ISSN: 0973-7510
E-ISSN: 2581-690X
The COVID-19 pandemic has primarily been controlled by testing for SARS-CoV-2 infections. Despite vaccines, testing will remain crucial for surveillance and screening, allowing for the detection of new variants in a timely manner and to isolate the infected people to lower the danger of the disease spreading further. The research study attempts to found out the efficiency of Reverse-transcriptase polymerase chain reaction (RT-PCR) and Rapid antigen tests in symptomatic COVID-19 patients at tertiary care hospitals. The research was performed on 1000 patients, both In-patients and Out-patients, who presented with COVID-19 symptoms. SARS-COV-2 nucleocapsid protein antigen was detected qualitatively with rapid antigen test in human nasal specimens through the immuno-chromatographic assay. The rapid test results were compared with a molecular test RT-PCR in which FAM, HEX, and ROX were the indicator dyes for the RdRp gene, E gene, and the internal control (RNAse P), respectively. Nearly 322 cases were positive with both RT-PCR and rapid antigen test methods. Fifty-nine samples yielded negative results with the rapid antigen test and positive with PCR. Three samples were negative with RT-PCR and positive with the rapid antigen test. The findings from our study show that the common symptoms are fever 92.2% and cough 74.1% in the reported test population. But in confirmed cases of RT-PCR showed cough at 74.1% was more prevalent, followed by fever at 41.3%. Rapid antigen test showed a overall sensitivity and specificity of 85.3% and 99.5%. According to World Health Organization, rapid antigen detection tests meet the minimum performance requirements of ≥80% sensitivity and ≥97% specificity. Hence, the present study meets this criterion and may perhaps be a probable tool for point-of-care in hospital settings.
COVID-19, RT-PCR, Rapid Test, Ct-score, Specificity
“Severe Acute Respiratory Syndrome Corona Virus 2 (SARS-COV-2) causes acute respiratory illness known as Corona Virus Disease (COVID-19)”1 and it has resulted in the worst financial catastrophe ever documented since World War II and an explosive, devastating pandemic that has claimed many people. The disease spread explosively and quickly, and even after it was designated a global pandemic in March 2020, cases continued to be reported in some nations. The epidemiological data, especially the geographic distribution, is predicted to alter over time1 because this explosive pandemic is still happening. According to the most recent WHO data release (Jan 2023), the cumulative number of reported cases was 761,402,282, and the cumulative total of deaths was 6,887,000. During December 2022 and January 2023, over 14.5 million new cases and over 46,000 new fatalities were reported globally. Globally, about 6.6 million fatalities and over 656 million confirmed cases had been documented as of January 2023. The Majority of instances were in the USA, followed by India, Brazil, the UK, and France. The second-highest number of Corona cases is reported from India. The pandemic’s growth trajectory was slower in India, where it peaked. Three waves of an exponential spike in cases were observed in India, the first beginning around September 2020, the second in April 2021, and the third in January 2022. According to WHO statistics from 2023, there have been 44,707,525 cases with confirmed COVID-19 and 530,841 reported deaths in India. The COVID-19 virus genome is constantly changing, and when these genetic alterations become considerable, they may cause the emergence of new virus strains known as variations.1 Many mutant strains including Alpha, Beta, Gamma, Delta, and Omicron, have been reported till date. The disease caused by the delta variety is more severe and more contagious. This delta variation was primarily to blame for the second wave in India. The Omicron variant is more likely to spread but is linked to comparatively milder illness. It has caused the explosive third wave in India. The primary transmission means for the COVID-19 virus are respiratory droplets and direct contact. Viral transmission can also occur during operations that produce aerosols, such as endotracheal intubation. It is essential to regularly wash your hands after potential contact exposure to stop this kind of transmission. Average incubation times for COVID-19 are five to six days; even though last up to 2 weeks. Patients with COVID-19 may exhibit symptoms such as fever, coughing up expectorants, exhaustion, and shortness of breath, myalgia, rhinorrhea, sore throat, and diarrhoea. Sometimes the development of respiratory symptoms is preceded by a loss of taste or smell sensation. Atypical symptoms such as fatigue, diminished alertness, limited mobility, diarrhoea, loss of appetite, delirium, and absence of fever were found in immune-compromised hosts.1 The COVID-19 pandemic has been controlled mainly by testing for SARS-CoV-2 infections.
Despite vaccines, testing will remain crucial for surveillance and screening, allowing for the detection of new variants in a timely manner and to isolate the infected people to lower the danger of the disease spreading further. A key component of controlling this pandemic is quickly detecting SARS-CoV-2 in the patient sample.2,3 The standard test for diagnosing SARS-CoV-2 is called RT-PCR. Other antigenic tests (AT) have also been created. Compared to RT-PCR testing, their turnaround time for findings is considerably less, at around 15 minutes. Even though RT-PCR tests continue to be the gold standard for diagnosis, Rapid tests (RADTs) have helped to expand testing capabilities globally.4,5 The quick turnaround time, increased accessibility, and lower RADT test costs make them a desirable option for workplace screening and public monitoring. The user can administer the rapid antigen test kits without extensive ongoing equipment maintenance and calibration and with little to no training. One issue with RADT testing is that, compared to RT-PCR, they have a higher percentage of false positives and false negatives. Performances of RT-PCR and RADTs have been compared in a few research.6 According to WHO, it is advised that, Rapid tests should have minimum sensitivity of 80% and a minimum specificity of 97%.
Additionally, it has been noted that specific N protein mutations can cause some RT-qPCR kits to provide false-negative results. As a result, there has been some debate regarding the usefulness of RT-PCR and rapid antigen test for infection detection and prevention. The present study attempts to determine the efficacy of RT-PCR and rapid antigen tests in symptomatic COVID-19 patients at tertiary care hospitals.
Study design and participants
It is a prospective study conducted on 1000 patients, both IP and OP, who presented with COVID-19 symptoms. Informed consent was taken from each individual before the collection of swabs. The study was undertaken after obtaining approval from the Institutional Ethics Committee. All the patient demographic data like age, sex, and history of any illness have been collected.
Inclusion and Exclusion criteria
The study included all symptomatic individuals who elected to undergo COVID-19 testing. Children under ten years old and patients unwilling to consent were also excluded from the study. Individuals with mental or psychiatric severe issues and those with active tuberculosis were not allowed to participate in the study.
Specimen collection and transport
Dacron or polyester flocked swabs were used to collect the patient nasopharyngeal swabs. Two swabs from each person were obtained to increase the viral load, and the VTM tube was then dipped into it. The samples were appropriately labeled, triple-packed in three layers, and then delivered to the lab while maintaining a good cold chain. Upon receiving the specimens they should be stored at an appropriate temperature (4°C for < 5 days and 77°C for 5 days).
Rapid Antigen test
Using the use of an immuno-chromatographic assay approach, SARS-COV-2 nucleocapsid protein antigen will be qualitatively detected in human nasal specimens. The test protocol for Rapid Antigen test through Standard Q COVID-19 antigen test (SD Biosensor, Inc, Korea) was followed according to the kit protocol. Depending on the amount of SARS-CoV-2 antigen is present in the sample, the intensity of the coloured test line will change.
Real-time RT-PCR testing
RT-PCR is the gold-standard test for COVID-19 diagnosis. Current guidelines by WHO and the government of India recommend that commercial kits target at least two genes SARS-COV-2, among the following genes: Spike protein (S), an Envelope protein (E), Membrane protein (M), RNA-dependent RNA polymerase (RdRp), Nucleocapsid protein (N), Open reading frames (ORF 1a and 1b). The Nasopharyngeal samples for RT-PCR were transported in a VTM immediately after collection and the specimens were processed in a BSL-2 Molecular virology laboratory for SARS-COV-2 testing using PROMEA therapeutics (QIAGEN, Germany). FAM, HEX, and ROX were the indicator dyes for the RdRp gene, E gene, and the internal control (RNAse P), respectively. The procedure was followed according to manufacturer’s instructions. Cycle threshold values (Ct) below 35 were considered positive, and anything above 35 was deemed negative.
Statistical analysis
Unpaired student ‘t’ test was used to find out the statistical significance between the study variables. P < 0.05 was taken as significant.
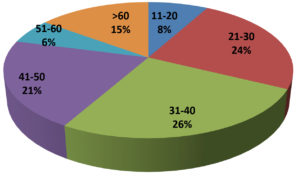
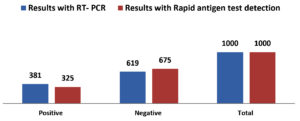
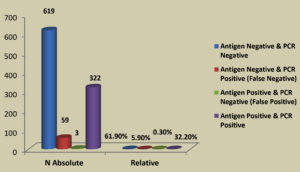
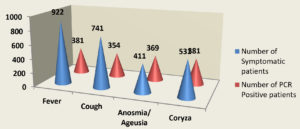
Table 1 summarizes the demographic data of the tested population. In 1000 total samples, males 60.2% were more than Females 39.8%. By comparing the two methods rapid and RT-PCR method with respect to gender and age statistically (Table 1 and Figure 1) the results were insignificant (P=0.093) with paired ‘t’ test analysis. The number of COVID-19 positives was more in males 38.9% than in females 36.9% and the results were statistically insignificant. Figure 1 depicts the age-wise distribution of cases. Among the different age groups observed during the study period, more cases were identified in the age group 31-40 years (26%) followed by 21-30 years (24%) and the results were statistically. Figure 2 shows the results obtained by the RT-PCR and Rapid antigen test. Of 1000 samples subjected to the rapid antigen test method, 32.5% were positive, and the rest were negative, whereas, with RT-PCR, the positive percentage was 38.1%. By comparing the results of both methods statistically they were insignificant P=1.00 (Table 2). Figure 3 compares the results by standard gold technique RT-PCR and rapid antigen test. Nearly 322 cases were positive with both RT-PCR and rapid antigen test methods. Fifty-nine samples yielded negative results with the rapid antigen test and positive with PCR. Three samples were negative with RT-PCR and positive with the rapid antigen test. This indicates that higher rates of false negative results occurred with the rapid antigen test than with RT-PCR when the subjects had a low viral load (Figure 3). The findings from our study show that the common symptoms are fever 92.2% and cough 74.1% in the reported test population and the results were statistically insignificant P=0.089 But in confirmed cases of RT-PCR showed cough at 74.1% was more prevalent, followed by fever at 41.3% (Figure 4). By comparing the two methods rapid and RT-PCR method with respect to symptomatic nature the results were statistically insignificant (P=0.089) (Table 3). The Majority of cases encountered in our study were mild. No severe cases or deaths were observed during the study period. The current study’s overall sensitivity and specificity are 85.3% and 99.5%, respectively. Concordance is 94.1%, Discordance is 5.9%, and Kappa value is 0.87. This indicates that the results almost agreed between the two methods. Table 4 showed the Ct values of the E gene and RdRp gene Ct values of RT-PCR, and the results showed Ct values of the E gene were 27.32± 6.30, and the RdRp gene Ct value was 25.82 ± 6.41.
Table (1):
Gender-wise distribution of cases
| Gender | Total samples (n=1000) and percentage | Total samples positive and percentage | Total samples negative and percentage | Standard deviation | Standard error mean | ‘t’ value | ‘P’ value |
|---|---|---|---|---|---|---|---|
| Males | 602 (60.2%) | 234 (38.9%) | 368 (61.1%) | 94.75 | 67.00 | 6.800 | 0.093 (Insignificant) |
| Females | 398 (39.8% | 147 (36.9%) | 251 (63.1%) | 73.53 | 52.00 |
Table (2):
Test results of RT-PCR and Rapid antigen detection
| Test result | Results with RT- PCR | Results with Rapid antigen test detection | Standard deviation | Standard error mean | ‘t’ value | ‘P’ value |
|---|---|---|---|---|---|---|
| Positive | 381 | 325 | 247.48 | 175.00 | 0.00 | 1.00 |
| Negative | 619 | 675 | 168.29 | 119.00 |
Table (3):
COVID-19 symptoms prevalence of the study population
| Symptoms | Number of Symptomatic patients | Number of PCR Positive patients | ‘P’Value |
|---|---|---|---|
| Fever | 922 | 381(41.3%) | 0.089 |
| Cough | 741 | 354(47.7%) | |
| Anosmia/ Ageusia | 411 | 369(89.78%) | |
| Coryza | 531 | 381(71.75%) |
Table (4):
Statistical results of RT-PCR results
Results of RT-PCR assay |
Mean ± SD |
|---|---|
The CT value of the E gene |
27.32± 6.30 (Min 12.53 Max 36.06) |
The CT value of RdRp (RNA-dependent RNA polymerase ) |
25.82 ± 6.41 (Min 12.96 Max 36.54) |
In the present study, more number of males was reported than the females and the most common age group affected was 31-40 years and the results were found to be statistically significant (P<0.05) with age and insignificant with Sex (P>0.05). Whereas Samer and Harake7 showed significant sociodemographic differences between males and females (P< 0.05). For example, a significantly higher proportion of males (vs. females) were aged ≥70-year-old in their study. Similar prevalence was identified from the previous studies.8-10 In this study, we compared the diagnostic accuracy of rapid immunochromatography test with RT-PCR for diagnosing SARS-CoV-2 using a prospective analytical approach. We found that the rapid antigen test and RT-PCR somewhat have the same agreement (estimated pooled Cohen’s kappa statistic of 0.87). The current study’s overall sensitivity and specificity are 85.3% and 99.5%. Out of 1000 samples, nearly 322 cases were positive with both RT-PCR and rapid antigen test methods, and 59 samples yielded negative results with the rapid antigen test and positive with PCR but the results were statistically insignificant. Similar percentage of overall sensitivity and specificity of RADT and RT-PCR was reported by Mandal et al.11 but their results showed significant difference (P value <0.001) between Ag- RDT+ and Ag- RDT− results when compared to Cq values obtained from RT- PCR. Three samples were negative with RT-PCR and positive with the rapid antigen test. This indicates that some false negative results occurred with the rapid antigen test than RT-PCR when the patients have a less viral load. In comparison to molecular testing, the WHO recommends RADTs should have minimum performance standards of 80% sensitivity and 97% specificity. The European Center for Disease Prevention and Control (ECDPC), however, suggests utilizing assays with performance more akin to RT-PCR, i.e., 90% sensitivity and 97% specificity.3,6,12,13 Our study showed sensitivity and specificity of rapid antigen test values 85.3% and 99.5%, which are within the limit according to WHO recommended guidelines. Still, the sensitivity is less, according to ECDPC. False negative results from RADT found in our investigation may have resulted from faulty sample collection or low viral load, among other possibilities. According to Lee et al. research,14 the rapid antigen test’s pooled sensitivity was somewhat higher than the RADTs in general. In our findings, we got the optimum sensitivity and specificity percentage with a standard COVID-19 Ag test, so the results aligned with the findings of Lee et al.14 Lee et al14 identified 24 studies (multicenter studies) comprising 14,188 patients using a metanalysis approach. The overall pooled sensitivity, specificity, and DOR of RADTs for diagnosis of SARS-CoV-2 were 0.68. The pooled sensitivity of RADTs was significantly increased in subjects with viral load of Ct-value ≤25 or in those within 5 days after symptom onset than it was in subjects with lower viral loads or longer symptom duration. In their study they showed the study parameters like age, gender, RADT and RT-PCR results, symptomatic nature were statistically significant whereas in our study the results were insignificant as we have a less sample size and it is a single centre study. Similar sensitivity and specificity of 82.1% and 99.1% were shown by Abdulrahman et al.15 Ashok Kumar et al.16 showed less sensitivity of 61% and a specificity of 94.4%. Chutikarn Chaimayo et al.17 showed the highest sensitivity, 98.3%, compared to our study. The rapid antigen test frequently returns a negative result in PCR-positive samples with Ct values more than 24-28, as shown by Platten et al.18 Moreover, 0.09% of swabs that tested positive for Ag but negative for RT-PCR showed a contradiction. This discrepancy may have been somewhat influenced by errors that may have occurred during the pre-analytical phase (such as sample collection) or the data collecting (e.g., subjective RADT reading). The difference may have also been caused by testing at various times, either early or late in the infection cycle. Because a significant section of the population contracts SARS-CoV-2 infection with an asymptomatic presentation, caution must be used while interpreting the results of RAD tests in these individuals. The results of our study indicated that the E gene’s Ct value was 27.32± 6.30, and the RdRp gene’s Ct value was 25.82 ± 6.41. Selvabai et al.19 showed similar findings in their study the Ct values of the E gene and RdRp gene were within 20 to 30, and some of the samples showed CT values for the RdRp gene were greater than 30. Similar results were reported by Perez-Garcia et al. indicating that for Pan bio and SD RAT’s, when the CT value was up to 20, the sensitivity exhibited by both the diagnostic kits was found to be 100%.20 Consequently, our evaluation clearly shows that the sensitivity of RAT is good for both the E and RdRp genes when the CT values are higher than 10 and up to 30. Hence, RADT can aid in the fast detection of SARS-CoV-2 positivity within 15 to 20 minutes and further direct the isolation of the positive patients. RAT can be utilized as a point-of-care diagnostic in remote locations without access to advanced SARS-CoV-2 devices. However, when the CT values are higher than 30, the sensitivity of RAT significantly declines, and it even fails to identify SARS-CoV-2 infection, resulting in false negativity. As a result, a key factor affecting the test’s sensitivity is the viral load in the sample.
Therefore, the sensitivity and specificity of the rapid antigen test kit are respectively 85.3% and 99.5%. RADTs had achieved minimum performance standards of 80% sensitivity and 97% specificity advised by WHO. As a result, the current study satisfies this requirement and may be useful for point-of-care testing. The SARS-CoV-2 RT-PCR discovered CT values for the E and RdRp genes demonstrate that the sensitivity range of the kit closely matches the viral load. To reduce the transmission of infection and develop infection prevention strategies, RAT nonetheless has the potential to be used as a point-of-care and screening test, particularly in hospital settings and isolated places with high infection rates.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, Dr Patnam Mahender Reddy Institute of Medical Sciences, Chevella, India.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141-154.
Crossref - Wells CR, Pandey A, Moghadas SM, et al. Comparative analyses of eighteen rapid antigen tests and RT-PCR for COVID-19 quarantine and surveillance-based isolation. Commun Med. 2022;2:84.
Crossref - World Health Organisation. Antigen-detection in the Diagnosis of SARS-CoV-2 Infection Using Rapid Immunoassays.2020. https://www.who.int/publications-detail-redirect/antigen-detection-in-the-diagnosis-of-sars-cov-2infection-using-rapid-immunoassays (accessed December 9, 2020).
- Ferte T, Ramel V, Cazanave C, et al. Accuracy of COVID-19 rapid antigenic tests compared to RT-PCR in a student population: The StudyCov study. J Clin Virol. 2021;141:104878.
Crossref - FDA. Genetic variants of SARS-CoV-2 may lead to false negative results with molecular tests for detecting SARS-CoV-2-letter to Clinical Laboratory Staff and Health Care Providers. FDA; 2021.
- Jeewandara C, Guruge D, Pushpakumara PD, et al. Sensitivity and specificity of two WHO-approved SARS-CoV2 antigen assays in detecting patients with SARS-CoV2 infection. BMC Infect Dis. 2022;22(1):276.
Crossref - Samer AK, Harake MDE. Sex-differences in COVID-19 diagnosis, risk factors and disease comorbidities: A large US-based cohort study. Front Public Health 2022;10:1029190.
Crossref - Singh S, Chowdhry M, Chatterjee A, Khan A. Gender-based disparities in COVID-19 patient outcomes: a propensity-matched analysis. medRxiv. 2020;20079046.
Crossref - Jin JM, Bai P, He W, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152.
Crossref - Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720.
Crossref - Mandal DK, Bhattarai BR, Pokhrel S, et al. Diagnostic Performance of SARS-CoV-2 Rapid Antigen Test in relation to RT-PCR Cq Value. Adv Virol. 2022;9245248.
Crossref - European centre for disease prevention and control, options for the use of rapid antigen tests for COVID-19 in the EU/EEA and the UK, Available from: https://www.ecdc.europa.eu/en/publications-data/options-use-rapid-antigen-tests-covid-19-eueea-and-uk, 2021 (assessed August 19 2021).
- World Health Organization (WHO). COVID-19 target product profiles for priority diagnostics to support response to the COVID-19 pandemic v.1.0. https://www.who.int/publications/m/item/covid-19-target-product-profiles-for-priority-diagnostics-tosupport-response-to-the-covid-19-pandemic-v.0.1 (Accessed August 13 2021).
- Lee J, Song JU, Shim SR. Comparing the diagnostic accuracy of rapid antigen detection tests to real time polymerase chain reaction in the diagnosis of SARS-CoV-2 infection: A systematic review and meta-analysis. J Clin Virol. 2021;144:104985.
Crossref - Abdulrahman A, Mallah SI, Alqahtani M. COVID-19 viral load not associated with disease severity: findings from a retrospective cohort study. BMC Infect Dis. 2021;21(1):688.
Crossref - Kumar A, Singh R, Kaur J, et al. Wuhan to World: The COVID-19 Pandemic. Front Cell Infect Microbiol. 2021;11:1-21.
Crossref - Chaimayo C, Kaewnaphan B, Tanlieng N, et al. Rapid SARS-CoV-2 antigen detection assay in comparison with real-time RT-PCR assay for laboratory diagnosis of COVID-19 in Thailand. J Virol. 2020;17(1):177.
Crossref - Platten M, Hoffmann D, Grosser R, et al. SARS-CoV-2, CT-values, and infectivity-conclusions to be drawn from side observations. Viruses. 2021;13(8):1459.
Crossref - Selvabai RAP, Koshy LV, Shanmugam P. Diagnostic Efficacy of COVID-19 Rapid Antigen Detection Card in Diagnosis of SARS-CoV-2. J Lab Physicians. 2022;14(3):324-328.
Crossref - Perez-Garcia F, Romanyk J, Moya Gutierrez H, et al. Comparative evaluation of Panbio and SD Biosensor antigen rapid diagnostic tests for COVID-19 diagnosis. J Med Virol. 2021;93(9):5650-5654.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.