ISSN: 0973-7510
E-ISSN: 2581-690X
Xylanase plays a crucial role in the degradation of hemicellulose and holds significant promise across a variety of biotechnological industries. This study investigates the effects of culture medium composition and initial pH on xylanase production by Aspergillus niger. Five different media; Mandels’, Vogel’s, Marciel’s, Okafor’s, and a modified Czapek-Dox, were evaluated for their ability to support enzyme synthesis. Additionally, initial pH levels ranging from 4.0 to 8.0 were tested to identify optimal production conditions. Among the tested media, Mandels’ medium supported the highest xylanase activity, while the optimal pH range for enzyme production was between 6.0 and 7.0. These findings provide valuable insights for optimizing large-scale xylanase production using A. niger, contributing to the development of more efficient and cost-effective bioprocesses. Notably, this study incorporates biomass-specific productivity metrics (IU/g biomass), allowing for a more accurate evaluation of the strain’s enzymatic efficiency. This quantitative approach may help inform future efforts to develop cost-effective and scalable bioprocesses, particularly when applied to low-cost agro-industrial substrates.
Xylanase, Hemicellulose Degradation, Culture Medium Optimization, Biomass-specific Productivity
Hemicellulose, a major polysaccharide component of plant biomass, represents a significant renewable resource for the production of value-added bioproducts.1,2 Xylan, the most abundant form of hemicellulose,3 is composed of a α-1,4-linked xylose backbone with various side groups and requires a synergistic set of enzymes for its complete hydrolysis. Among these, xylanases (endo-β-1,4-xylanases; EC 3.2.1.8) catalyze the random cleavage of the xylan backbone, making them critical for the efficient degradation of lignocellulosic biomass.4 Owing to their efficiency and versatility, xylanases are widely employed in several industrial sectors, including pulp and paper processing (for environmentally friendly bleaching), animal feed improvement, food and beverage clarification, textile processing, and biofuel production.5,6
Given the growing interest in sustainable bioprocessing, microbial production of xylanase has received considerable attention. Filamentous fungi, particularly Aspergillus species, are regarded as prolific producers due to their high secretion capacity and well-established fermentation profiles.7,8 Aspergillus niger has emerged as a leading organism for industrial enzyme production8 due to its Generally Recognized as Safe (GRAS) status, robust growth on diverse substrates, and ease of genetic manipulation.9 However, the production yield and activity of fungal xylanases are highly sensitive to cultivation conditions, especially the composition of the culture medium10,11 and environmental pH.12,13
Among these variables, the composition of the culture medium plays a decisive role in modulating enzyme synthesis, not only by supplying essential nutrients but also by influencing regulatory pathways involved in enzyme induction or repression.14 For instance, the utilization of agro-industrial residues like wheat bran and corn cob has been shown to enhance xylanase production in A. niger strains under solid-state fermentation conditions.15,16 Additionally, pH plays a crucial role in enzyme production17 by influencing nutrient solubility, membrane transport, enzyme stability, and the activity of regulatory proteins.18,19 Studies have demonstrated that A. niger can produce xylanase over a broad pH range, with optimal activity observed in both acidic and alkaline conditions, depending on the strain and cultivation parameters.20,21
Although numerous studies have investigated xylanase production, comprehensive comparative analyses of the effects of different culture media and pH conditions on A. niger remain limited. In this study, five culture media; Mandels’, Vogel’s, Marciel’s, Okafor’s, and a modified Czapek-Dox medium, were evaluated for their ability to support xylanase production by A. niger. In addition, the influence of initial pH (4.0-8.0) was examined to determine optimal conditions for enzyme synthesis. The findings provide valuable insights for the biotechnological optimization of A. niger xylanase production, with potential applications in diverse industrial sectors.
Strain and inoculum preparation
A. niger ATCC 16888 was used as the enzyme-producing strain. It was cultivated on potato dextrose agar (PDA) at 30 °C for 7 days. Spores were harvested using sterile water, and the spore suspension was adjusted to 108 spores/mL.
Culture media and pH conditions
Five liquid media were tested: Mandels’ medium, Vogel’s medium, Marciel’s medium,22 Okafor’s medium,23 and modified Czapek-Dox medium (CDM)24 as presented in Table 1. Birchwood xylan (1.0% w/v) was used as the carbon source. Initial pH was set to 5.6. To assess pH influence, Mandels’ medium was adjusted to 4.0, 5.0, 6.0, 7.0, and 8.0 using acetate buffer (pH 4.0-5.0) and phosphate buffer (pH 6.0-8.0).
Table (1):
Composition of the media for A. niger cultivation
| Composition (g/L) | Mandels’ | Vogel’s | Marciel’s | Okafar’s | Modified CDM |
|---|---|---|---|---|---|
| Urea | 0.30 | – | – | – | – |
| Peptone | 0.75 | – | – | – | – |
| Yeast extract | 0.25 | – | – | – | 5.00 |
| (NH4)2SO4 | 1.40 | – | – | – | – |
| NH4NO3 | – | 2.00 | – | – | – |
| KH2PO4 | 2.00 | 5.00 | – | 1.00 | 1.00 |
| K2HPO4 | – | – | 0.23 | – | 1.00 |
| MgSO4·7H2O | 0.30 | 0.20 | 0.05 | – | 0.50 |
| CaCl2·2H2O | 0.40 | 0.10 | 0.01 | – | – |
| NaNO3 | – | – | 0.05 | 3.00 | 3.00 |
| KCl | – | – | – | 0.50 | 0.50 |
| Na3-citrate | – | 2.50 | – | – | – |
| Trace elements (mg/L) | |||||
| FeSO4·7H2O | 5.00 | 0.75 | 9.00 | 10.00 | 10.00 |
| ZnSO4 | 1.40 | 3.00 | 2.00 | 1.00 | – |
| MnSO4 | 1.60 | 0.50 | 12.00 | 12.5 | – |
| CoCl2·6H2O | 20.00 | – | – | – | – |
| CuSO4·5H2O | – | 0.30 | – | 0.50 | – |
| H3BO3 | – | 0.05 | – | – | – |
Cultivation technique
Fifty milliliters of sterilized culture medium (e.g., Mandels’ medium) were dispensed into 250 mL Erlenmeyer flasks, adjusted to the desired initial pH (ranging from 4.0 to 8.0), and autoclaved at 121 °C for 15 minutes. After cooling, the media were inoculated with A. niger spore suspension at a concentration of 107 spores/mL. The cultures were incubated at 30 °C for 5 days with shaking at 120 rpm to ensure adequate aeration. Samples were collected at regular intervals to assess biomass accumulation and xylanase activity. All experimental conditions were tested in triplicate to ensure accuracy and reproducibility.
Enzyme extraction and assay
Samples were centrifuged at 6,000 rpm for 10 minutes, after which the supernatant was collected for enzyme assays, and the biomass dry weight was measured. Xylanase activity was determined according to the method of Bailey et al.25 using birchwood xylan (1% w/v) as the substrate. The reaction mixture contained 0.5 mL of appropriately diluted enzyme solution and 0.5 mL of substrate prepared in 50 mM sodium citrate buffer (pH 6.0). The mixture was incubated at
50 °C for 10 minutes.
The amount of reducing sugars released was quantified using the 3,5-dinitrosalicylic acid (DNS) method,26 with absorbance measured at 540 nm. One unit (IU) of xylanase activity was defined as the amount of enzyme required to release 1.0 µmol of xylose per minute under the assay conditions.
Determination of biomass
Fungal biomass obtained from the cultivation was harvested by filtration using pre-weighed Whatman No. 1 filter paper, then washed thoroughly with distilled water to remove residual medium. The biomass was dried at 80 °C for 24 hours or until a constant weight was achieved and subsequently weighed to determine dry biomass. All measurements were performed in triplicate to ensure accuracy and reproducibility.
Data analysis
All results are expressed as mean ± standard deviation (SD). Statistical significance was evaluated using one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test for multiple comparisons at a 95% confidence level (p <0.05). All statistical analyses were performed using Minitab 19 software (Minitab Inc., State College, PA, USA).
Effect of culture media composition on the biomass and xylanase production
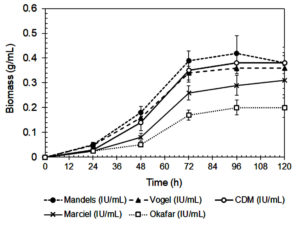
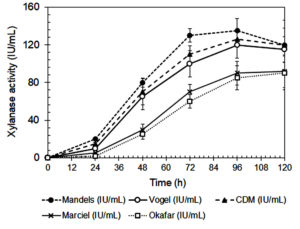
The composition of the culture medium had a significant impact on xylanase activity and fungal biomass production by A. niger, as shown in Figures 1 and 2. Among the tested media, Mandels’ medium produced the highest biomass production and xylanase activity at 72 and 96 hours, respectively. This suggests that its balanced nutrient composition effectively supports both fungal growth and enzyme biosynthesis.27 Mandels’ medium includes multiple nitrogen sources, including urea, peptone, yeast extract, and ammonium sulfate, that together provide a rich mixture of amino acids, peptides, and vitamins, all of which promote extracellular enzyme production.28,29
Modified Czapek-Dox (CMD) medium also supported relatively high xylanase activity. However, its dependence on sodium nitrate as the sole nitrogen source may have reduced nitrogen uptake efficiency.30 In contrast, Vogel’s, Marciel’s, and Okafor’s media yielded significantly lower enzyme activity. This can be attributed to their limited nitrogen diversity and the lower bioavailability of their nitrogen sources. Vogel’s medium primarily uses ammonium nitrate, while Marciel’s and Okafor’s formulations rely heavily on sodium nitrate.
Inorganic nitrogen sources like nitrate (NO3–) require enzymatic reduction to nitrite (NO0–) and then to ammonium (NH4+) before the fungus can assimilate them.30 This multi-step reduction, mediated by nitrate and nitrite reductases, consumes energy and may delay nitrogen assimilation during early fungal growth and enzyme synthesis.31 As a result, media that rely mainly on nitrate may not support optimal xylanase production.
In contrast, organic nitrogen sources, such as those found in Mandels’ medium are more easily metabolized and require less energy for assimilation. They supply readily available building blocks for protein and enzyme biosynthesis, which enhances both fungal growth and secondary metabolite production.32 The lowest biomass yield and xylanase activity were observed in Okafor’s medium, further underscoring the importance of nitrogen source composition and bioavailability in supporting efficient enzyme production.
These results are consistent with earlier studies showing that complex nutrient sources enhance xylanase secretion, especially when agro-industrial residues are used as carbon sources. For example, Izidoro and Knob33 reported that brewer’s spent grain significantly increased xylanase production by A. niger, particularly in nutrient-rich media. Similarly, Fasiku et al.34 found that A. niger GIO achieved the highest xylanase activity during solid-state fermentation (SSF) using alkaline-pretreated maize straw. In that study, optimal enzyme activity occurred at 40 °C and pH 5.0, demonstrating that agro-waste pretreatment can improve nutrient availability and enzyme yield.
The carbon source’s structure and concentration also play a critical role. Tan et al.35 showed that corn cob xylan at 3.5 g/L resulted in higher xylanase activity than empty fruit bunches during A. niger fermentation. This highlights the importance of substrate accessibility and composition in inducing enzyme production.
In addition to macronutrients, trace elements such as iron, zinc, and manganese are essential for xylanase biosynthesis.36-38 These act as cofactors that maintain enzyme stability and support catalytic function.39 Mandels’ and CMD media, which produced higher enzyme activity, included a more balanced profile of these trace elements. In contrast, Vogel’s medium contained fewer essential minerals, which may explain its lower enzyme yields.
Recent advances in strain improvement have also demonstrated the potential for enhancing xylanase production beyond medium optimization. Sharma et al.40 reported that A. niger mutants developed through UV irradiation and 5-bromouracil treatment exhibited up to a fourfold increase in xylanase activity compared to wild-type strains. These improved mutants were selected based on colony morphology, stability, amino acid requirements, and enzyme output. Combining genetically enhanced strains with nutrient-rich media, such as Mandels’, offers a synergistic approach to improving industrial xylanase yields.
Marciel’s medium, which lacks nitrogen source diversity, showed the lowest enzyme activity among the tested media. Although Okafor’s medium contains a relatively high concentration of sodium nitrate, it was less effective than Mandels’ or CMD in supporting xylanase production. This is likely due to the slower uptake of nitrate compared to ammonium and organic nitrogen sources like peptone and yeast extract, as nitrate must be enzymatically reduced before it can be assimilated.41
Mandels’ and CMD media also provided a better balance of trace elements, likely contributing to enhanced xylanase stability and catalytic efficiency. In contrast, Vogel’s medium, with fewer essential minerals, resulted in lower enzyme production. These findings support the use of A. niger as a preferred microorganism for enzyme production. Its GRAS status, rapid growth, and ability to metabolize lignocellulosic substrates make it highly suitable for industrial-scale fermentation.40 Enzymes from A. niger are widely applied in the paper and pulp industry, food processing, and biofuel production, emphasizing the importance of process optimization strategies such as those presented here.
The data summarized in Table 2 demonstrates the xylanase production capacity, expressed as enzyme activity per gram of biomass (IU/g), provides a more accurate assessment of the fungus’s efficiency in enzyme secretion independent of biomass quantity. Based on Table 2, the highest specific xylanase productivity was observed in Okafor’s medium (425.0 IU/g biomass at 96 h), despite its low overall xylanase activity and biomass yield. This indicates that under nitrogen-limited conditions, A. niger may redirect metabolic energy toward secondary metabolite synthesis rather than growth, a phenomenon often reported in filamentous fungi during nutrient stress.40 Mandels’ medium, which supported the highest overall xylanase activity and biomass production, showed a moderate xylanase production capacity. While the total enzyme output is greater, the enzyme yield per unit biomass is slightly lower than that in Okafor’s medium, likely due to the nutrient-rich conditions favoring both primary and secondary metabolism. CMD medium also exhibited relatively balanced performance with a xylanase activity of 331.6 IU/g biomass, suggesting efficient enzyme production under semi-defined nutrient conditions. Vogel’s and Marciel’s media had lower capacities, consistent with their limited nitrogen availability and reduced metabolic flexibility.
Table (2):
Summary of maximum biomass production and xylanase activity, and xylanase production capacity of A. niger cultivated in different media
| Media | Maximum biomass (g/mL), Time (h) | Maximum xylanase activity (IU/mL), Time (h) | Xylanase production capacity of A. niger (IU/g biomass) | |
|---|---|---|---|---|
| 72 h | 96 h | |||
| Mandels | 0.39 ± 0.04a, 72 h | 135.8 ± 7.1a, 96 h | 333.3a | 378.2b |
| Vogel | 0.34 ± 0.05b, 72 h | 120.1 ± 4.0c, 96 h | 294.1d | 333.3c |
| CDM | 0.35 ± 0.03b, 72 h | 126.0 ± 4.6b, 96 h | 314.3c | 331.6c |
| Marciel | 0.26 ± 0.03c, 72 h | 90.2 ± 12.6d, 96 h | 269.2d | 310.3d |
| Okafar | 0.17 ± 0.02d, 72 h | 85.4 ± 2.8e, 96 h | 352.9a | 425.0a |
Note: Data are presented as mean ± standard deviation. Different superscript letters in the same row indicate significant differences (p < 0.05).
These findings underscore the inverse relationship that can sometimes occur between biomass yield and enzyme productivity per biomass unit. Therefore, optimizing culture conditions should not focus solely on maximizing biomass or enzyme titer alone but rather on enhancing specific productivity, particularly in industrial settings where enzyme yield per input biomass is economically critical.
Effect of culture pH on the biomass and xylanase productions of A. niger
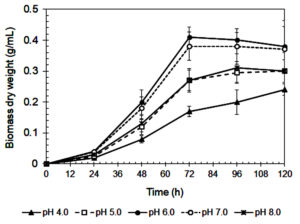
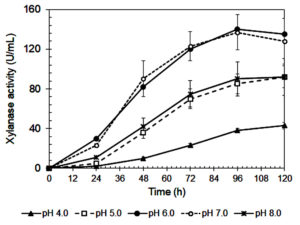
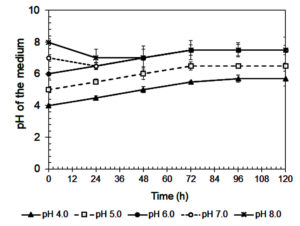
The influence of initial pH on xylanase production by A. niger is shown in Figure 3. The highest enzyme activity (141 IU/mL) occurred at pH 6.0, consistent with previous studies.42 Enzyme activity declined sharply at more acidic pH values (≤ 5.0), likely due to poor fungal growth (Figure 4) and reduced enzyme stability.43,44 A similar reduction at pH 8.0 suggests that alkaline conditions are also unfavorable for enzyme synthesis.45
Figure 3. Xylanase activity of A. niger during cultivating in the Mandels’ medium at various initial pHs
Figure 4. Biomass production of A. niger during cultivating in the Mandels’ medium at various initial pHs
Xylanase showed optimal stability and productivity in a slightly acidic to neutral pH range, which is ideal for food industry applications, such as baking and beverages, where controlled pH is important.46 The steep decrease in activity at pH 4.0-5.0 may be due to enzyme denaturation, conformational changes, and inhibited fungal metabolism.29,31 On the other hand, reduced enzyme production at pH ≥7.0 could result from suppressed fungal growth or disruption of metabolic pathways.46
Interestingly, although maximum activity was recorded at pH 6.0, xylanase production remained relatively stable between pH 6.0 and 7.0.47 This suggests that small pH fluctuations within this range may not significantly affect enzyme output, a beneficial trait for industrial processes where exact pH control is difficult.45
The tendency for fermentation pH to stabilize between 6.2 and 6.4 during cultivation, regardless of the starting pH (Figure 5), further supports process robustness. This buffering effect is likely caused by fungal metabolism and the release of organic acids or bases.46 In addition, changes in colony color (black under acidic and white under alkaline conditions) suggest that pH affects fungal physiology, possibly influencing sporulation or metabolic behavior.43
As summarized in Table 3, pH also influenced the efficiency of enzyme production in relation to fungal biomass. Although pH 6.0 led to the highest xylanase titer and biomass yield, pH 7.0 produced the highest xylanase productivity per gram of biomass (325.3 IU/g), suggesting more efficient enzyme synthesis at this pH. In contrast, extreme acidic (pH 4.0) and alkaline (pH 8.0) conditions resulted in much lower specific productivity (195.9 and 277.0 IU/g, respectively). These findings confirm that maintaining culture pH between 6.5 and 7.0 is ideal for maximizing enzyme production efficiency in submerged fermentation systems.
Table (3):
Summary of maximum biomass production and xylanase activity, and xylanase production capacity of A. niger cultivated in Mandels’ medium at different pH values
pH |
Maximum biomass (g/mL) |
Maximum activity (IU/mL) |
Fermentation time (h) |
Xylanase production capacity of A. niger (IU/g biomass) |
|---|---|---|---|---|
4.0 |
0.22 ± 0.02c |
43.1 ± 3.4c |
120 |
195.9c |
5.0 |
0.27 ± 0.04b |
70.3 ± 9.8b |
72 |
292.9a |
6.0 |
0.41 ± 0.03a |
120.0 ± 9.6a |
72 |
292.7a |
7.0 |
0.38 ± 0.05a |
123.6 ± 14.7a |
72 |
325.3a |
8.0 |
0.27 ± 0.03b |
74.8 ± 13.5b |
72 |
277.0b |
Note: Data are presented as mean ± standard deviation. Different superscript letters in the same row indicate significant differences (p <0.05)
This study underscores the critical influence of culture medium composition and initial pH on xylanase production by Aspergillus niger. Among the evaluated media, Mandels’ formulation proved most effective, primarily due to its balanced combination of organic and inorganic nitrogen sources and essential trace elements. Optimal enzyme yields were achieved within a pH range of 6.0-7.0, conditions that also supported robust fungal growth and enhanced enzyme productivity. These findings provide a solid framework for scaling up fermentation processes, with potential to increase process efficiency, lower production costs, and ensure consistent xylanase output.
Given the broad industrial relevance of xylanase, the implications of this work are far-reaching. In the pulp and paper industry, A. niger derived xylanase enables eco-friendly bio-bleaching and improves fiber quality. In food and feed sectors, it enhances product texture, nutrient release, and digestibility. Furthermore, in lignocellulosic biorefineries, xylanase is vital for the hydrolysis of hemicellulose into fermentable sugars, facilitating the sustainable production of biofuels and bio-based chemicals.
The demonstrated compatibility of A. niger with low-cost agro-industrial substrates, its resilience under mildly acidic to neutral pH, and its GRAS (Generally Recognized as Safe) status reinforce its value as a workhorse for industrial enzyme production. Overall, this study contributes to the optimization of key bioprocess parameters and supports the development of scalable, sustainable, and economically viable strategies for industrial xylanase manufacturing.
ACKNOWLEDGMENTS
The author gratefully recognizes the Department of Food Engineering, School of Engineering, King Mongkut’s Institute of Technology Ladkrabang for providing equipment.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
TC conceptualized the study and applied methodology. PP and TC performed formal analysis. TC performed data curation, visualization, validation and supervision. TC wrote the manuscript. TC and PP reviewed and edited the the manuscript. Both authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Mujtaba M, Fraceto LF, Fazeli M, et al. Lignocellulosic biomass from agricultural waste to the circular economy: A review with focus on biofuels, biocomposites and bioplastics. J Clean Prod. 2023;402:136815.
Crossref - Awasthi S, Mishra A, Pal DB. Production of hemicellulose from biomass and agricultural/food waste contains lignocellulosic materials. In: Pal DB, Bansal SL, (eds). Fungal waste biomass management for energy, environment and value-added products. Cham: Springer. 2025:27-42.
Crossref - Knob A, Terrasan CRF, Carmona EC. β-Xylosidases from filamentous fungi: An overview. World J Microbiol Biotechnol. 2009;26(3):389-407.
Crossref - Sharma M, Kumar A. Xylanases: An overview. Br Biotechnol J. 2013;3(1):1-28.
Crossref - Bajpai P. Industrial applications of xylanases. In: Elsevier eBooks. Amsterdam: Elsevier; 2022:149-211.
Crossref - Kumar V, Shukla P. Functional aspects of xylanases toward industrial applications. In: Shukla P, (eds). Industrial enzymes: Structure, function and applications. New Delhi: Springer India. 2016:157-165.
Crossref - Liu L, Feizi A, Osterlund T, Hjort C, Nielsen J. Genome-scale analysis of the high-efficient protein secretion system of Aspergillus oryzae. BMC Syst Biol. 2014;8:73.
Crossref - Cairns TC, Barthel L, Meyer V. Something old, something new: Challenges and developments in Aspergillus niger biotechnology. Essays Biochem. 2021;65(2):213-225.
Crossref - Pel HJ, de Winde JH, Archer DB, et al. Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat Biotechnol. 2007;25(2):221-231.
Crossref - Kavya V, Padmavathi T. Optimization of growth conditions for xylanase production by Aspergillus niger in solid state fermentation. Pol J Microbiol. 2009;58(2):125-130.
- de Sousa Paredes R, da Rocha Olivieri de Barros R, Inoue H, Yano S, Bon EPS. Production of xylanase, α-L-arabinofuranosidase, β-xylosidase, and β-glucosidase by Aspergillus awamori using the liquid stream from hot-compressed water treatment of sugarcane bagasse. Biomass Convers Biorefin. 2015;5(3):299-307.
Crossref - Javed U, Aman A, Qader SAU. Utilization of corncob xylan as a sole carbon source for the biosynthesis of endo-1,4-β-xylanase from Aspergillus niger KIBGE-IB36. Bioresour Bioprocess. 2017;4:19.
Crossref - Ramanjaneyulu G, Sridevi A, Seshapani P, et al. Enhanced production of xylanase by Fusarium sp. BVKT R2 and evaluation of its biomass saccharification efficiency. 3 Biotech. 2017;7(5):351.
Crossref - Fawole OB, Odunfa SA. Some factors affecting production of pectic enzymes by Aspergillus niger. Int Biodeterior Biodegradation. 2003;52(4):223-227.
Crossref - Ali S, Noor P, Ahmed MU. Kinetics of cellulase-free endo xylanase hyper-synthesis by Aspergillus niger using wheat bran as a potential solid substrate. BMC Biotechnol. 2024;24:69.
Crossref - Desai DI, Iyer BD. Optimization of medium composition for cellulase-free xylanase production by solid-state fermentation on corn cob waste by Aspergillus niger DX-23. Biomass Convers Biorefin. 2022;12:1153-1165.
Crossref - Mamo J, Kangwa M, Fernandez-Lahore HM, Assefa F. Optimization of media composition and growth conditions for production of milk-clotting protease (MCP) from Aspergillus oryzae DRDFS13 under solid-state fermentation. Braz J Microbiol. 2020;51(2):571-581.
Crossref - Poutanen K, Puls J. Characteristics of Trichoderma reesei beta-xylosidase and its use in the hydrolysis of solubilized xylans. Appl Microbiol Biotechnol. 1988;28:425-432.
Crossref - Haltrich D, Nidetzky B, Kulbe KD, Steiner W, Zupko I. Production of fungal xylanases. Bioresour Technol. 1996;58(2):137-161.
Crossref - Azzouz Z, Bettache A, Boucherba N, Prieto A, Martinez MJ, Benallaoua S, de Eugenio LI. Optimization of β-1,4-endoxylanase production by an Aspergillus niger strain growing on wheat straw and application in xylooligosaccharides production. Molecules. 2021;26(9):2527.
Crossref - Fasiku SA, Bello MA, Odeniyi OA. Production of xylanase by Aspergillus niger GIO and Bacillus megaterium through solid-state fermentation. Access Microbiol. 2023;5(6):acmi000506.v5.
Crossref - Maciel GM, de Souza Vandenberghe LP, Haminiuk CWI, et al. Xylanase production by Aspergillus niger LPB 326 in solid-state fermentation using statistical experimental designs. Food Technol Biotechnol. 2008;46(2):183-189.
- Okafor UA, Okochi VI, Onyegeme-Okerenta BM, Nwodo-Chinedu S. Xylanase production by Aspergillus niger ANL 301 using agro-wastes. Afr J Biotechnol. 2007;6(14):1710-1714.
- Pal A, Khanum F. Characterizing and improving the thermostability of purified xylanase from Aspergillus niger DFR-5 grown on solid-state medium. J Biochem Technol. 2010;2(4):203-209.
Crossref - Bailey MJ, Biely P, Poutanen K. Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol. 1992;23(3):257-270.
Crossref - Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31(3):426-428.
Crossref - Fouda A, Alshallash KS, Atta HM, et al. Synthesis, optimization, and characterization of cellulase enzyme obtained from thermotolerant Bacillus subtilis F3: An insight into cotton fabric polishing activity. J Microbiol Biotechnol. 2024;34(1):207-219.
Crossref - Nakamura A, Takahashi H, Sulaiman S, et al. Evaluation of peptones from chicken waste as a nitrogen source for micro-organisms. Lett Appl Microbiol. 2021;72(4):408-414.
Crossref - Barbieri GS, Bento HBS, de Oliveira F, et al. Xylanase production by Talaromyces amestolkiae valuing agroindustrial byproducts. Biotech. 2022;11(2):15.
Crossref - Miki S, Sakai K, Nakagawa T, et al. Analysis of nitrogen source assimilation in industrial strains of Aspergillus oryzae. J Biosci Bioeng. 2024;137(4):231-238.
Crossref - Deb S, Lewicka-Szczebak D, Rohe L. Microbial nitrogen transformations tracked by natural abundance isotope studies and microbiological methods: A review. Sci Total Environ. 2024;926:172073.
Crossref - Lima VH, Matugawa AT, Mascarin GM, Fernandes EKK. Complex nitrogen sources from agro-industrial byproducts: Impact on production, multi-stress tolerance, virulence, and quality of Beauveria bassiana blastospores. Microbiol Spectr. 2024;12(6):e04040-23.
Crossref - Izidoro SC, Knob A. Production of xylanases by an Aspergillus niger strain in waste grains. Acta Sci Biol Sci. 2014;36(3):313-319. doi: 10.4025/actascibiolsci.v36i3.20567
- Fasiku SA, Bello MA, Odeniyi OA. Production of xylanase by Aspergillus niger GIO and Bacillus megaterium through solid-state fermentation. Access Microbiol. 2023;5(6):000506.v5.
Crossref - Tan CZ, Chang WT, Tarrsini M, et al. Xylanase production via Aspergillus niger: Effect of carbon source and composition. J Phys Conf Ser. 2021;2080(1):012001.
Crossref - Rahman MA, Choi YH, Pradeep GC, et al. An alkaline and metallo-protein type endo xylanase from Streptomyces sp. CSWu-1. Biotechnol Bioprocess Eng. 2014;19(2):311-319.
Crossref - Ruzicka FJ, Wedekind JE, Kim J, Rayment I, Frey PA. Galactose-1-phosphate uridylyltransferase from Escherichia coli, a zinc and iron metalloenzyme. Biochemistry. 1995;34(16):5610-5617.
Crossref - Do TT, Quyen DT, Dam TH. Purification and characterization of an acid-stable and organic solvent-tolerant xylanase from Aspergillus awamori VTCC-F312. ScienceAsia. 2012;38(2):157-162.
Crossref - Bibi Z, Nawaz MA, Irum-us-Salam, Waqas M, Aman A, Ul Qader SA. Significance of metal ions, solvents and surfactants to improve the xylan degrading behavior of β-1,4-D-xylanohydrolase from Geobacillus stearothermophilus KIBGE-IB29. Biocatal Agric Biotechnol. 2019;17:242-246.
Crossref - Sharma A, Jaitly AK, Rai PK. Increased xylanase activity in Aspergillus niger through mutation. Curr Sci. 2021;121(7):966-968.
Crossref - Krappmann S, Braus GH. Nitrogen metabolism of Aspergillus and its role in pathogenicity. Med Mycol. 2005;43(Suppl 1):31-39.
Crossref - Dahiya S, Kumar A, Malik V, Kumar V, Singh B. Biochemical characterization and enhanced production of endoxylanase from thermophilic mould Myceliophthora thermophila. Bioprocess Biosyst Eng. 2021;44(7):1539-1555.
Crossref - Kapoor V, Nandan D. Optimization of physico-chemical parameters for the production of endoxylanase using combined response surface method and genetic algorithm. In: Laha V, Marechal P, Mishra SK, (eds). Optimization, variational analysis and applications. Cham: Springer. 2021:307-323.
Crossref - Wang Q, Xia T. Enhancement of the activity and alkaline pH stability of Thermobifida fusca xylanase A by directed evolution. Biotechnol Lett. 2008;30(5):937-944.
Crossref - Maheshwari P, Sankar PM, Ramyabharathi SA. Production, purification, and characterization of endoxylanase. In: Biotic Elicitors: Production, Purification, and Characterization. Amin D, Amaresan N, Ray S, eds. Springer Protocols Handbooks. Humana; 2022:25-34.
Crossref - Nagar S, Jain RK, Thakur VV, Gupta VK. Biobleaching application of cellulase-poor and alkali-stable xylanase from Bacillus pumilus SV-85S. 3 Biotech. 2013;3(4):277-285.
Crossref - Beliën T, Van Campenhout S, Van Acker M, Robben J, Courtin CM, Delcour JA, Volckaert G. Mutational analysis of endoxylanases XylA and XylB from the phytopathogen Fusarium graminearum reveals comprehensive insights into their inhibitor insensitivity. Appl Environ Microbiol. 2007;73(14):4602-4608.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.