ISSN: 0973-7510
E-ISSN: 2581-690X
Fluorescent Pseudomonas species have a number of traits like antifungal, siderophores, phosphate solubilization, lytic enzymes, HCN, ammonia and plant growth regulators that allow them to act as plant growth promoting and biocontrol agents. They competitively colonize plant roots, play important role in enhancing the growth of plants and in plant disease control. These may be used for soil improvement and to increase soil fertility hence for management of replant problem of apple in Himachal Pradesh. To ensure the sustained availability of PGP and biocontrol agent’s in soil formulation development protocol has to be standardized. On the basis of different PGPR activities three indigenous strains viz., Pseudomonas aeruginosa C, Pseudomonas fluorescens M and Pseudomonas putida L. were selected for field studies. In all the treatments with individual (L, M and C) and consortia strains (L+M, L+C, M+C, L+M+C) there was an 8.0 to 86.5 per cent increase in plant height as compared to control. The performance of replanted apple was much better in terms of root colonization capacity, plant establishment and increase in plant growth in terms of plant height, number of nodes and branches over their respective control after fifteen months of plantation.
Pseudomonas sp., bioformulation, apple, replant site.
Apple (Malus domestica Borkh.) is one of the most important fruit crop grown and consumed all around the world and has been distributed almost across the whole temperate region in the Northern and Southern hemispheres.1
Replant problem refers to the poor growth of replanted young trees on the old sites. It is distributed worldwide and is often encountered in establishing new orchards.2 The disease is a complex syndrome that reduces growth, survival and yield of replanted tree. Replant problem is caused by biotic and abiotic factors.3 The biotic factors includes the rhizosphere microflora (bacteria, fungi, actinomycetes, nematodes and their interactions) and abiotic factors includes phytotoxins, nutrient imbalance, low or high pH, soil structure and damage, and lack of excess of moisture.4
Fluorescent Pseudomonas species are the most diverse and versatile group of plant growth promoting rhizobacteria. Their potential to synthesize different secondary metabolites with diverse biological activities is the important function of soil fertility and sustainability of crops.5 The integration of their important traits like production of antifungal antibiotics, iron chelating siderophores, lytic enzymes, plant growth regulators, phosphate solubilization, ammonia and HCN production with ecological fitness of the strains will be prerequisite for designing useful, efficient and effective novel bioagent. The large scale application of indigenous plant growth promoting fluorescent Pseudomonas sp. may be able to manage replant problem of fruit crops especially apple.
The main objective of this research was to exploit the indigenous potential fluorescent Pseudomonas strains viz., P. aeruginosa, P. fluorescens and P. putida in individual and consortial combination to act as biofertilizer which can become one of the most promising biotechnologies to solve apple replant disease and can also improve production with low inputs in fertilizer. The aim of the study was to select more effective combination of fluorescent Pseudomonas sp. which can be used for biofertilizer development for management of apple replant problem.
Isolation of fluorescent Pseudomonas sp. from apple rhizosphere
The fluorescent Pseudomonas sp. was isolated from the rhizosphere of apple plants in normal and replant sites of Shimla district (Himachal Pradesh). The rhizosphere soil loosely adhering to apple roots were gently teased out with small root pieces in polythene bags and immediately transported to laboratory under cold conditions (4ºC) for further process. The serial dilution agar plate method was used to isolate Pseudomonas sp. on King’s B medium.6 The composition of the medium was (g/l-1): Peptone, 20.0; K2HPO4, 1.5; MgSO4.7H2O, 1.5; Glycerol, 15.0 ml. All the twelve isolates from apple rhizosphere were morphologically, physiologically and biochemically characterized so as to select the isolates belonging to P. aeruginosa, P. putida and P. fluorescens group.
Molecular characterization and in-vitro screening of fluorescent Pseudomonas sp. for plant growth promoting activities
All the twelve fluorescent Pseudomonas sp. were characterized for plant growth promoting activities viz., phosphate solubilization,7 siderophore production,8 HCN production,9 ammonia production10 and plant growth regulator production11,12,13 according to their respective methods. Three potential fluorescent Pseudomonas isolates L, M and C were selected and molecularly characterized by 16S rRNA technique using Pseudomonas specific primers viz., FP-1 (GGTCTGAGAGGATGATCAGT) and RP-1 (TTAGCTCCACCTCGCGGC) in MJ Mini BIORAD personal thermal cycler-100 (PTC-100).14 The PCR amplification was as follows: denaturation at 94°C for 1 min, annealing at 55°C for 2 min, and elongation at 72°C for 2 min with a total of 35 cycles. For DNA sequencing, eluted amplified DNA products of fluorescent Pseudomonas isolates L, M and C was purified followed by sequencing in Bioserve Private limited (Hyderabad, India). After sequencing the obtained sequence were analyzed by Basic local alignment search tool (BLAST) for their identification with the NCBI data base.15 On submission of partial sequence of Pseudomonas putida L, Pseudomonas fluorescens M and Pseudomonas aeruginosa C to GenBank database (NCBI) the accession no. assigned was KF751235, KF751236 and KJ871666
Replant sites for field experiment
The field experiment was conducted with apple rootstocks (MM793 and MM111) in ARD (apple replant disease) infected sites of Shimla distt. (Himachal Pradesh). The two sites at Maggota and Sharontha were selected for conducting field experiment on the bases of age of orchard and symptoms of ARD. A total of eighty apple rootstocks (MM793 and MM111) were purchased from KVK (Rohru) by farmers.
Field application of Pseudomonas putida L, Pseudomonas fluorescens M and Pseudomonas aeruginosa C in replant sites of Maggota and Sharontha (Shimla distt.)
The field experiment was conducted to investigate the effect of individual and consortial fluorescent Pseudomonas strains on the growth and establishment of apple rootstock planted in replant sites of Maggota and Sharontha. The fluorescent Pseudomonas sp. viz., Pseudomonas putida L, Pseudomonas fluorescens M and Pseudomonas aeruginosa C were grown in nutrient broth at 28±2 °C for 48-72 hr (adjusted to inoculum density 1 x 108cfu/ml) and used for the treatment of plant roots and soil of pits individually and in different combinations viz., L, M, C, L+M, L+C, M+C, L+M+C and control. The consortial combination viz., L+M, L+C, M+C, L+M+C was made in 1:1 and 1:1:1 ratio. The control plants were treated with 1/10 diluted nutrient broth.
Five rootstocks of each treatment were planted in replant site of Maggota and Sharontha after treating roots according to different treatment combination. The plants were treated with fluorescent Pseudomonas suspension by dipping their roots in liquid inoculum for 15-30 minutes before planting them in replant site pits pretreated with Pseudomonas suspension.16 The cyclic treatment for successive twelve months were given at monthly interval in rhizosphere of plants by adding 100-150 ml of inoculum (1×108 cfu/ml) to the basin of plants. The results were compared with uninoculated control. Effect on plant and soil parameters like plant height, number of nodes/leaves, branches and NPK content of soil were studied. The survival percentage of plants, total microbial and Pseudomonas count was also determined from the rhizosphere soil after every month of the cyclic treatment. The experiment was carried out in a randomized block design (RBD). The experimental data was analyzed statistically using ANOVA.
Twelve plant growth promoting rhizobacteria were isolated from apple rhizosphere and characterized as belonging to fluorescent Pseudomonas sp. on the bases of biochemical and physiological characteristics. Reynolds17 (2004) also characterized isolates on the bases of biochemical tests including oxidase, catalase, gelatin hydrolysis, nitrate reduction, growth at 4 and 41°C and identified them as P. fluorescens.
The screening strategy was carried out to find out an effective PGP fluorescent Pseudomonas strain that act through the combination of several different mechanisms. The screening resulted in a group of bacteria able to produce phosphate solubilizing activity, siderophore production, HCN production, ammonia production and growth hormone production thus allowing us to select Pseudomonas strains showing multifarious plant growth promoting activities. All the fluorescent Pseudomonas sp. showed significant production of phosphate solubilizing activity, siderophores, HCN, ammonia, and growth hormones (Table 1). Overall result showed that fluorescent Pseudomonas strains L, M and C produced maximum number of plant growth promoting activities in-vitro and also inhibited major fungal pathogens of apple.
Table (1):
Characterization of fluorescent Pseudomonas sp. for plant growth promoting activities.
| fluorescent Pseudomonas isolates | Plant growth promoting traits | ||||||
|---|---|---|---|---|---|---|---|
| Phosphate solubilization* (Pi), (µg/ml) | Siderophore production** % SU | HCN production | Ammonia production | Plant growth regulators (µg/ml) | |||
| Auxins | Gibberellins | Cytokinins | |||||
| An-1-Jub | 17 | 10.5 | ++++ | +++ | 17 | 150 | 18 |
| An-3-Jub | 36 | 10.5 | ++ | + | 19 | 70 | 30 |
| An-4-Jub | 26 | 10.5 | ++++ | +++ | 19 | 90 | 22 |
| An-5-Jub | 47 | 2.1 | ++++ | +++ | 14 | 120 | 30 |
| Ar-1-Jub | 31 | 10.5 | – | ++ | 13 | 100 | 25 |
| An-1-Sh | 20 | 5.2 | – | ++ | 21 | 180 | 28 |
| An-2-Sh | 54 | 5.2 | – | + | 07 | 60 | 35 |
| Ar-1-Sh | 50 | 10.5 | – | +++ | 22 | 80 | 28 |
| Ar-2-Sh | 25 | 15.7 | + | ++ | 21 | 110 | 28 |
| L | 39 | 23.0 | + | +++ | 16 | 135 | 20 |
| M | 41 | 22.4 | + | +++ | 11 | 140 | 24 |
| C | 30 | 50.8 | + | +++ | 36 | 160 | 16 |
*Phosphate solubilizing activity expressed in terms of tricalcium phosphate solubilization which in turn represents µg/ml of soluble inorganic phosphate(Pi) in supernatant as calibrated from the standard curve of KH2PO4 (10-100 µg/ml).
**The siderophore unit (% SU) expressed as percent reduction in blue color of chrome azurol-S as compared to reference i.e. % SU= (Ar-As)/Ar×100
where, Ar = Absorbance of reference solution at 630 nm; As = Absorbance of test solution at 630 nm
Plant growth promoting effect of PGPR strains in different crops were clearly demonstrated.18 Bacterial inoculants are able to increase plant growth, germination rate, improve seedling emergence, responses to external stress factors and protect plants from diseases.19 This present investigation confirms the earlier work. In this study, inoculation of fluorescent Pseudomonas sp. increased all growth and soil parameters as compared to control plants. The performance of replanted apple rootstocks after fifteen months of cyclic treatment with individual and consortium strains of P. putida, P. fluorescens and P. aeruginosa along with control at two different replant sites is detailed in Table 3. The details of replant site, age of orchard and rootstocks used for field experiment were presented in Table 2. The data was presented as per cent increase over control for plant height, number of nodes and available NPK content of soil.
Table (2):
Detail of replant sites and rootstocks used in field experiment.
Sr. no |
Replant site |
Age of orchard |
Rootstock replanted |
Total rootstock replanted |
|---|---|---|---|---|
1 |
Maggota (Shimla distt.) |
>25 years |
MM793 |
40 |
2 |
Sharontha (Shimla distt.) |
>35 years |
MM111 |
40 |
Table (3):
Effect of cyclic application of individual and consortium strains of P. putida, P. fluorescens and P. aeruginosa on growth of replanted rootstocks after 15 months at Maggota and Sharontha (Shimla distt.).
| Replant site | Treatments (1×108cfu/ml) | Plant height cm, (%I) | Number of nodes (%I) | Number of branches |
|---|---|---|---|---|
| Maggota | L | 40.5 | 43.7 | 6.2 |
| M | 8.0 | 58.0 | 6.4 | |
| C | 86.1 | 109.5 | 6.6 | |
| L+M | 40.9 | 68.0 | 6.6 | |
| L+C | 86.5 | 144.5 | 6.4 | |
| M+C | 42.9 | 106.5 | 6.4 | |
| L+M+C | 33.2 | 104.3 | 6.7 | |
| Sharontha | L | 48.5 | 55.6 | 6 |
| M | 51.1 | 51.5 | 6 | |
| C | 65.7 | 69.3 | 6.6 | |
| L+M | 27.4 | 68.8 | 6.6 | |
| L+C | 28.4 | 56.2 | 7.2 | |
| M+C | 31.6 | 60.1 | 7.4 | |
| L+M+C | 38.1 | 17.4 | 7.6 |
% I= percent increase over control
The growth performance was studied on the basis of plant height, number of nodes/leaves, chlorophyll content and number of branches. On an average in all the treatments with liquid bioformulation of individual (L, M and C) and consortial strains (L+M, L+C, M+C, L+M+C) of Pseudomonas sp. there was an increase in plant height as compared to control after 15 months of replantation of apple rootstocks in the range of 8.0 to 86.5 % increase at Maggota and 27.4 to 65.7 % increase at Sharontha as compared to control plants respectively. At Maggota maximum per cent increase in terms of plant height and number of nodes was recorded in formulation with consortia of two strains L+C (86.5 % and 144.5 %) followed by individual strain C (86.1 % and 109.5 %) whereas at Sharontha maximum per cent increase in plant height and number of nodes was observed in formulation C (65.7 % and 69.3 %) Table 3. The maximum number of branches was observed in consortium of three strains viz., L+M+C at both the replant sites. The present experiment revealed that rhizosphere inoculation with Pseudomonas sp. resulted in an increased plant height, number of nodes, branches and available NPK content of rhizospheric soil. Similar results have shown previously by Verma et al., (2014)20 who reported that cyclic treatment of fluorescent Pseudomonas sp. resulted in significant increase in various plant and soil parameters. Similar increase in plant height was observed in different crops inoculated with Pseudomonas, Azospirillum and Azotobacter strains by other workers.21-24
The chlorophyll content of leaves of replanted apple rootstocks was also estimated. Results in Table 4 showed that there was not a significant increase in chlorophyll content (a, b and total) in all the treatments as compared to control. The available N, P and K of rhizosphere soil were also assessed before and after the cyclic application of fluorescent Pseudomonas sp. (Table 5).The results showed that after application of Pseudomonas sp. in the field, there was a considerable increase in available NPK content of rhizosphere soil as compared to control plants. Our results were supported by the findings of Verma et al., (2014)20
Table (4):
Effect of cyclic application of individual and consortium strains of P. putida, P. fluorescens and P. aeruginosa on chlorophyll content of leaves of replanted rootstocks after 15 months at Maggota and Sharontha (Shimla distt.).
| Replant site | Treatments (1×108cfu/ml) | Chlorophyll ‘a’ | Chlorophyll ‘b’ | Total Chlorophyll |
|---|---|---|---|---|
| Maggota | Control | 0.16 | 0.23 | 0.3 |
| L | 0.16 | 0.25 | 0.4 | |
| M | 0.18 | 0.24 | 0.4 | |
| C | 0.17 | 0.24 | 0.4 | |
| L+M | 0.17 | 0.24 | 0.4 | |
| L+C | 0.17 | 0.23 | 0.4 | |
| M+C | 0.17 | 0.24 | 0.4 | |
| L+M+C | 0.17 | 0.23 | 0.4 | |
| Sharontha | control | 0.15 | 0.21 | 0.3 |
| L | 0.17 | 0.25 | 0.4 | |
| M | 0.18 | 0.23 | 0.4 | |
| C | 0.17 | 0.25 | 0.4 | |
| L+M | 0.16 | 0.22 | 0.3 | |
| L+C | 0.17 | 0.23 | 0.4 | |
| M+C | 0.16 | 0.22 | 0.3 | |
| L+M+C | 0.16 | 0.23 | 0.3 |
Table (5):
Effect of cyclic application of liquid bioformulation of L, M, C and their consortial formulations on available N, P, K content of rhizospheric soil of Maggota and Sharontha.
| Treatments (1×108cfu/ml) | Available macronutrients NPK( kg/ha) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % increase over control (%I) after 15 month | ||||||||||||
| Maggota | Sharontha | |||||||||||
| N | % I | P | % I | K | % I | N | % I | P | % I | K | % I | |
| L | 313 | 11.7 | 54 | 35.0 | 146 | 10.6 | 290 | 2.1 | 35 | 16.6 | 145 | 13.2 |
| M | 295 | 5.3 | 48 | 20.0 | 140 | 6.0 | 296 | 4.2 | 46 | 53.3 | 142 | 10.9 |
| C | 325 | 16.0 | 58 | 45.0 | 157 | 18.9 | 324 | 14.0 | 38 | 26.6 | 138 | 7.8 |
| L+M | 316 | 12.8 | 67 | 67.5 | 164 | 24.2 | 298 | 3.5 | 34 | 13.3 | 137 | 7.0 |
| L+C | 290 | 3.5 | 69 | 72.5 | 148 | 12.1 | 328 | 15.4 | 42 | 40.0 | 146 | 14.0 |
| M+C | 336 | 20.0 | 58 | 45.0 | 154 | 16.6 | 312 | 9.8 | 54 | 80.0 | 154 | 20.3 |
| L+M+C | 340 | 21.4 | 66 | 65.0 | 142 | 7.5 | 295 | 3.8 | 47 | 56.6 | 148 | 15.6 |
| Control | 280 | 40 | 132 | 284 | 30 | 128 | ||||||
After cyclic application of liquid formulations of both individual and consortial strains (L, M, C, L+M, L+C, M+C, L+M+C) in field the decrease in total bacterial and fungal population was observed with a gradual increase in fluorescent Pseudomonas population (Figure 1). After fifteen months of plantation, there was an increase of 20 to 46 cfu/g of soil in total fluorescent Pseudomonas sp. count in all the bioformulation treated rootstocks at both the sites. The results indicated towards the possibility of sufficient increase in number and establishment of Pseudomonas species in rhizosphere of apple plants planted in replant sites of apple orchards. They also might have decreased the deleterious microflora up to some extent.
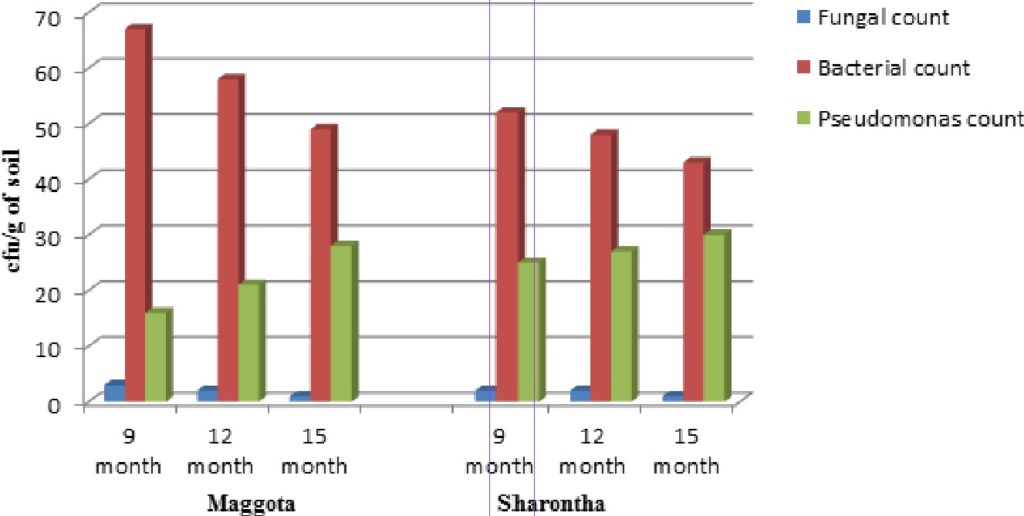 Fig 1. Effect of cyclic application of fluorescent Pseudomonas sp. on rhizobacterial and fungal population in apple rhizosphere
Fig 1. Effect of cyclic application of fluorescent Pseudomonas sp. on rhizobacterial and fungal population in apple rhizosphere Burd et al., (2000)25 reported that plant growth promoting rhizobacteria might enhance plant height and productivity by synthesizing phytohormones, increasing the local availability of nutrients, facilitating the uptake of nutrients by the plants decreasing the heavy metal toxicity in the plants antagonizing plant pathogens. The increased plant height and number of nodes/leaves as compared to control plants clearly showed the beneficial role of Pseudomonas as a rhizobacteria. Such an improvement might be attributed to phosphate solubilising capacity of bacteria as well as the ability of these microorganisms to produce growth promoting substances.26
The results of this study suggest that plant growth promoting fluorescent Pseudomonas sp. isolated from apple rhizosphere has potential to be used successfully for replant problem of apple. There was a considerable increase in various plant and soil parameters after fifteen months of cyclic treatment of fluorescent Pseudomonas formulation. So it can be concluded from the present study that the individual and consortium of fluorescent Pseudomonas strains can be further exploited for bio fertilizer development to overcome the replant problem in apple orchards.
- Way, R.D., Aldwinckle, R.C, Rejman, A., Sansavini, S., Shen, T., Watkins, R., Westwood, M.N. and Yoshida, Y. Apples. In: Genetic resources temperate fruit and nut crops (Moore, J.N., Ballington, J.R. eds). Journal of Fruit and Ornamental Plant Research, 1990; 2: 5-6.
- Mai, W.F., Abawi, G.S. Controlling replant disease of pome and stone fruits in northeastern United States by replant fumigation. Plant Diseases, 1981; 11: 859-864.
- Utkhede, R.S. Smith, E.M. Biotic and abiotic causes of replant problems of fruit trees. Acta Horticulturae, 1994; 363: 25-31.
- Castka, V., Vancura, V., Hudska, G. Prikryl, Z. Rhizosphere microorganisms in relation to the apple replant problem. Plant and Soil, 1982; 69: 187-197.
- Malik, V.S. Genetics and biochemistry of secondary metabolites Advanced Applied Microbiology, 1982; 28: 28-101.
- King, E.O., Ward, M.K. and Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescein. Journal of Laboratory and Clinical Medicine, 1954; 44: 301-307.
- Pikovsakaya, R.E. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Microbiologia, 1948; 17: 362-370.
- Schwyan, B. and Neilands, J.B. Universal chemical assay for the detection and determination of siderophore. Analytic Biochemistry, 1987; 28(8): 751-759.
- Bakker, A.W. and Schippers, B. Microbial cyanide production in the rhizosphere in relation to total yield reduction and Pseudomonas sp. mediated plant growth stimulation. Soil Biology and Biochemistry, 1987; 19: 451-457.
- Lata and Saxena, A.K. Characterization of plant growth promoting rhizobacteria. In: Training manual on biofertilizer technology (Saxena, A.K. ed). Delhi: IARI, 2003; pp. 24-25
- Gorden and Paleg, L.G. Quantitative measurement of IAA. Plant Physiology, 1957; 10: 37-38.
- Holbrook, A.A, Edge, W.J.W and Bailey, F. Spectrophotometric method for determination of gibberellic acid in gibberellin. ACS, 1961: Washington. pp. 159-167
- Letham. Regulator of cell division in plant tissue. XII. A cy6tokinin bioassay using excised radish cotyledons. Physiologia Plantarum, 1971; 25: 391-396
- Widmer, F., Seidler, R. J., Gillevet, P.M., Watrud, L.S. and DiGiovanni, G.D. Highly selective PCR protocol for detecting 16S rRNA genes of the genus Pseudomonas (sensu stricto) in environmental samples. Applied and Environmental Microbiology, 1998; 64(7): 2545-2553.
- Altschul, S.F., Gish, W., Miller, W., Myers, E.W. and Lipman, D.J. Basic local alignment search tool. Journal of Molecular Biology, 1990; 215: 403-441.
- Verma, P.P, Sharma, S. and Kaur, M. Effect of indigenous strains of fluorescent Pseudomonas sp. on growth of apple plants in replant site of Himachal Pradesh. Indian Journal of Applied Research, 2014; 4(7):63-68.
- Reynolds, J. Lab procedures manual: Biochemical tests. Richland College. http://www.rlc. dcccd.edu/mathsci/Reynolds/micro/lab_manual/TOC.html. 2004
- Wu, S.C., Cao, Z.H., Li, Z.G., Cheung, K.C. and Wong, M.H., 2005. Effects of biofertilizer containing N-fixer, P and K solubilizers and AM fungi on maize growth: a greenhouse trial. Geoderma, 2005; 125: 155– 166.
- Lugtenberg, B., Chin-a-Woeng and Bloemberg, G. Microbe-plant interactions: principles and mechanisms. Antonie van Leeuwenhoek, 2002; 81: 373-383.
- Verma, P.P., Sharma, S. and Kaur, M. Effect of indigenous strains of fluorescent Pseudomonas sp. on growth of apple plants in replant site of Himachal Pradesh. Indian Journal of Applied Research, 2014; 4(7): 432-437.
- Martinez-Toledo, M.V., Gonzalez-Lopez, J., de La Rubia, T., Moreno, J., Ramos-Cormenzana, A. Effect of inoculation with Azotobacter chroococcum on nitrogenase activity of zea mays roots grown in agricultural soils under aseptic and non-sterile conditions. Biology and Fertility of Soils, 1988; 6: 170-73.
- Shaukat, K., Affrasayab, S., Hasnain, S. Growth responses of Helianthus annus to plant growth promoting rhizobacteria used as a biofertilizer. Journal of Agriculture Research, 2006a; 1(6): 573-81.
- Shaukat, K., Affrasayab, S., Hasnain, S. Growth responces of Triticum aestivum to plant growth promoting rhizobacteria used as a biofertilizer. Research Journal of Microbiology, 2006b; 1(4): 330-38.
- Siddiqui, I.A., Shaukat, S.S. Mixtures of plant disease suppressive bacteria enhance biological control of multiple tomato pathogens. Biology and Fertility of Soils, 2002; 36: 260-68.
- Burd, G.J., Dixon, D.G., Glick, B.R. Plant growth promoting rhizobacteria that decrease heavy metal toxicity in plants. Canadian Journal of Microbiology, 2000; 33: 237-45.
- Salantur, A., Ozturk, A., Akten. Growth and yield response of spring wheat (Triticum aestivum L.) to inoculation with rhizobacteria. Plant, Soil and Environmental Agricultural Journal, 2006; 52(3): 111-118.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


