ISSN: 0973-7510
E-ISSN: 2581-690X
Methicillin-resistant Staphylococcus aureus (MRSA) infections are a primary health concern. They are commonly differentiated as hospital-acquired methicillin-resistant Staphylococcus aureus (HA-MRSA) and community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) infections, based on their epidemiology, susceptibility findings, and molecular typing patterns. Therefore, appropriate contact precautions and isolation measures should be implemented. CA-MRSA mostly causes skin and soft-tissue infections, but the probability and incidence of it causing sepsis and invasive infections have increased dramatically in recent years. In this study, we report a case of CA-MRSA pneumonia with pan-pneumonic effusion in a 59-year-old male diabetic patient with preexisting comorbidities such as diabetic ketoacidosis and non-ST elevated myocardial infarction. The early reporting of the organism’s identity and its antimicrobial susceptibility, as well as timely initiation of antibiotic therapy, aided in the successful management and cure of the patient.
CA-MRSA, Methicillin Resistance, Pneumonia, Parapneumonic Effusion
Staphylococcus aureus commonly presents as normal skin flora and is harbored by most healthy adults at any given point in time. It usually colonizes the anterior nares, axilla, pharynx, vagina, perineum, or abraded skin. Symptomatic infections usually occur following a breach of the mucosal or cutaneous barriers. It causes a wide range of clinical infections, ranging from skin and soft tissue infections to life-threatening conditions such as sepsis, endocarditis, and toxic shock syndrome.1
Staphylococcal strains resistant to methicillin are encoded by the mec A gene. Methicillin resistance is defined by the Clinical Laboratory Standards Institute (CLSI) as an oxacillin minimum inhibitory concentration (MIC) ≥4 µg/mL, whereas MICs ≤2 µg/mL are considered susceptible.2 The European Committee on Antimicrobial Susceptibility Testing breakpoint differs from that of CLSI and defines the resistance of oxacillin as a MIC of >2 µg/mL.3 Oxacillin is a semisynthetic penicillin that has surpassed the use of methicillin as methicillin is not currently available commercially.
Other methods for detecting methicillin resistance include disk diffusion tests using cefoxitin or polymerase chain reaction to detect the presence of mec A gene. Strains resistant to methicillin or oxacillin were also found to be resistant to most β-lactam antibiotics, including cephalosporins. Exceptions include the fifth-generation cephalosporins, ceftaroline, and ceftobiprole.2 Such resistance patterns have led to gross treatment failure, thereby worsening clinical outcomes.
The prevalence of methicillin-resistant Staphylococcus aureus (MRSA) infection on a worldwide basis ranges from 13% to 74%.4 In India, studies have shown that it is more prevalent in the southern areas, with approximately 50% prevalence rates, compared with the western areas which have prevalence rates of approximately 25%.5 Although MRSA is responsible for hospital-acquired pneumonia (HAP), only a few reports are available on the organisms that cause community-acquired pneumonia (CAP).6 The estimated prevalence of community-acquired MRSA (CA-MRSA) is approximately 0.51–0.64 per 100,000 cases.7 Although rare, CAP caused by MRSA can lead to debilitating illnesses and mortality. The clinical outcomes are worse than those of CAP caused by Pneumococci. The mortality rate associated with CA-MRSA pneumonia is high, ranging from 56 – 63%.7
Many cases of CA-MRSA are associated with an Influenza viral infection, and most cases occur after a viral infection. This often leads to improper use of empirical antibiotics, thereby leading to poor clinical outcomes. Most cases of CA-MRSA are also associated with the Panton Valentine Leukocidin (PVL) gene, which is an important virulence-coding gene. It can cause dangerous symptoms such as multilobular infiltration, lung necrosis, hemoptysis, and severe sepsis, thereby leading to high mortality rates.8
In this article, we report a case of CA-MRSA pneumonia in a 59-year-old man with diabetes complicated by parapneumonic effusion, diabetic ketoacidosis, and non-ST elevated myocardial infarction (NSTEMI).
Case Report
A 59-year-old male patient with a previous diagnosis of diabetes was brought to our hospital with complaints of right-sided chest pain, breathlessness, fever, and dry cough for the previous week. The patient had experienced the above mentioned symptoms intermittently for the past six months, and they had worsened in the previous week. On examination, the patient was afebrile and tachypneic with tachycardia, and his vital signs were as follows: pulse rate, 135/minute; BP is 180/100 mm Hg; respiratory rate, 26/min; capillary blood glucose, 415 mg/dL; temperature-97.1 F; GCS, 15/15, and oxygen saturation on room air, 95%. He had been diagnosed with diabetes for the past six years and was receiving treatment with metformin 500 mg and glimepiride 1 mg. He had been a chronic smoker for the past 30 years. The patient reported that he had contracted COVID-19 in 2020, but it was managed at home only with no history of hospitalization or positive test results.
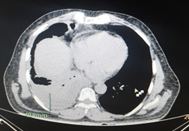
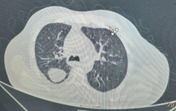
A complete blood count (CBC) performed on admission revealed neutrophilic leukocytosis (15,000 cells/mm3). Radiography showed signs of pleural effusion with blunting of the costophrenic angle on the right side. Plain computed tomography (CT) showed signs of parapneumonic effusion with pleural effusion fluid of approximately 570–650 mL and consolidation changes consistent with pneumonia in the right lower lobe. (Figure 1, 2) A contrast-enhanced chest CT was suggested to rule out empyema. ECG showed signs of NSTEMI with sinus tachycardia for which the patient was immediately given a stat dose of clopidogrel (300 mg), aspirin (300 mg), and atorvastatin (80 mg). The patient was immediately transferred to the intensive care unit (ICU) for further management by a pulmonologist.
Figure 2. Plain CT Chest of the patient showing Pneumonia with consolidation in the right lower lobe. Figure 1 and Figure 2 both together suggestive of Para-pneumonic Effusion.
In the ICU, the patient was started on nasal oxygen at 2 L/min and regular nebulization, along with strict cardiovascular monitoring. Oral azithromycin (500 mg) was administered once daily for seven days, and intravenous cefoperazone/sulbactam was started. Abdomen ultrasonography showed tiny B/L renal calculi requiring no urgent intervention, along with right-sided pleural effusion with basal consolidation. ABG showed a picture of metabolic acidosis. The blood sugar levels were very high, fluctuating between 300 and 450 mg/dL. Hence, diabetic ketoacidosis was suspected, and urinary ketones were found to be positive. A CBC performed two days later showed that the leukocytosis had settled, and the leukocyte count was 13,000 cells/mm3. Air entry was reduced in the infraaxillary and infrascapular areas. Hence, diagnostic thoracocentesis was performed, and fluid cytology revealed signs of neutrophil-rich effusion. Since tuberculosis was suspected clinically, both AFB staining and GeneXpert were performed, both of which were found to be negative. Adenosine deaminase levels, erythrocyte sedimentation rate, and absolute eosinophil count were found to be elevated. Intermittent fever was present along with dry cough, and the patient was unable to expectorate sputum. The antibiotic was then changed to intravenous piperacillin/tazobactam, as no clinical signs of improvement were noted.
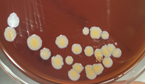
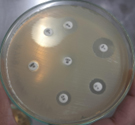
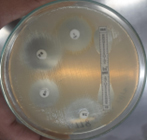
Pleural fluid was aspirated and found to be slightly turbid and was, therefore, sent for Gram staining as well as culture and antibiotic sensitivity testing to the microbiology laboratory. Gram staining revealed multiple pus cells and gram-positive cocci in pairs and clusters. The culture yielded moderate growth of yellowish opaque colonies. The colonies were medium-sized, smooth, convex with regular edges and margins, and had no characteristic odor. (Figure 3, 4). Smears made from the colonies showed gram-positive cocci in clusters that were catalase-positive and both tube and slide coagulase-positive. Antibiotic sensitivity testing (Kirby-Bauer disc diffusion method) was performed as per CLSI Guidelines, and the organism was identified as MRSA. The organism was resistant to penicillin, cefoxitin (a surrogate marker for methicillin), and gentamicin; moderately sensitive to ciprofloxacin; and sensitive to cotrimoxazole, tetracycline, chloramphenicol, erythromycin, clindamycin, vancomycin, teicoplanin, and linezolid. The D-test for inducible clindamycin resistance was negative. E-strips were used for vancomycin and teicoplanin sensitivity. (Figure 5, 6).
The concerned care team was immediately alerted about the report. Appropriate contact precautions and isolation measures were also implemented. The patient was then switched to oral vancomycin and intravenous clindamycin, following which he started showing signs of improvement.
Soon after, the fever spikes settled, with symptomatic improvement, manifested by a decrease in breathlessness and coughing. Repeat urine ketone tests were negative. Capillary blood glucose tests performed at this point showed improved glycemic control at 126 mg/dL. Vitals were as follows: oxygen saturation on room air, 97%; pulse rate, 96/min; blood pressure, 140/70 mm Hg; and respiratory rate, 16/min. Repeat chest radiography showed reduced pulmonary infiltrates, suggestive of improvement. The Mantoux test performed at this point was negative. The patient improved symptomatically and was discharged with oral antibiotics. He was asked to review after a week at the outpatient department.
The first identification of MRSA occurred in a hospital in the United Kingdom in the early 1960s. This occurred shortly after the discovery of methicillin. Initially, MRSA was identified as a nosocomial-acquired pathogen, with most strains being highly resistant to most antibiotics. These were named hospital-acquired MRSA (HA-MRSA) infections.9 Later, between the 1980s and 1990s, several cases of MRSA were reported in young children and healthy adults who previously had no significant history of hospitalization. These isolates are now known to cause CA-MRSA infections.9 These strains are said to have better antibiotic sensitivity than the highly resistant HA-MRSA strains.
CA-MRSA and HA-MRSA are differentiated in many ways, such as their method of acquisition (community/hospital), their antibiotic sensitivities, and more recently, by genotyping techniques.10 Specific genes like Staphylococcal chromosomal cassette mec (SCCmec) gene types 1, 2, and 3 are responsible for conferring methicillin resistance in HA-MRSA strains, while it is SCCmec types 4 and 5 in CA-MRSA strains. The SCCmec genes carried by HA-MRSA strains confer additional resistance to more antibiotics like the non-beta lactams, and this accounts for the increased sensitivity of CA-MRSA isolates to antimicrobial agents compared with HA-MRSA isolates.11 Newer strains, such as vancomycin-sensitive Staphylococcus aureus (VSSA), vancomycin-resistant Staphylococcus aureus (VRSA), vancomycin-intermediate Staphylococcus aureus (VISA), and heterogeneous, vancomycin-intermediate Staphylococcus aureus (hVISA), have also been identified.12
The CDC definition of CA-MRSA infection is as follows: positive MRSA cultures isolated within 48 hours of admission to the hospital, no presence of indwelling catheters or medical devices that are permanently placed in the skin, no prior history of MRSA colonization, and no significant history of hospitalization in the recent past.13 In this case, we isolated an MRSA strain from the patient’s pleural fluid, which was sent for culture and antibiotic-sensitivity testing. The isolated MRSA strain satisfied all the above-mentioned criteria and was identified as a CA-MRSA strain and not HA-MRSA. Antibiotic susceptibility findings also substantiated this. Appropriate contact precautions for both healthcare workers and patient attendees and strict isolation measures for patients were carried out.
The patient’s clinical outcome was complicated by other significant findings such as parapneumonic effusion, diabetic ketoacidosis, and NSTEMI, all of which were identified only after admission to the hospital. The patient was immediately started on oral vancomycin and IV clindamycin, which is the recommended treatment of choice for MRSA infections. Linezolid was reserved for the worsening cases.14 Although our patient responded well and improved following treatment, the severity of CA-MRSA infections can be assessed using factors such as the need for ICU admission, the presence of necrotizing pneumonia with infiltrations, and the presence of any impending emphysematous changes.15
Although our patient initially required ICU admission and care, he improved clinically when treated with appropriate antibiotics, after which he was transferred to the hospital ward. The patient did not respond well to cefoperazone sulbactam and piperacillin/tazobactam combinations, thereby proving that for every organism causing infection and sepsis, treatment must be initiated with the appropriate antibiotics of choice. This is aided by the microbiology laboratory, which performs and reports antibiotic sensitivity testing. This necessitates the need for early antimicrobial susceptibility testing and reporting and the use of suitable antibiotics, which will ultimately help improve the patient’s clinical condition and could also be lifesaving in some instances.16
It is necessary to acknowledge that CA-MRSA strains can lead to lethally dangerous pneumonia, even in previously immunocompetent individuals. Therefore, the best solution would be to recognize this infection as early as possible by considering MRSA infections in a clinical picture of pneumonia and timely antimicrobial therapy with appropriate antibiotics to improve the clinical prognosis of the disease.17
ACKNOWLEDGMENTS
The authors would like to thank Dr. Gurubharath I, Department of Radiology, Chettinad Hospital and Research Institute, Tamil Nadu, India, for helping us obtain the radiological images.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
SR and PS contributed in conceptualization, study design, literature review, data collection, data analysis, interpretation, writing original draft and editing. MN contributed in data collection. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Human Ethics Committee for Faculty Research (CARE IHEC-II), Chettinad Academy of Research and Education, Tamil Nadu, India (IEC:189/2020).
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Ananthanarayan R, Paniker C. Textbook of Microbiology. 11th ed. Orient Longman. 1980.
- Clinical and Laboratory Standards Institute. M100 Performance Standards for Antimicrobial Susceptibility Testing. CLSI, Wayne, PA. 2021;31(1).
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 9.0, 2019. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/
Breakpoint_tables/v_9.0_Breakpoint_Tables.pdf - Hassoun A, Linden PK, Friedman B. Incidence, prevalence, and management of MRSA bacteremia across patient populations-a review of recent developments in MRSA management and treatment. Crit Care. 2017;21(1):211.
Crossref - Indian Network for Surveillance of Antimicrobial Resistance (INSAR) group, India. Methicillin resistant Staphylococcus aureus (MRSA) in India: prevalence & susceptibility pattern. Indian J Med Res.
Crossref - Loewen K, Schreiber Y, Kirlew M, Bocking N, Kelly L. Community-associated methicillin-resistant Staphylococcus aureus infection: Literature review and clinical update. Can Fam Physician. 2017;63(7):512-520. Erratum in: Can Fam Physician. 2017;63(8):596. PMID: 28701438.
- Xia H, Gao J, Xiu M, Li D. Community-acquired pneumonia caused by methicillin-resistant Staphylococcus aureus in a Chinese adult: A case report. Medicine. 2020;99(26):e20914.
Crossref - Yonezawa R, Kuwana T, Kawamura K, Inamo Y. Invasive Community-Acquired Methicillin-Resistant Staphylococcus aureus in a Japanese Girl with Disseminating Multiple Organ Infection: A Case Report and Review of Japanese Pediatric Cases. Case Rep Pediatr. 2015;2015:291025.
Crossref - Martino JL, McMillian WD, Polish LB, Dixon AE. Community-acquired methicillin-resistant Staphylococcus aureus pneumonia. Respir Med. 2008;102(6):932-934.
Crossref - Otto M. Community-associated MRSA: what makes them special? Int J Med Microbiol. 2013;303(6-7):324-330.
Crossref - Kateete DP, Bwanga F, Seni J, et al. CA-MRSA and HA-MRSA coexist in community and hospital settings in Uganda. Antimicrob Resist Infect Control. 2019;8:94.
Crossref - Olson DP, Soares S, Kanade SV. Community-acquired MRSA pyomyositis: case report and review of the literature. J Trop Med. 2011;2011:970848.
Crossref - Sowash MG, Uhlemann AC. Community-associated methicillin-resistant Staphylococcus aureus case studies. Methods Mol Biol. 2014;1085:25-69.
Crossref - Goundan PN, Mehrotra A, Mani D, Varadarajan I. Community acquired methicillin-resistant Staphylococcus aureus pneumonia leading to rhabdomyolysis: a case report. Cases J. 2010;3:61.
Crossref - Salgado CD, Farr BM, Calfee DP. Community-Acquired Methicillin-Resistant Staphylococcus aureus: A Meta-Analysis of Prevalence and Risk Factors. Clin Infect Dis. 2003;36(2):131-139.
Crossref - Alshengeti A, Alamri R, Tharwat R, et al. An Unusual Presentation of Community-Acquired Methicillin-Resistant Staphylococcus aureus Infection in a Child Treated With Linezolid. Cureus. 2021;13(10):e18830.
Crossref - Rutenberg D, Zhang Y. MRSA complicating a parapneumonic effusion despite timely antibiotics. CHEST J. 2020;157(6):A108.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.