ISSN: 0973-7510
E-ISSN: 2581-690X
Utilisation of fungus and bacteria in bioremediation research is a very common phenomenon in recent time, in this effort, we have also tried to find out the same but in a comparative manner. During this experiment, Trametes versicolor and Bacillus subtilis 1133 were taken into the consideration because of the laccase production ability will give additional advantage to them to detoxify more amounts of toxicants than other non-laccase producers. First, we have seen that Trametes versicolor has significant higher laccase production ability than Bacillus subtilis 1133 determined from the peak obtained in the FPLC, latter on in the degradation of BPA (using ESI-MS), decolourization of industrial dyes (using spectrophotometer) Trametes versicolor showed its significant activity than Bacillus subtilis 1133. At the end we have checked the heavy metal (cadmium) removal activity; here also Trametes versicolor manifested more resistance, adsorption, adaptive nature (in terms of recovering viability), absorption and accumulation of cadmium when compared to Bacillus subtilis 1133.
Laccase, ESI-MS, FPLC, cadmium, FT-IR.
Industrial development is one of the glorious sides of the triumph of science and technology, but recently different industrial toxic pollutants are giving serious threats to the environment. Not only industry, some toxic chemicals are coming to the environment from our daily domestic life, as a whole, the pollutants from industry or domestic sources causing serious health issues for not only human, different living organisms are also affected severely. In this respect bioremediation using microbes can play a key role in the detoxification and removal of those toxicants because microbes can utilise different types of chemicals for their nutritional source using different types of metabolic enzymes. After utilisation as nutrition, microbes either oxidise or reduce the toxicants and most of the cases, toxicants lose its toxicity. Laccase is a well studied economically important oxidoreductase enzyme with a wide range of oxidation ability.1 Laccase predominantly found in fungus but very few bacterial species were reported having laccase production ability. Among white rot fungus Trametes versicolor is a common and predominantly found wood rotting fungus with ample amount of laccase synthesis ability, another side among very few Bacillus subtilis 1133 also secretes the same.2 In this era endocrine disrupting chemicals become one of the most common threats for all living organisms, some endocrine disrupting chemicals are synthesising in our daily house hold life, BPA is one of them.3 Low-quality plastics can generate BPA after moderate exposure to the water and prolong exposure of BPA to living organisms can cause different types of serious health issues like decrement in sperm counts as well as abnormalities in female reproductive system, breast cancer in case of human and early maturation in female fishes due to its estrogenic activity.4 In other side toxic industrial dyes are one of the major causes of water pollution, generally textile industries are using several types of dyes that are not at all degradable due to photolysis but their presences are destabilizing the aquatic environment by causing serious health issues in aquatic organisms as well as altering the physical parameters of the aquatic environment.5 Apart from that, heavy metals are the another toxic chemicals giving environmental threats decades after decades, industries like electroplating industry, paint industry, tannery industry are solely responsible for heavy metal pollution.6 Bioremediation can play an effective role against all of those types of pollutants, intense research and development can able to reduce the environmental destabilisation occurred due to those risks. In this effort, we have tried to find out the efficiency of fungus or bacteria in bioremediation using a comparative analysis, which can give us a clear idea about the efficient bioremediating agent more suitable for our environment.
Protein purification
Trametes versicolor was isolated from Pondicherry7 and Bacillus subtilis 1133 obtained from MTCC (microbial type culture collection centre), Chandigarh, India. A fresh culture of Trametes versicolor and Bacillus subtilis 1133 grown in potato dextrose broth and nutrient broth respectively. Trametes versicolor was incubated at 28°C for seven days and Bacillus subtilis 1133 was incubated at 37°C for 24 hours maintaining 150 rpm for both incubation periods. Supernatant from both the cultures were collected after centrifugation at 13,000 rpm for 15 minutes. The supernatant was taken and ammonium sulphate precipitation of extracellular laccase enzyme at 75% saturation level was performed. Precipitated sample was dialysed for 2 hours, 4 hours and overnight against sterile distilled water. After complete removal of salt using dialysis, the sample was allowed to run through DEAE- anion exchange prepacked column using fast protein liquid chromatography.8 Tris-HCl pH-8.0 buffer and a gradient of [0.1-1(M)] NaCl was used during running the sample through fast protein liquid chromatography. The individual peak appeared in NaCl gradient line was collected using fraction collector and concentrated using vacuum evaporator. The fraction containing laccase was determined after incubating with laccase specific substrate ABTS; specific green colour formation will confirm the laccase-ABTS reaction. Estimation of laccase was performed using Lowry method9 to determine the concentration difference of laccase obtained from Trametes versicolor and Bacillus subtilis 1133.
Degradation of BPA and industrial dyes
Trametes versicolor and Bacillus subtilis 1133 inoculated in their requisite liquid media mentioned previously along with 200 ppm concentration of BPA, 1000 ppm concentration of industrial dyes (basic red, reactive blue, acid firaza, acid reactive blue, direct blue, direct firaza) separately after maintaining 28°C, 37°C respectively with 120 rpm for 7 days. After one-week, degradation was determined after comparing with control (without enzyme treatment). Supernatant from both the cultures were taken after centrifugation (at 13000 rpm for 15 minutes) and an equal volume of ethyl acetate (analytical grade) added in the supernatant followed by vigorous shacking and careful removal of the organic part. Organic part kept in the room temperature for complete evaporation, after adding MS grade methanol to dissolve the precipitate and analysed using ESI-MS7. The similar way both the organism grown separately in presence of different industrial dyes and maintained the same incubation condition mentioned previously. In the case of industrial dyes after 7 days, culture filtrates were collected along with controls used to determine the decolourization using spectrophotometer (625 nm for blue dye and 510nm for red dye were used while taking the reading in a spectrophotometer)7,10. Degradation of BPA and decolourization of industrial dyes in presence of fungal and bacterial strains were statistically analysed using one-way ANOVA
Activity against heavy metal
Trametes versicolor, Bacillus subtilis 1133 inoculated in their respective media mentioned previously (potato dextrose broth and nutrient broth respectively) in presence of different concentration of cadmium separately to determine the minimal inhibitory concentration after seven days of incubation (concentration of cadmium from 100 to 6000 ppm used for Trametes versicolor and 25 to 1000 ppm concentration of cadmium used for Bacillus subtilis 1133). Trametes versicolor and Bacillus subtilis 1133 grown in presence of 200 ppm concentration of cadmium and after requisite incubation biomass was collected from both of the organisms after centrifugation at 13000 rpm for 15 minutes. Biomass of both fungus, bacteria was lyophilized and used for atomic absorption spectroscopy along with FT-IR to find out the removal of cadmium along with functional groups involved during adsorption of cadmium on the surface of the both organisms (Amutha and Abhijit 2014). During AAS analysis the sample was diluted 10 times for that reason the removal value of cadmium multiplied by 10 further to get actual removal value of cadmium. Viability test also performed with already grown cultures of both fungus and bacteria after treatment of 200 ppm concentration of cadmium and determined after every day interval using alamar blue.11,12 Centrifuged biomass of fungus and bacteria separately kept in phosphate buffer saline (pH-7.4) and the content was ultrasonicated at 50MHZ and 40 MHZ with 40% amplitude for 10 cycles (2minutes/cycle with 1.5 seconds on 0.5 seconds off) and the lysed cells were kept undisturbed for 10 minutes at 4R”C followed by centrifugation at 13000 rpm for 1 hour at 4R”C. The supernatant was lyophilized and prepared according to Choudhary et al. 2011,13 for analysis using AAS to determine the concentration of cadmium absorbed by both the organism. All the experiment performed three times in a triplicate manner. Overall removal, absorption of cadmium by Trametes versicolor and Bacillus subtilis 1133 was statistically analysed by one-way ANOVA. Generally, microbes can accumulate heavy metals, for this reason biomass from 200 ppm cadmium-treated Trametes versicolor and Bacillus subtilis 1133 were taken and lyophilized to determine the presence of significant cadmium deposition peak using powder XRD after comparing the 2¸ value with existing JCPDS cadmium file
Protein purification
Specific peak obtained from FPLC was concentrated using vacuum evaporator and the green colour product formation confirmed laccase- ABTS reaction. The laccase specific peak obtained from FPLC is much higher in case of Trametes versicolor when compared to Bacillus subtilis 1133 (figure: 1). Estimation of laccase protein using Lowry method revealed that 100 ml of media containing Trametes versicolor and Bacillus subtilis 1133 contained 176 µg/ml, 45 µg/ml concentration of laccase produced by Trametes versicolor and Bacillus subtilis 1133. This comparative analysis of laccase production explained that due to huge body mass laccase production from the fungus is much higher than bacteria.
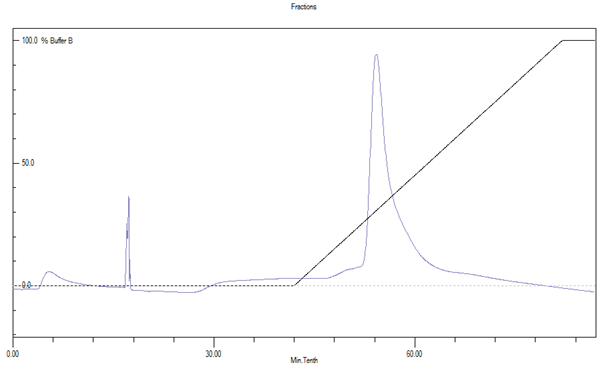
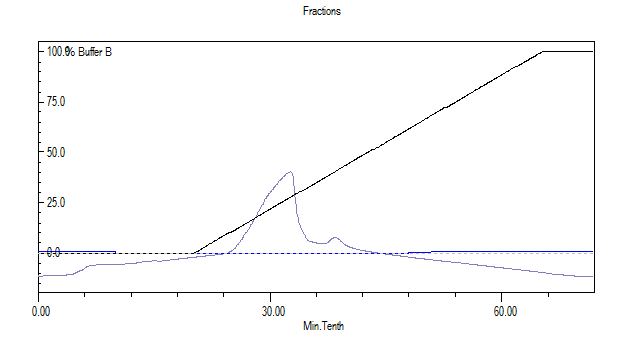 Fig. 1. Protein concentration difference
Fig. 1. Protein concentration difference Degradation of BPA and industrial dyes
ESI-MS data revealed that the BPA degradation happened in presence of Trametes versicolor (50%) was greater than the degradation happened in presence of Bacillus subtilis 1133 (30%) (figure: 2). In another side, Trametes versicolor showed more degradation capability of different industrial dyes than Bacillus subtilis 1133 (table:1) and after statistical analysis, it was clear that degradation of BPA and industrial dyes manifested significant degradation capability difference in favour of Trametes versicolor when compared to Bacillus subtilis 1133 (p<0.001). The reason behind the superior degradation capability of Trametes versicolor can be correlated with laccase production ability. In previous steps, we have compared the laccase production capability of Trametes versicolor and Bacillus subtilis 1133, comparison reviled that Trametes versicolor contained more laccase production ability than Bacillus subtilis 1133. Being an oxidoreductase enzyme laccase can oxidise wide range of chemical substances and it helps Trametes versicolor to achieve huge degradation ability like BPA and industrial dyes than Bacillus subtilis 1133.
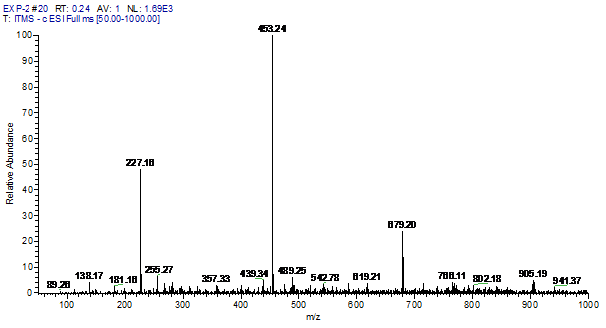
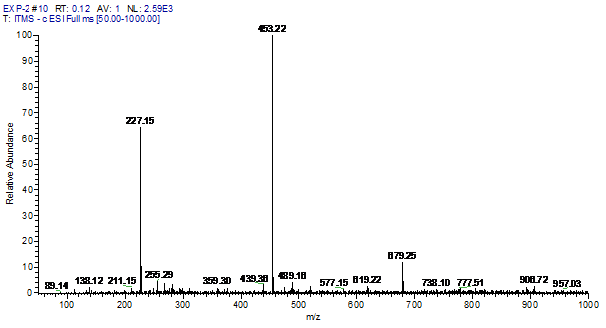 Fig. 2. Degradation comparison of BPA
Fig. 2. Degradation comparison of BPATable (1):
Determination of decolourization of industrial dyes after 7 days.
No. |
Name of the dye |
% of degradation by Trametes versicolor |
% of degradation by Bacillus subtilis |
p-value |
|---|---|---|---|---|
1 |
Basic red |
82 |
26 |
<0.001 |
2 |
Reactive blue |
94 |
54 |
<0.001 |
3 |
Acid firaza |
98 |
46 |
<0.001 |
4 |
Acid reactive blue |
96 |
53 |
<0.001 |
5 |
Direct blue |
98 |
59 |
<0.001 |
6 |
Direct firaza |
98 |
63 |
<0.001 |
Activity against heavy metal:
MIC result clearly revealed that Trametes versicolor can resist cadmium stress up to 5000 ppm but Bacillus subtilis 1133 manifested the MIC value only up to 800 ppm (figure: 3a). FT-IR analysis manifested that hydroxyl groups present on the surface of the microbes played a significant role during adhesion of cadmium; Trametes versicolor exhibited more hydroxyl group stretching at the 3200-3500 cm-1 range when compared to Bacillus subtilis 1133 after seven days of cadmium (200 ppm) treatment. Trametes versicolor being a fungus with higher biomass compared to Bacillus subtilis 1133 can adhere more amount of cadmium because of a higher amount of body surface area and that was determined from the data obtained from the FT-IR (hydroxyl group stretching) and from AAS data it was determined that removal was 70.5 ppm and 120 ppm respectively in presence of Trametes versicolor and Bacillus subtilis 1133 (figure: 3b & 3c). In this regard viability of the organisms will be an important phenomenon because during cadmium stress condition if viability will be less then absorption will be reduced, viability assay using alamar blue clearly revealed that due to 200 ppm stress viability of the Bacillus subtilis 1133 was gradually reduced up to seventh day but in other side after some short of viability reduction Trametes versicolor resisted the cadmium stress (figure: 3d). Absorption detection showed that Trametes versicolor absorbed almost 85 ppm of cadmium after seven days of cadmium (200 ppm) stress but after same days Bacillus subtilis 1133 absorbed almost 10 ppm of cadmium, this result was statistically analysed and it showed the statistical significant difference (p<0.001) (figure: 3e). At the end the accumulation of the cadmium was analysed using X-ray diffraction (figure: 3f), this study clearly manifested that the peak appeared at the 2¸ value of 44.21 was compared with JCPDS-CdS file-050640 and it was clear that the cadmium accumulation peak appeared due to Trametes versicolor was much greater when compared to Bacillus subtilis 1133. In all cases, Trametes versicolor showed a significant result that Bacillus subtilis 1133 and it usually happened due to their higher biomass, huge growth rate and resistant capability against heavy metal like cadmium.
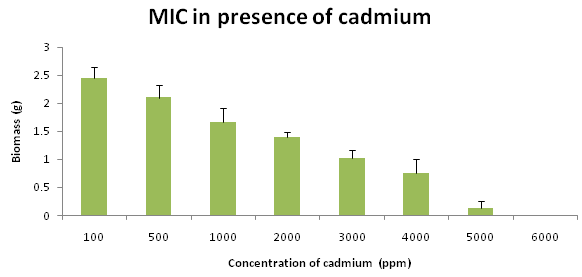
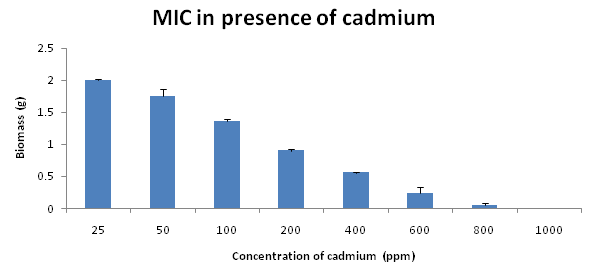 Fig. 3a. MIC presence of cadmium
Fig. 3a. MIC presence of cadmium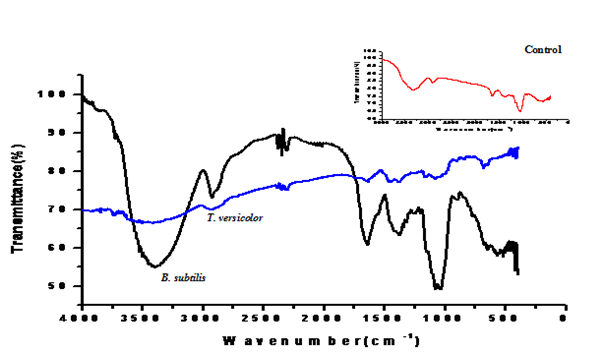 Fig. 3b. Determination of cadmium adhesion on the surface
Fig. 3b. Determination of cadmium adhesion on the surface
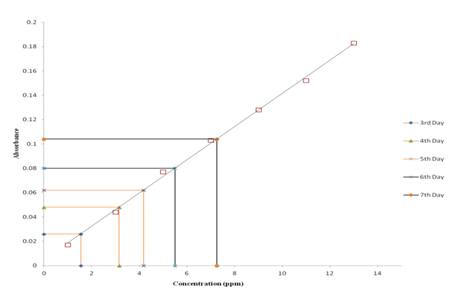 Fig. 3c. Removal of cadmium
Fig. 3c. Removal of cadmium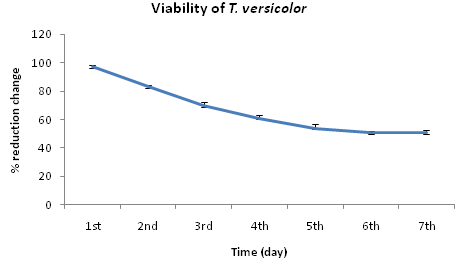
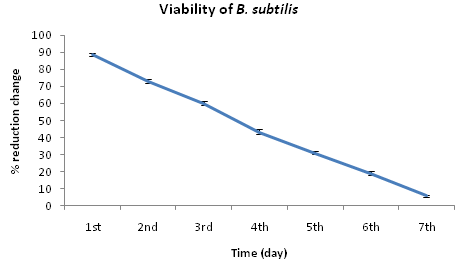 Fig. 3d. Viability of Trametes versicolor and Bacillus subtilis 1133 in presence of 200ppm cadmium
Fig. 3d. Viability of Trametes versicolor and Bacillus subtilis 1133 in presence of 200ppm cadmium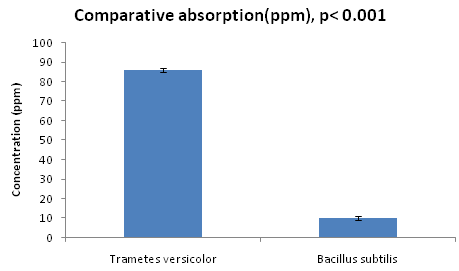 Fig. 3e. Absorption difference of cadmium
Fig. 3e. Absorption difference of cadmium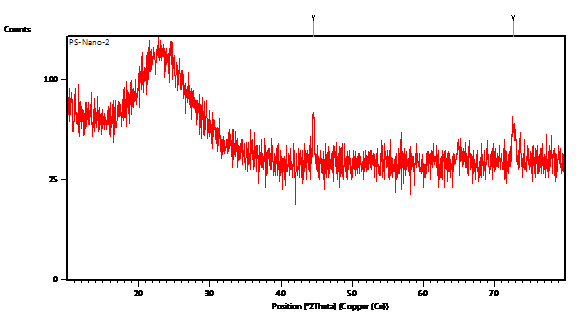
 Fig. 3f. Deposition of cadmium
Fig. 3f. Deposition of cadmiumIn this comparative analysis, we have chosen Trametes versicolor and Bacillus subtilis 1133 as representative of fungus and bacteria having laccase synthesising ability. Laccase being an oxidoreductase enzyme can oxidise wide variety of chemical toxicants so laccase producers contain an additional advantage when compared to other microbial organisms regarding detoxification of toxicants. At the beginning, we have executed the experiment showing comparative analysis regarding laccase synthesis by Trametes versicolor and Bacillus subtilis 1133 because higher laccase synthesis will signify the better detoxification ability and indirectly we can correlate that due to higher synthesis ability of laccase, Trametes versicolor is more effective against the detoxification of toxicants than Bacillus subtilis 1133. Apart from the laccase production activity when the degradation of BPA as well as toxic industrial dyes were checked using whole organisms, in every case Trametes versicolor showed more degradation ability than Bacillus subtilis 1133 and this happened because of the wide range of oxidation ability generated from the synthesised laccase. In another side, while we are talking about the heavy metal removal activity, Trametes versicolor exhibited the significant heavy metal tolerance, adsorption, viability, absorption and accumulation ability than Bacillus subtilis 1133 due to its more growth rate and huge biomass. At the end, we can conclude that Trametes versicolor contains higher laccase production, detoxification of toxicants; removal capability of heavy metal like cadmium than Bacillus subtilis 1133 and it can play a significant role for the environment in terms of detoxification of the pollutants.
At the end we can conclude that not only Trametes versicolor more effective in bioremediation activity than Bacillus subtilis 1133, but as whole fungi are more efficient in the bioremediation activity than bacteria because of higher biomass, high quantity of effective enzyme secretion.
ACKNOWLEDGMENTS
The financial support of Department of Biotechnology-Interdisciplinary program in Life science (DBT-IPLS PhD programme, India) and University grant commission (UGC) are gratefully acknowledged.
- Viswanath, B., Rajesh, B., Janardhan, A., Praveen Kumar, A. Narasimha G. Fungal Laccases and Their Applications in Bioremediation. Enzyme. Research., 2014; doi.org/10.1155/2014/163242.
- Sheikhi, F., Roayaei Ardakani M Enayatizamir, N., Rodriguez-Couto, S. The Determination of Assay for Laccase of Bacillus subtilis WPI with Two Classes of Chemical Compounds as Substrates. Indian. J. Microbiol., 2012; 52: 701–707.
- Vandenberg, L. N, Maffini, M. V, Sonnenschein, C, Rubin, B. S., Soto, A. M. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocrine. rev., 2009; 30: 75-95.
- Arboleda, C., Cabana, H., De Pril, E., Peter Jones, J., Jiménez, G. A, Mejía, A. I., Agathos, S. N., Penninckx, M. J. Elimination of Bisphenol A and Triclosan Using the Enzymatic System of Autochthonous Colombian Forest Fungi., ISRN. Biotechnol., 2013; 12.
- Verma, Y. Acute toxicity assessment of textile dyes and textile and dye industrial effluents using Daphnia magna bioassay. Toxicol. Ind. Health., 2008; 24: 491-500.
- Amutha, C., Subramanian, P. Cadmium alters the reproductive endocrine disruption and enhancement of growth in the early and adult stages of Oreochromis mossumbicus, Fish Physiol. Biochem., 2013; 39: 351-361.
- Amutha, C., Abhijit, M. Screening and Isolation of Laccase Producers, Determination of Optimal Condition for Growth, Laccase Production and Choose the Best Strain. J. Bioremed. Biodeg., doi: 2015; 10.4172/21556199.100029.
- Chefetz, B., Chen, Y., Hadar, Y. Purification and Characterization of Laccase from Chaetomium thermophilium and Its Role in Humification. Appl. Environ. Microbiol., 1968; 64: 3175–3179.
- Lowry, H., Rosebrough, N. J., Farr, A. L., Randall, R. L. Protein measurement with the folin phenol reagent. J. Biol. Chem., 1951; 193: 265-275.
- Olusola, M., Julius, O., Hari Deka, B., Charles, A., Arjit, B. Maina Borah Extraction and purification of extracellular laccase from wild, mutants and hybrid strains of two white-rot fungus and its applications in decolourization and ligninolysis. Journal of Microb. Biotech., 2012; 13: 998-1016.
- Karthikeyan, R., Vijayalakshmi, S., Balasubramanian, T. Seasonal distribution of heavy metals in the sediments from Uppanar estuary (East Coast of India). J.Aqua.Biol., 2004; 19:112-119.
- Alamar Blue assay. U.S patent no: 5,501,959.
- Choudhary, S., Thakur, R. A, Ray Chaudhuri, S. Novel microbial consortium for laboratory scale lead removal from city effluent. J. Env. Sci. Tech., 2011; 4: 41-54.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


