ISSN: 0973-7510
E-ISSN: 2581-690X
The prevalence of Dermatophytosis is similar in several continents of the Asian country. The varied state of weather is favourable for mycosis that may lead to various clinical symptoms and infection spreads speedily if left untreated with time. The aim of the present study is to determine the clinical and mycological pattern of dermatophyte infections in patients arriving at our dermatology outpatient department and also to correlate the formal clinical diagnosis with KOH positivity and culture positivity. This descriptive observational study was conducted among 300 patients presenting with dermatophyte infections who came to the dermatology outpatient Department of SRM Medical College and research centre, Kattankulathur, Chengalpattu. The clinical specimens were put through direct microscopy by Potassium Hydroxide (KOH) mount and with the culture on Sabouraud’s Dextrose Agar (SDA). The study showed males were affected mostly and most of the participants were students. The common symptoms observed were Itching, Scaling and Discolouration. Commonly, patients had a primary diagnosis of Tinea corporis infection followed by T. cruris. Trichophyton mentagrophytes were the commonly isolated organism followed by Trichophyton rubrum and Trichophyton violaceum. This study indicates the need for personal hygiene and the disadvantage of as only few participants had zoophilic infections of Tinea species. These methods of diagnosing and identification will further aid in better patients management.
Tinea cruris, Tinea corporis, Trichophyton Mentagrophytes, Dermatophytic Infections
Dermatophytosis is one of the most common diseases in the world, caused by the dermatophytic fungi such as Trichophyton, Microsporum, and Epidermophyton colonising keratinized tissue of the hair, skin, and nails.1,2 Infections of the foot are the most frequent type of dermatophytosis, with an estimated lifetime risk of 10-20% worldwide. They were commonly known as ringworm or Tinea and obstructed to the non-living corniculate layers due to their incapability to go deeper in the immunecompetent host.3,4 Pruritis was found to be the commonest indication which was seen in humans with dermatophyte infection as it depends on the host’s reaction and environmental components. Also, the infection may occur from mild forms like seborrheic dermatitis to critical forms like favus.5,6
The epidemiological shift in dermatophyte growth patterns that gives them an advantage of better survival and persistence, an evolution in the genetic makeup of the fungi that increases their virulence and pathogenicity, and the rapid emergence of drug-resistant species as a result of the widespread use of insufficient doses of potent antifungals are all possible causes of the rising trend of recalcitrant dermatophytoses.7
These infections can be confused with other skin disorders; thus, a preliminary laboratory diagnosis is useful at the beginning of the treatment.8 Although most of the dermatophytic infection is non-interventive and straight forward to cure, its extensive universal nature, social embarrassment, disablement of quality of life and harm to the lucrative standing because of the price of the treatment square measure major public considerations.9 The aim of the present study is to determine the clinical and mycological pattern of dermatophyte infections in patients arriving at our dermatology outpatient department and also to correlate the formal clinical diagnosis with KOH positivity and culture positivity.
It was an illustrative study which was taken to estimate the clinical-mycological profile of dermatophytic infections. The specimens were gathered from January 2019 to June 2020 from the individuals who are clinically diagnosed with Dermatophytosis. Ethical approval was obtained from the Institute Ethical Committee (Human Studies) on 2nd February 2017 (1138/IEC/2017). Patients who have applied any kind of topical medications including steroids, Antifungals, antibacterials, antiseptics, native medications for the past one month or on current use are excluded.
An Informed and written consent form has been acquired from all the study participants in this current investigation. In case of children, permission was taken from their parents. A detailed history was taken with emphasis on age, gender, occupation, site, type, duration of symptoms, personal hygiene, type of clothing, footwear habits, associated diseases, sharing of Fomites, contact with pets, history of recurrence, history of similar previous episodes, duration of previous episodes, treatment taken from either over the counter or physician or dermatologist, duration of treatment taken, presence of any symptom free period were all noted.
Sample collection
The samples were collected depending on the site of infection. If it is skin, scrapings from the edge of the lesion and clipping of nail and under the nail debris in case of nail infection were collected. Impaired hair was plucked from the base using clean aseptic forceps along with scrapings. Specimens from infected sites were collected aseptically and cleaned the area using 70% isopropyl alcohol and allowed to dry. A sample of the skin was scrapped from the active fringe of the lesion; clippings of nails and subungual junk were gathered and infected hair with the base was taken for examination. The direct microscopic examination of hair and skin was processed in 10% Potassium hydroxide solution and for nails in 40% Potassium hydroxide solution to search for arthrospores.
Isolation of dermatophytes on culture
The dermatophytic culture was separated into a group of tubes of Sabouraud’s dextrose agar (SDA) where a set was mixed with chloramphenicol (0.05mg/ml) and another with chloramphenicol and cycloheximide (0.5mg/ml). One will be incubated at 25°C where as the other at 37°C for 4 weeks and ascertained twice a week. Those grown were observed for the growth rate of colonies, megascopic characters like pigment and texture.10-12
Species identification
Macroscopic characters were observed through lactophenol cotton blue stain and micro-slide culture. Identification will be finalized based on various arrangements of hyphae (coiled, pencil, spindle or club form, their septate, nodular organ), the agamogenesis pattern and the presence of microconidia and macroconidia. Biochemical identification tests such as urea hydrolysis and hair perforation are performed wherever needed. Tubes were examined for the presence of growth after 4 weeks and the results were recorded.
Statistical analysis
Descriptive analysis is dispensed through the mean and variance for quantitative, proportion and frequency for unconditioned variables. Data collected were plotted through suitable diagrams like a pie and diagram and box plots. The coalition between diagnosis, culture & KOH positive was evaluated by cross tabulation and percentage comparison. Chi square test is used to examine the analytical significance. P values< 0.05 were taken as importance. IBM SPSS version 13 was used for arithmetical analysis.
In this study, the mean age was 28.47 ± 15.23 (Table 1) with 194 (64.70%) male and 106 (35.50%) female participants. Out of 300 participants, 26 (8.70%) were agriculturists, 141(47%) participants were students, 47 (15.70%) participants were housewives, 36 (12%) participants were a labourer and 50 (16.70%) participants were others. Itching, scaling and discoloration are the most common symptoms with 114 (38.00%) participants having a recurrence and 81 (27%) participants had a similar family history. 131 (43.70%) participants had pets. Among the study population, 36 (12%) participants had type II diabetes mellitus, 3 (1%) participants had systemic hypertension, 4 (1.30%) participants had bronchial asthma, and 5 (1.70%) participants had hypothyroidism.
Table (1):
Descriptive analysis of age group in the study population (N=300)
Age group |
No. of cases |
Percentage |
|---|---|---|
Up to 10 |
21 |
7.0% |
11 to 20 |
99 |
33.00% |
21 to 30 |
78 |
26.00% |
31 to 40 |
39 |
13.00% |
41 to 50 |
34 |
11.30% |
51 to 60 |
13 |
4.30% |
61 and above |
16 |
5.30% |
About 16% of participants had a groin infection. The proportion of scalp, Abdomen+ Groin, Abdomen +Back, face and abdomen were 8.3%, 7.3%, 8%, 6.7% and 6%, respectively. The majority of the study participants had infections caused by Tinea corporis, as it accounts for about 31.30%, followed by T.cruris (16%), T.capitis (8.30%), T.faciei (6.70%), and T.pedis (5%) respectively. Among the Tinea corporis infected individuals, 83 (27.7%) participants had an annular type, 5 (1.7) participants had an eczematous type, 4 (1.3%) participants had a Black dot tinea, and 1 (0.3%) participant had crusted type and psoriasiform type. Among the Tinea capitis, 13 (4.3%) participants had black, 8 (2.7%) participants had a grey patch, and 4 (1.3%) participants had kerion.
Among the Tinea barbae, 7 (2.3%) participants had a superficial type, and 4 (1.3%) participants had a Circinate type. Among the Tinea pedis infected individuals, 11 (3.7%) participants had a chronic intertriginous type, and 4 (1.3%) participants had a vesicular or vesiculobullous type. Among the Tinea manuum infected individuals, 9 (3%) participants had non-inflammatory squamous form. Among the study population, 48 (16%) participants had Tinea cruris. Among the study population, 20 (6.7%) participants had Tinea faciei. Among the mixed type, majority proportion 8.7% participants had an Annular type (Tinea corporis) + Tinea cruris. The proportion of Annular type (Tinea corporis) + chronic intertriginous type (Tinea pedis) and Tinea cruris, chronic intertriginous type (Tinea pedis) was 8.7% and 2%, respectively (Table 2).
Table (2):
Descriptive analysis of morphological patterns with the site of involvement of the disease in the study population (N=300)
Subtype |
No. of cases |
Percentage |
|---|---|---|
Tinea corporis (N=94) |
||
Annular type |
83 |
27.7% |
Eczematous type |
5 |
1.7% |
Plaque type |
4 |
1.3% |
Crusted type |
1 |
0.3% |
Psoriasiform type |
1 |
0.3% |
Tinea Capitis (N=25) |
||
Black Dot |
13 |
4.3% |
Grey patch |
8 |
2.7% |
Kerion |
4 |
1.3% |
Tinea barbae (N=11) |
||
superficial type |
7 |
2.3% |
Circinate type |
4 |
1.3% |
Tinea pedis (N=15) |
||
chronic intertriginous type |
11 |
3.7% |
vesicular or vesiculobullous type |
4 |
1.3% |
Tinea unguium (N=14) |
||
DLSO |
11 |
3.7% |
Total dystrophic onychomycosis |
3 |
1.0% |
Tinea manuum (N=9) |
||
Non inflammatory Squamous form |
9 |
3.0% |
Tinea cruris(N=48) |
48 |
16.0% |
Tinea faciei (N=20) |
20 |
6.7% |
Mixed types |
||
Annular type (Tinea corporis) + Tinea Cruris |
26 |
8.7% |
Annular type (Tinea corporis) + chronic intertriginous type (Tinea pedis) |
6 |
2.0% |
Tinea cruris, chronic intertriginous type(Tinea pedis) |
6 |
2.0% |
Tinea cruris + Non inflammatory Squamous form (Tinea manuum) |
4 |
1.3% |
Non inflammatory Squamous form (Tinea manuum)+ DLSO(Tinea unguium) |
4 |
1.3% |
chronic intertriginous type (Tinea pedis) +DLSO(Tinea unguium)) |
4 |
1.3% |
Tinea faciei + superficial type (Tinea barbae) |
3 |
1.0% |
plaque type (Tinea corporis) + Tinea cruris |
3 |
1.0% |
Annular type (Tinea corporis) + Tinea cruris + DLSO(Tinea unguium)) |
3 |
1.0% |
Psoriasis form (Tinea corporis) + Tinea cruris |
2 |
0.7% |
Tinea faciei + Inflammatory type (Tinea barbae) |
2 |
0.7% |
Tinea faciei + circinate type (Tinea barbae) |
1 |
0.3% |
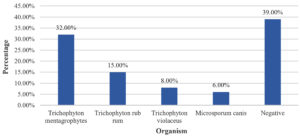
Among the study population, 156 (52%) participants had KOH positive + Culture positive, 27 (9%) participants had KOH negative + Culture positive, 104 (34.70%) participants had KOH positive + Culture negative and 13 (4.30%) participants had Culture + KOH negative (Table 3). Amidst the study population, 96 (32%) participants had Trichophyton mentagrophytes, 45 (15%) participants had Trichophyton rubrum, 24 (8%) participants had Trichophyton violaceus, and 18 (6%) participants had Microsporum Canis (Figure).
Table (3):
Descriptive analysis of KOH and culture in the study population (N=300)
KOH and culture |
No. of cases |
Percentage |
|---|---|---|
KOH positive+culture positive |
156 |
52.00% |
KOH negative+culture positive |
27 |
9.00% |
KOH positive+culture negative |
104 |
34.70% |
KOH negative + culture negative |
13 |
4.30% |
The prevalence of Dermatophyte infection differs with totally divergent geographic region and weather. Despite in depth development in life science, fungal infection is the rifest disease that includes individuals of any cohort, sex and working area and causes monumental money and emotional disturbances. Fungal infection continues to be thought-about collectively as the main public health issue in several components of the planet. The current study was conducted with 300 dermatophytosis patients who were clinically diagnosed, to study the clinical and the mycological patterns of dermatophyte infections. This study also assessed the correlation of clinical diagnosis with both culture and KOH positivity.
In the present study, the majority of the study participants were aged between 11 to 20 years (33%) followed by 21 to 30 years (26%). Our study findings were comparable with that of Singh et al. who have reported people in the age group of 16-30 years to be the commonly affected group by superficial dermatophyte infections.13 The bulk of the literature shows a majority of the affected subjects to be in the age range of 20 to 40 years, with relatively minor proportion in the paediatric and elderly population groups. This can be attributed to the involvement of the economically productive age groups in physical labour, often in the hot and humid environments leading to excessive sweating and wet body surfaces
The most affected population was students, this condition was because of the undeveloped immune system, progressive exhibition to in apparent suppuration carried in the home and school, incapability to sustain hygiene, which makes them prone to get current infections by Fungi. Like our study Kucheria et al. shown that a larger no. of patients were students (28%) and housewives (25%) followed by labourers and service class people.14 But contrary to our study, Pandit et al. have reported that majorly 32.6% of patients were housewives followed by labours (20%) and the proportion of students was only 14%.15
In our study, 38.00% of study participants had a recurrence. Deepasri et al. study also reported nearly similar finding where recurrence was seen in 31.3% of cases.16 There are very few studies, which have reported the recurrence of infection in the literature. There is a clear need to conduct more studies on this aspect to understand the factors associated with recurrence so that effective measures can be taken to prevent it. Improper treatment often received over the counter, inadequate treatment, poor compliance of the affected subject are the factors which can be strongly associated with recurrence.
KOH and culture results suggest that 54% (n=156) of the cases were both KOH positive and culture positive. About 34% of the cases were KOH positive and culture negative followed by 9% showed KOH negative and culture positive results and 4.30% of the study participants showed KOH negative and culture negative results. Therefore, the present study findings were in accordance with the study findings of Deepasri et al. which reported that nearly 63% of the cases were both KOH positive and culture positive.16
Among the study population, type II diabetes mellitus, has been most frequent disease association, in accordance with Kucheria et al. study, whose findings showed that 30% of the dermatophytosis were diabetic patients.14 In the present study majority proportion, 31.30% participants had Tinea corporis. In a study by Das et al., Tinea corporis (21.4%), onychomycoses (14.7%) and Tinea capitis (6.2%) were the most common in laboratory diseases.17 In a study by Maraki et al Tinea unguium followed by Tinea pedis were the commonly sites of dermatophyte infections in contrast to the current study findings.18
Our study findings were contrary to some of the existing studies, in Kucheria et al. Trichophyton rubrum (46.4%) was the commonest dermatophyte isolated followed by Trichophyton mentagrophytes (30.4%). In a study by Maraki S et al, Trichophyton rubrum was the very often segregated dermatophyte amounting 48% of the infections, continued by Microsporum canis (17.9%), Trichophyton mentagrophytes var. inter digitale (14.2%) and Epidermophyton floccosum (6%). Gupta et al. have reported that the very commonly isolated organism was Trichophyton verrucosum (35.5%).
The proportionof Trichophyton mentagrophytes was 30.8%, and Trichophyton rubrum was 20.8% to the current study findings.18,19 Even though there are variations in the relative proportion of different fungi contributing to the overall dermatophyte infection, Trichophyton rubrum and Trichophyton mentagrophytes were indisputably the two common fungi responsible. Observation of the findings reported by studies from India, conducted over last 10 to 15 years shows that Trichophyton mentagrophytes is gradually replacing, Trichophyton rubrum as the most common fungus isolated. The changing demographic profile of the affected population, changes occurring in the fungi, the pattern of drug therapies and susceptibility of fungi to them may be responsible for this changing trend.18 This study provides details on the present state of the illness in a specific region. The epidemiology of dermatophytoses may alter over time. We now understand more about the mycological pattern of dermatophytoses in this area because of this study.
There may be a geographical variation among the dispersal of dermatophyte species which are visible from studies from distinct places of India. Our study shows Trichophyton mentagrophyte to be the chief organism to cause dermatophytosis. A toxicological shift in the type of fungus has been perceived by so many researchers within the era of dermatophytosis. Preliminarily, Trichophyton rubrum was very common organism, whereas now, Trichophyton mentagrophytes is isolated as the common species. This is the cause of recurrent and recalcitrant dermatophytosis. The dermatologists and different clinicians treating the dermatophyte infections has to be sensitized regarding the dynamic profile of fungi inflicting dermatophytes and acceptable ways of diagnosis and management.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institute Ethical Committee, SRM Medical College Hospital & Research Centre, India, with reference number 1138/IEC/2017.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Bhatia VK, Sharma PC. Epidemiological studies on Dermatophytosis in human patients in Himachal Pradesh, India. Springerplus. 2014;3:134.
Crossref - Balakumar S, Rajan S, Thirunalasundari T, Jeeva S. Epidemiology of dermatophytosis in and around Tiruchirapalli, Tamilnadu, India. Asian Pac J Trop Dis. 2012;2(4):286-289.
Crossref - Dei Cas E, Vernes A. Parasitic adaptation of pathogenic fungi to mammalian hosts. Crit Rev Microbiol. 1986;13(2):173-218.
Crossref - King RD, Khan HA, Foye JC, Greenberg JH, Jones HE. Transferrin, iron, and dermatophytes. I. Serum dematophyte inhibitory component definitively identified as unsaturated transferrin. J Lab Clin Med. 1975;86(2):204-212.
- Nweze EI. Dermatophytosis in Western Africa: a review. Pak J Biol Sci. 2010;13(13):649-656.
Crossref - Begum J, Mir NA, Lingaraju MC, Buyamayum B, Dev K. Recent advances in the diagnosis of dermatophytosis. J Basic Microbiol. 2020;60(4):293-303.
Crossref - Singh BSTP, Tripathy T, Kar BR, Ray A. Clinicomycological Study of Dermatophytosis in a Tertiary Care Hospital in Eastern India: A Cross-sectional Study. Indian Dermatol Online J. 2019;11(1):46-50.
Crossref - Mushtaq S, Faizi N, Amin SS, Adil M, Mohtashim M. Impact on quality of life in patients with dermatophytosis. Australas J Dermatol. 2020;61(2):e184-e188.
Crossref - Vanapalli S, Turpati NR, Gopal KVT, Krishnam Raju PV, Devi BG. A Clinico-mycological, Antifungal Drug Sensitivity and Therapeutic Study of Extensive Dermatophytosis in Coastal Andhra Pradesh. Indian Dermatol Online J. 2022;13(6):747-753.
Crossref - Osman M, Kasir D, Rafei R, et al. Trends in the epidemiology of dermatophytosis in the Middle East and North Africa region. Int J Dermatol. 2022;61(8):935-968.
Crossref - Verma SB, Panda S, Nenoff P, et al. The unprecedented epidemic-like scenario of dermatophytosis in India: II. Diagnostic methods and taxonomical aspects. Indian J Dermatol Venereol Leprol. 2021;87(3):326-332.
Crossref - Rokas A. Evolution of the human pathogenic lifestyle in fungi. Nat Microbiol. 2022;7(5):607-619.
Crossref - Singh S, Beena PM. Profile of dermatophyte infections in Baroda. Indian J Dermatol Venereol Leprol. 2003;69(4):281-283.
- Kucheria M, Gupta SK, Chhina DK, Gupta V, Hans D, Singh K. Clinico-mycological profile of dermatophytic infections at a tertiary care hospital in North India. Int J Commun Health Med Res. 2106;2(2):17-22.
Crossref - Pandit VS, Mehta H. A hospital based cross-sectional clinical and mycological study of dermatophytoses in a tertiary care centre. J Pak Assoc Dermatol. 2017;27(4): 375-380
- Deepasri1 A, Maheswari PKU, Mangayarkarasi V. Clinical and species profile of dermatophytosis in Tertiary Healthcare Centre. Int J Curr Microbiol Appl Sci. 2018;7(5):2221-2225.
Crossref - DAS S, Goyal R, Bhattacharya SN. Laboratory-based epidemiological study of superficial fungal infections. J Dermatol. 2007;34(4):248-253.
Crossref - Maraki S, Nioti E, Mantadakis E, Tselentis Y. A 7-year survey of dermatophytoses in Crete, Greece. Mycoses. 2007;50(6):481-484.
Crossref - Gupta AK, Mohan A, Singh SK, Pandey AK. Studying the clinic mycological pattern of the dermatophytic infection attending OPD in Tertiary Care Hospital in eastern uttar pradesh and bihar. Int J Res Dermatol. 2018;4(2).
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.