
Our findings suggested the potential of M. indica, P. granatum, and P. dioica methanolic extracts as a source of effective inhibitors of QS-mediated virulence factors. These results indicate the presence of QS inhibitory compounds that might be interfering with either the synthesis or reception of autoinducers. Thus, they may disrupt the intercellular communication process by suppressing the synthesis of violacein in
C. violaceum without affecting its growth.
Though the discovery of antibiotics has greatly benefited human civilization, a review of the effectiveness of the antimicrobials in use is necessary due to the rising prevalence of multidrug-resistant bacteria. Antibacterial agents that interfere with the QS system in bacteria, affecting their virulence factor production, can be a potential way to reduce the menace of antibiotic resistance. Given that quorum sensing triggers the activation of virulence genes in various pathogens, agents capable of disrupting bacterial communication can be utilized as antipathogenic drugs. Therefore, this further opens up an opportunity to evaluate their bioactive compounds systematically as drugs adjunct to standard antibiotics. An enhanced comprehension of the mechanism of action of these QS inhibitors on microbial cell communication has the potential for innovative therapeutic applications in combating the pathogen. The findings of this work demonstrate the potential of Indian medicinal plants as a source of anti-QS phytochemicals. Additionally, it emphasizes the significance of assessing the untapped diversity of these plants for their ability to exhibit such activity. Further studies that elucidate the phytochemicals present in these plants, along with their molecular mechanism associated with such appreciable anti-infective activity, are warranted. Such investigations may pave the way for the development of novel drug candidates for quorum sensing inhibition and associated complications.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Leela Iyengar, Adjunct Professor, Jain (Deemed-to-be University), Bengaluru, India, and Chief Scientific Officer (Retd.), I.I.T. Kanpur, India, for her suggestions and critical revision of the manuscript.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Zahin M, Hasan S, Aqil F, Khan MSA, Husain FM, Ahmad I. Screening of certain medicinal plants from India for their anti-quorum sensing activity. Indian J Exp Biol. 2010;48(12):1219-1224.
- Sule D, Baris G, Ismail S, Ahmet D, Sesal NC. Anti-quorum sensing and anti-biofilm activities of Hypericum perforatum extracts against Pseudomonas aeruginosa. J Ethnopharmacol. 2019;(235):293-300.
Crossref - Patel P, Joshi C, Kothari V. Antipathogenic potential of a polyherbal wound-care formulation (Herboheal) against certain wound-infective gram-negative bacteria. Adv Pharmacol Sci. 2019;2019:1739868.
Crossref - Gupta K, Singh SP, Manhar AK, et al. Inhibition of Staphylococcus aureus and Pseudomonas aeruginosa Biofilm and Virulence by Active Fraction of Syzygium cumini (L.) Skeels Leaf Extract: In-Vitro and In Silico Studies. Indian J Microbiol. 2019;59(1):13-21.
Crossref - Verbeke F, De Craemer S, Debunne N, et al. Peptides as quorum sensing molecules: Measurement techniques and obtained levels In vitro and In vivo. Front Neurosci. 2017;11:1-18.
Crossref - Tamfu AN, Ceylan O, Fru GC, Ozturk M, Duru ME, Shaheen F. Antibiofilm, antiquorum sensing and antioxidant activity of secondary metabolites from seeds of Annona senegalensis, Persoon. Microb Pathog. 2020;144:104191.
Crossref - Maver T, Maver U, Stana Kleinschek K, Smrke DM, Kreft S. A review of herbal medicines in wound healing. Int J Dermatol. 2015;54(7):740-751.
Crossref - Ouyang J, Sun F, Feng W, et al. Quercetin is an effective inhibitor of quorum sensing, biofilm formation and virulence factors in Pseudomonas aeruginosa. J Appl Microbiol. 2016;120(4):966-974.
Crossref - Ugurlu A, Yagci AK, Ulusoy S, Aksu B, Bosgelmez-Tinaz G. Phenolic compounds affect production of pyocyanin, swarming motility and biofilm formation of Pseudomonas aeruginosa. Asian Pac J Trop Biomed. 2016;6(8):698-701.
Crossref - Dimitrova PD, Damyanova T, Paunova-Krasteva T. Chromobacterium Violaceum: A Model for Evaluating the Anti-Quorum Sensing Activities of Plant Substances. Sci Pharm. 2023;91(3):33.
Crossref - Pathirana CK, Madhujith T, Eeswara J. Bael (Aegle marmelos L. Correa), a Medicinal Tree with I mmense Economic Potentials. Adv Agric. 2020;2020:8814018.
Crossref - Namasivayam SKR, Vivek JM. Anti quorum sensing activities of medicinal plant extracts against quorum sensing mediated virulence factors of Pseudomonas aeruginosa. Der Pharm Lettre. 2016;8(8):412-423.
- Yeo SSM, Tham FY. Anti-quorum sensing and antimicrobial activities of some traditional chinese medicinal plants co mmonly used in South-East Asia. Malays J Microbiol. 2012;8(1):11-20.
Crossref - Shashi A, Jain SK, Verma A, Kumar M, Mahor A, Sabharwal M. Plant profile, phytochemistry (Shatavari ): A review and pharmacology of A sparagus racemosus. 2013;3(3):242-251.
Crossref - Singh N, Mishra S, Mondal A, Sharma D, Jain N, Aseri GK. Potential of Desert Medicinal Plants for Combating Resistant Biofilms in Urinary Tract Infections. Appl Biochem Biotechnol. 2023;195(9):5568-5582.
Crossref - Mishra R, Gwalani K, Nashikkar N, Bundale S. Antibacterial and Anti-Quorum Sensing Studies of Extracellularly Synthesized Silver Nanoparticles from Azadirachta indica (Neem) Leaf Extract. Biosci Biotechnol Res Asia. 2022;19(4):1065-1078.
Crossref - Ghosh T, Maity TK, Dash DK, Boss A. A study on wound healing activity of Bacopa monnieri Linn. aerial parts. Orient Pharm Exp Med. 2007;7(2):150-156.
Crossref - Parai D, Islam E, Mitra J, Mukherjee SK. Effect of Bacoside A on growth and biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa. Can J Microbiol. 2016;63(2): 169-178.
Crossref - Kumar H, Jain SK, Singh N, Dixit V, Singh P. Wound healing activity of the plant of Baliospermum montanum Willd. Int J Pharm Sci Res. 2011;2(4):1073-1076.
Crossref - Faujdar C, Priyadarshini. Comparative study of hydroalcoholic extracts of Bryophyllum pinnatum and Macrotyloma uniflorum for their antioxidant, antiurolithiatic, and wound healing potential. J Appl Biol Biotechnol. 2022;10(1):196-205.
Crossref - Tanimu H, Vijayan NK, Bindhu OS. Evaluation of Biofilm Inhibitory Efficacy of Bacopa monnieri, Acalypha indica and Calotropis gigantea Extracts and their Combination Against Wound Colonizing Bacteria Pseudomonas aeruginosa and Staphylococcus Aureus. Int J Life Sci Pharma Res. 2022;12(6):198-206.
Crossref - Peerzada Z, Kanhed AM, Desai KB. Effects of active compounds from Cassia fistula on quorum sensing mediated virulence and biofilm formation in Pseudomonas aeruginosa. RSC Adv. 2022;12(24):15196-15214.
Crossref - Pandith H, Zhang X, Liggett J, Min KW, Gritsanapan W, Baek SJ. Hemostatic and Wound Healing Properties of Chromolaena odorata Leaf Extract. ISRN Dermatol. 2013;2013:168269.
Crossref - Yahya MFZR, Ibrahim MSA, Zawawi WMAWM, Hamid UMA. Biofilm killing effects of Chromolaena odorata extracts against Pseudomonas aeruginosa. Res J Phytochem. 2014;8(3):64-73.
Crossref - Mosaib MG, Maruf MAA, Islam R, et al. Antibacterial Activity of Cissus quadrangularis Stem Extract on the Pathogenic and Industrial Waste Watered Bacteria. Eur J Med Heal Sci. 2020;2(2):28-38.
Crossref - D’Almeida RE, Sued N, Arena ME. Citrus paradisi and Citrus reticulata essential oils interfere with Pseudomonas aeruginosa quorum sensing in vivo on Caenorhabditis elegans. Phytomedicine Plus. 2022;2(1):100160.
Crossref - Ghareeb MA, Refahy LA, Saad AM, et al. In vitro antimicrobial activity of five Egyptian plant species. J Appl Pharm Sci. 2015;5(Suppl 2):45-49.
Crossref - Joo HS, Deyrup ST, Shim SH. Endophyte-produced antimicrobials: a review of potential lead compounds with a focus on quorum-sensing disruptors. Phytochem Rev. 2021;20(3):543-568.
Crossref - Bhargava N, Singh SP, Sharma A, Sharma P, Capalash N. Attenuation of quorum sensing-mediated virulence of Acinetobacter baumannii by Glycyrrhiza glabra flavonoids. Future Microbiol. 2015;10(12):1953-1968.
Crossref - Samreen, Qais FA, Ahmad I. Anti-quorum sensing and biofilm inhibitory effect of some medicinal plants against gram-negative bacterial pathogens: in vitro and in silico investigations. Heliyon. 2022;8(10):e11113.
Crossref - Kannappan A, Santhakumari S, Srinivasan R, Pandian SK, Ravi AV. Hemidesmus indicus, a traditional medicinal plant, targets the adherence of multidrug-resistant pathogens to form biofilms. Biocatal Agric Biotechnol. 2019;21:101338.
Crossref - Anitha VT, Antonisamy JM, Jeeva S. Anti-bacterial studies on Hemigraphis colorata (Blume) H.G. Hallier and Elephantopus scaber L. Asian Pac J Trop Med. 2012;5(1):52-57.
Crossref - Husain FM, Ahmad I, Al-Thubiani AS, Abulreesh HH, AlHazza IM, Aqil F. Leaf extracts of Mangifera indica L. inhibit quorum sensing – Regulated production of virulence factors and biofilm in test bacteria. Front Microbiol. 2017;8:727.
Crossref - Jonnalagadda S, Deshabathini UK. Anti quorum sensing potential of Moringa oleifera seed extract. Int J Pharm Pharm Sci. 2016;8(1):76-82.
- Vasavi HS, Arun AB, Rekha PD. Inhibition of quorum sensing in Chromobacterium violaceum by Syzygium cumini L. and Pimenta dioica L. Asian Pac J Trop Biomed. 2013;3(12):954-959.
Crossref - Datta S, Jana D, Maity TR, Samanta A, Banerjee R. Piper betle leaf extract affects the quorum sensing and hence virulence of Pseudomonas aeruginosa PAO1. 3 Biotech. 2016;6(1):18.
Crossref - Tan LY, Yin WF, Chan KG. Piper nigrum, Piper betle and Gnetum gnemon– Natural Food Properties, Sources with Anti-Quorum Sensing. Sensors (Switzerland). 2013;13(3):3975-3985.
Crossref - Khan MA, Celik I, Khan HM, et al. Antibiofilm and anti-quorum sensing activity of Psidium guajava L. leaf extract: In vitro and In silico approach. PLoS One. 2023;18(12):e0295524.
Crossref - Hamrita B, Noumi E, Hafi F, Nazzaro F, Snoussi M. Phytochemical composition and antimicrobial, and anti-quorum sensing activities of Punica granatum L. methanolic extract. Iran J Microbiol. 2022;14(3):373-382.
Crossref - Mani A, Gayathri M. Effect of anti-biofilm potential of different medicinal plants: Review. Asian J Pharm Clin Res. 2017;10(2):24-32.
Crossref - Pellegrini MC, Ponce AG. Beet (Beta vulgaris) and Leek (Allium porrum) Leaves as a Source of Bioactive Compounds with Anti-quorum Sensing and Anti-biofilm Activity. Waste and Biomass Valorization. 2020;11(8):4305-4313.
Crossref - Al-Haidari RA, Shaaban MI, Ibrahim SRM, Mohamed GA. Anti-quorum sensing activity of some medicinal plants. Afr J Tradit Complement Altern Med. 2016;13(5):67-71.
Crossref - Adeyemo RO, Famuyide IM, Dzoyem JP, Lyndy Joy M. Anti-Biofilm, Antibacterial, and Anti-Quorum Sensing Activities of Selected South African Plants Traditionally Used to Treat Diarrhoea. Evid Based Complement Altern Med. 2022;2022:1307801.
Crossref - Kowalska-Krochmal B, Dudek-Wicher R. The minimum inhibitory concentration of antibiotics: Methods, interpretation, clinical relevance. Pathogens. 2021;10(2):165.
Crossref - Gadisa E, Gebru W, Kassu D, et al. Combined Antibacterial Effect of Essential Oils from Three Most Commonly used Ethiopian Traditional Medicinal Plants on Selected Multidrug Resistant Bacteria. 2019;19(1):24.
Crossref - Choo JH, Rukayadi Y, Hwang JK. Inhibition of bacterial quorum sensing by vanilla extract. Lett Appl Microbiol. 2006;42(6):637-641.
Crossref - Baloyi IT, Cosa S, Combrinck S, Leonard CM, Viljoen AM. Anti-quorum sensing and antimicrobial activities of South African medicinal plants against uropathogens. S Afr J Bot. 2019;122:484-491.
Crossref - Damte D, Gebru E, Lee SJ, Suh JW, Park SC. Evaluation of anti-quorum sensing activity of 97 indigenous plant extracts from korea through bioreporter bacterial strains Chromobacterium violaceum and Pseudomonas aeruginosa. J Microb Biochem Technol. 2013;5(2):42-46.
Crossref - Mehta GJ, Jadeja VJ. Assessment of anti-quorum sensing potential of selected medicinal plant extracts using Chromobacterium violaceum. Int J Pharm Res. 2019;11(4):601-611.
- Oh SK, Chang HJ, Chun HS, Kim HJ, Lee N. Pomegranate [Punica granatum L.) peel extract inhibits quorum sensing and biofilm formation potential in Yersinia enterocolitica. Korean J Microbiol Biotechnol. 2015;43(4):357-366.
Crossref - Yang Q, Wang L, Gao J, et al. Tannin-Rich Fraction from Pomegranate Rind Inhibits Quorum Sensing in Chromobacterium violaceum and Biofilm Formation in Escherichia coli. Foodborne Pathog Dis. 2016;13(1):28-35.
Crossref - Alrashidi AA, Noumi E, Snoussi M, De Feo V. Chemical Composition, Antibacterial and Anti-Quorum Sensing Activities of Pimenta dioica L. Essential Oil and Its Major Compound (Eugenol) against Foodborne Pathogenic Bacteria. Plants. 2022;11(4):540.
Crossref - Al-Hussaini R, Mahasneh AM. Microbial growth and quorum sensing antagonist activities of herbal plants extracts. Molecules. 2009;14(9):3425-3435.
Crossref - Hemeg HA, Moussa IM, Ibrahim S, et al. Antimicrobial effect of different herbal plant extracts against different microbial population. Saudi J Biol Sci. 2020;27(12):3221-3227.
Crossref - Nagalakshmi K, Angeline RM, Prasath GS. Phytochemical characterisation, antioxidant and anti-microbial efficacy of allspice Pimenta dioica. Res J Pharm Technol. 2023;16(12):5878-5873.
Crossref - Howell AB, D’Souza DH. The pomegranate: Effects on bacteria and viruses that influence human health. Evid Based Complement Altern Med. 2013;2013:606212.
Crossref - Joshi C, Patel P, Kothari V, et al. Anti-infective potential of hydroalcoholic extract of Punica granatum peel against gram-negative bacterial pathogens. F1000Res. 2019;8:70.
Crossref - Kumar M, Saurabh V, Tomar M, et al. Mango (Mangifera indica l.) leaves: Nutritional composition, phytochemical profile, and health-promoting bioactivities. Antioxidants. 2021;10(2):299.
Crossref - Diso SU, Ali M, Mukhtar SI, Garba M. Antibacterial Activity and Phytochemical Screening of Mangifera indica (Mango) Stem and Leaf Extracts on Clinical Isolates of Methicillin Resistant Staphylococcus aureus. J Adv Med Pharm Sci. 2017;13(1):1-6.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.