ISSN: 0973-7510
E-ISSN: 2581-690X
Acute Febrile illnesses are caused by diverse microbial pathogens and are associated with significant morbidity and mortality. The methodical evaluation of area-specific etiologies of AFI helps in the rapid diagnosis, treatment and prevention and control strategies. To investigate the causes of AFI, their clinical spectrum and the seasonal trend through the year. A facility based prospective study was conducted in a tertiary care hospital in Kolar, Karnataka, South India; between January 2016 to December 2016. A total of 432 patients with AFI were enrolled and screened for specific etiological agents. The etiological agents were identified in 351 (81.25%) of AFI cases. The etiological agent could not be established in 81(18.75%) of cases. Dengue was the most common cause of AFI (52.1%) followed by Scrub typhus (9.02%), Leptospirosis (6.48%), Chikungunya (5.32 %), Enteric fever (4.16%), Malaria (3.93%) and Brucellosis (0.23%). The most common symptoms reported by enrolled patients included headache (93.1%), malaise (89.4%), joint pain (80%), chills (73.7%). In our study, AFI occurred most commonly during rainy and autumn seasons accounting for 272 (77.5%) cases. Laboratory based syndromic data of AFI can make clinicians vigilant regarding the potential pathogens in local area and further aid to establish the epidemiologic database of different etiologies.
Acute febrile illness, Dengue, Scrub typhus
Acute Febrile illness (AFI) is associated with significant morbidity and mortality in developing countries. AFI is caused by diverse microbial pathogens. In developed countries AFI is often due to viral etiological agents; whereas in the developing countries, the etiologies of AFI includes potentially significant infections such as malaria, enteric fever, rickettsiosis, leptospirosis, brucellosis, dengue fever, chikungunya and Japanese encephalitis.1, 2
In addition to the great diversity of the pathogens, the burden of AFI is compounded by limited resources, low immunization rates and poor public health control measures. Further, AFI have similar signs and symptoms (i.e., fever, headache, joint pain, myalgia and splenomegaly) without any focal point of infection, making its diagnosis and management a very challenging affair. 3 Although the clinical management guidelines for acute febrile illness are available these are rarely supported by knowledge of the locally prevalent causative agents. The infectious agents causing AFI varies by different regions suggesting that diagnosis and management needs to be based on a methodical evaluation of area-specific etiologies.
The use of laboratory-based syndromic surveillance can alert clinicians and also provide the knowledge of common infectious etiologies circulating in the given region during a specific time period.4 Thus, it is important to maintain a proper epidemiological data of AFI which helps us in generating an evidence-based diagnosis and effective treatment guidelines as most of them are preventable deaths.
With this background, a hospital based observational study was carried out to investigate the causes of AFI, their clinical spectrum and the seasonal trend through the year.
A facility based prospective study was conducted in a tertiary care hospital in Kolar, Karnataka, South India; between January 2016 to December 2016.
All adult patients more than 18 years of age with fever lasting for less than 2 weeks with no evident focus of infection following initial clinical evaluation were included in the study. Patients with chronic illness, autoimmune disorders, bone marrow hypoplasia, malignancies, on immunosuppressant drugs, fever with definite source of infection like urinary tract infections; pneumonia, tuberculosis, and refusal to give informed consent were excluded from the study.
The study protocol was approved by the Institutional Ethical review committee and written informed consent was obtained from the patient. The detailed clinical history, physical examination findings and routine laboratory investigation results were recorded in the standardized format. The routine baseline investigations included complete blood count analysis, serum electrolytes, and liver and renal function tests.
Specific Investigations
Serological tests were performed at the discretion of the Physician advice based on the clinical picture. The serological tests were interpreted according to the manufacturer’s instruction as positive, equivocal, or negative. These included Dengue IgM Microlisa (J. Mitra & Co. Pvt Ltd, New Delhi, India), Scrub typhus IgM ELISA (InBios International, Inc., Seattle, WA, USA), leptospira IgM Microlisa (J. Mitra & Co. Pvt Ltd, New Delhi, India), IgM Chikungunya ELISA (J. Mitra & Co. Pvt Ltd, New Delhi, India) ,Widal test (Salmonella Antigen test, Arkray healthcare Pvt Ltd, Surat, Gujarat, India), Malaria rapid test (Advantage Mal card J. Mitra & Co. Pvt Ltd, New Delhi, India), Brucella Standard agglutination test (Micropath, Omega Diagnostics Ltd, Scotland, UK), and Weil felix test (Micropath, Omega Diagnostics Ltd, Scotland, UK).
Convalescent serological testing after 2-4 weeks was performed if the initial serological diagnosis was unclear and if the patient was willing. A 4-fold increase in titer from acute to convalescent sample, or a result change going from negative to positive, was considered indicative of seroconversions. A thin smear was also performed to detect malarial parasites. A single blood culture was obtained from patients in an aerobic BacT/Alert 3D (bioMerieux, Hazelwood, MO) bottle and incubated for up to 7 days in the BacT/Alert 3D blood culture system. All inpatients were followed up until discharge from the hospital.
Diagnostic criteria
Dengue
Clinical features of dengue with dengue IgM positive and other serologies and blood culture negative or seroconversion on convalescent sera
Scrub typhus
Clinical features of Scrub typhus + scrub IgM ELISA positive or Scrub IgM ELISA positive with other serologies and blood culture negative , Weil Felix test titres of OX K titres ≥ 1 : 160
Chikungunya
Clinical features of Chikungunya with Chikungunya IgM positive and other serologies and blood culture negative or seroconversion on convalescent sera
Leptospirosis
Clinical features of Leptospirosis with Leptospira IgM positive with other serologies and blood culture negative
Malaria
Clinical features of Malaria with rapid malaria card test or malaria parasites (Plasmodium falciparum, Plasmodium vivax or mixed) visualized on thin blood smears
Enteric fever
Clinical features of enteric fever with blood culture positive for Salmonella typhi or Salmonella paratyphi or 4-fold rise in titer on the Widal test in convalescent sera
Brucellosis
Clinical features of Brucellosis: with blood culture positive for Brucella Spp 4-fold rise in titer on the Standard Agglutination test in convalescent sera
Undiagnosed
After complete evaluation, a definitive diagnosis was not made. The patients who did not give convalescent sera or who had multiple serologies positive without fulfilling the diagnostic criteria.
Statistical Analysis
Data were entered using Microsoft Excel and analyzed using SPSS version 22. Distribution of AFI cases in the year and also seasonal variation were represented using line graphs. Clinical and lab parameters in each of the AFI cases were grouped according to the diagnosis and expressed as proportions (%).
During the study period of one year, a total of 487 patients presented to the hospital with AFI (both outpatient department and casualty). Of the 487 patients, 55 patients were excluded as they either refused to give consent for blood sample collection for confirmation of the diagnosis (16) or were loss to follow-up (39). Thus, a total of 432 patients with AFI were enrolled and evaluated as shown in Figure 1. Of these, 272 (63%) were males and 160 (37%) were females.

Fig. 1. Flow chart of patients presenting with AFI
The majority of the patients were from Karnataka (72.8%) and the neighboring states of Andhra Pradesh (18.4%) and Tamil Nadu (8.8%). Among 432 patients screened for specific etiological agents ,we could identify the etiological agents in 351 (81.25%) cases of AFI. Despite the extensive evaluation carried out, 81(18.75%) of AFI cases, the cause could not be determined as the serological tests were negative in 75 of patients and in six of them more than one serological test result was positive, probably due to cross-reactivity or may be due to dual infections. Dengue was the most common cause of AFI (52.1%) followed by Scrub typhus (9.02%), Leptospirosis (6.48%), Chikungunya (5.32 %), Enteric fever (4.16%), Malaria (3.93%) and Brucellosis (0.23%) as shown in Figure 1.
The most common symptoms reported by enrolled patients included headache (93.1%), malaise (89.4%), joint pain (80%), chills (73.7%) as shown in Table 1. The most common deranged hematological parameter was Thrombocytopenia (69.8%) followed by leucopenia (37.3%) and anemia (24.2%). The majority of thrombocytopenia is seen in patients with dengue fever. The Biochemical parameters also shows derangement of liver enzymes which are depicted in Table 2.
Table (1):
Clinical Parameters Associated With Common Aetiological Agents of Afi (all figures Mentioned as Percentage)
Parameters |
Leptospirosis N =28 |
Scrub Typhus N =39 |
Enteric Fever N =18 |
Chikungunya N = 23 |
Brucellosis N =1 |
Malaria N =17 |
Dengue N =225 |
Total N=351 |
|---|---|---|---|---|---|---|---|---|
Fever |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
Headache |
92.8 |
94.8 |
94.4 |
91.3 |
100 |
82.3 |
93.7 |
93.1 |
Malaise |
82.1 |
84.6 |
77.7 |
91.3 |
100 |
76.4 |
92.8 |
89.4 |
Chills |
67.8 |
35.8 |
11.1 |
60.8 |
100 |
64.7 |
88.0 |
73.7 |
Joint Pain |
14.2 |
30.7 |
38.8 |
100 |
100 |
41.1 |
99.1 |
80.0 |
Abd Pain |
64.2 |
15.3 |
94.4 |
13.0 |
0 |
23.5 |
6.2 |
17.6 |
Rash |
0 |
43.5 |
5.5 |
4.3 |
0 |
5.8 |
5.7 |
8.8 |
Jaundice |
92.8 |
10.2 |
61.1 |
13.0 |
0 |
29.4 |
1.7 |
17.9 |
Bleeding |
0 |
0 |
0 |
0 |
0 |
0 |
5.6 |
3.7 |
Splenomegaly |
0 |
71.7 |
88.8 |
0 |
100 |
88.2 |
1.7 |
17.9 |
Table (2):
Laboratory parameters associated with common aetiological agents of AFI(All figures mentioned as Percentage)
| Parameters | Leptospirosis N =28 | Scrub Typhus N =39 | Enteric FeverN =18 | Chikungunya N = 23 | Brucellosis N =1 | Malaria N =17 | Dengue N =225 | Total N=351 |
|---|---|---|---|---|---|---|---|---|
| Hematological | ||||||||
| Anaemia | 64.2 | 61.5 | 16.6 | 8.6 | 100 | 82.3 | 10.2 | 24.2 |
| Leucopenia | 42.8 | 35.8 | 88.8 | 13.0 | 100 | 52.9 | 33.7 | 37.3 |
| Thromocytopenia | 64.2 | 28.2 | 11.1 | 13.0 | 100 | 35.2 | 90.6 | 69.8 |
| Biochemical | ||||||||
| ALT | 92.8 | 35.8 | 44.4 | 13.0 | 100 | 35.2 | 14.2 | 25.6 |
| AST | 89.2 | 30.7 | 50.0 | 17.3 | 100 | 41.1 | 15.1 | 26.2 |
| ALP | 96.4 | 38.4 | 61.1 | 13.0 | 100 | 35.2 | 14.6 | 27.3 |
| Bilirubin | 92.8 | 33.3 | 38.8 | 8.6 | 100 | 41.1 | 9.3 | 21.9 |
| Albumin | 78.5 | 28.2 | 27.7 | 8.6 | 100 | 47.0 | 6.2 | 17.9 |
Seasons were classified as Summer (March to June), Rainy (July to September), Autumn (October to November) and Winter (December to February) according to the Indian Meteorological Department. In our study, the seasonal upsurge of AFI cases were observed and majority of AFI cases occurred in rainy (July, August, September) and Autumn seasons (October and November) accounting for 272 cases (77.5%) as shown in Figure 2, 3 and 4.
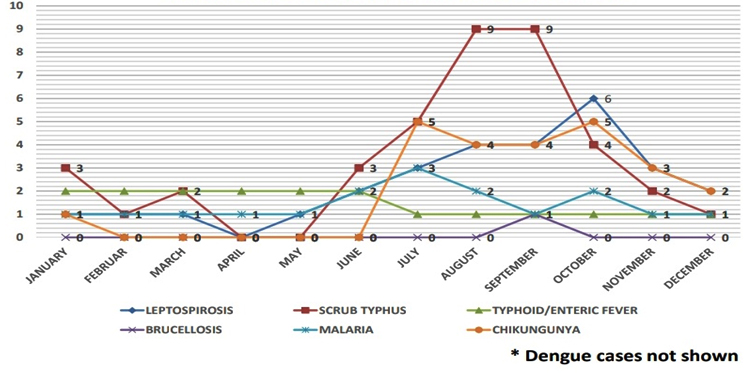
Fig. 2. Distribution of diagnosed AFI attending a tertiary care institute, Kolar in the year 2016*
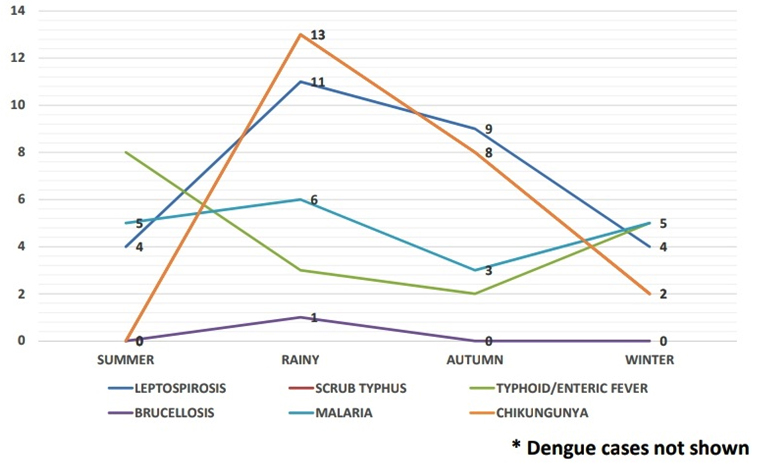
Fig. 3. Distribution of diagnosed AFI attending a tertiary care institute, Kolar in the year 2016*
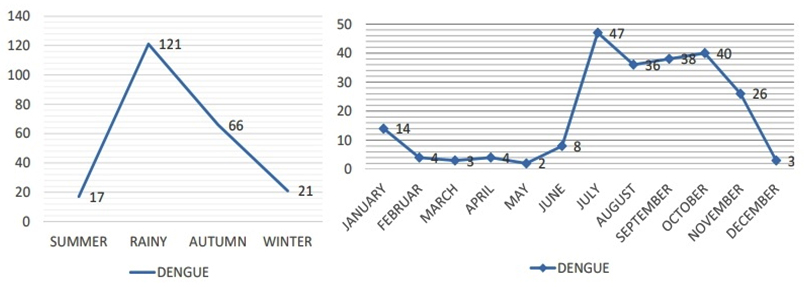
Fig. 4. Distribution of Dengue cases from a tertiary care institute, Kolar in the year 2016 (both seasonal and monthly distribution)
The causes of AFI are numerous and are region and country specific. AFI accounts for most of the preventable deaths in developing countries. AFI causes significant morbidity and mortality in developing countries. The majority of the patients in our study were males. The male preponderance may be due to more chance of exposure to vectors like mosquitoes and mites owing to their outdoor activities and also attributed to lower utilization of health care facilities by females due to socio-cultural reasons.5
Most of the patients were in the middle age group (20 – 45 years), in economically productive period during which they have a greater risk of having contact with contaminated environment.
Our study revealed that there is a heavy burden of dengue fever, followed by scrub typhus, leptospirosis, chikungunya, enteric fever, malaria cases in South Indian region. The previous regional studies had reported etiologies among patients with AFI, with some variations and distribution based on the sampled population or the pathogen diagnostic panel facilities. 6-10
Our finding was consistent with a similar study conducted by Garima Mittal et al., from a region of North India which showed that dengue was the most common cause of AFI followed by enteric fever, scrub typhus, malaria. A study by Abhilash K P et al. in 2013 from Southern India among inpatients had shown scrub typhus, dengue, malaria, and enteric fever to be the main etiologies.
We have identified the etiological agents in 81% of the cases. In the previous studies, the rates of identifying the etiology varied from 40% to 73.3%.11–13
In our study, the majority of the patients presented with non-specific symptoms, such as headache, malaise, joint pain, chills. A similar study conducted by Abhilash K P et al showed that the most common symptoms were generalized myalgia followed by headache, vomiting, abdominal pain and breathlessness. The symptoms and differential diagnoses of AFI are similar, often mimicking and making accurate clinical diagnosis is very difficult without laboratory confirmation.1,6,14
In our study, AFI occurred most commonly during rainy and autumn seasons accounting for 77.5% cases. The stagnant water as a result of rains compounded by poor drainage system in most of the areas in developing countries becomes a breeding ground for the mosquitoes helping them to transmit the diseases. Seasonal upsurge in fever is also a well-known documentation in previous studies.15, 16
The accurate diagnosis of AFI is complicated by a lack of knowledge of local pathogens, presence of similar signs and symptoms and unavailability of the extensive diagnostic panel leading to mismanagement of AFI cases. The infectious agents causing AFI varies by different regions suggesting that diagnosis and management need to be based on a methodical evaluation of area-specific etiologies based on the laboratory-based syndromic surveillance.
This study has few limitations. Our diagnostic testing panel did not cover the potential pathogens like H1N1, Hantan virus to name a few due to financial constraints and biohazard safety issues. Secondly, some patients did not turn up for follow up (second visit), so convalescent sera could not be obtained for confirmation of diagnosis. Thirdly, the study included only adult patients while pediatric cases would expect to have different febrile etiologies due to their different exposure and immunity status. So, the pattern of AFI observed in the present study could not be considered as representative cases of general population and thus cannot be generalized.
The similarity in clinical manifestations, diversity of etiological agents, lack of complete diagnostic panel poses a great challenge to Clinicians in diagnosis and management of AFI cases.
Laboratory based syndromic data of AFI can make clinicians vigilant regarding the potential pathogens in local area and further aid to establish the epidemiologic database of different etiologies of AFI in every region to anticipate epidemic preparedness in terms of resources and to provide quality health care facilities. This data can also be help to the local district health care officials to take action in major vector control and plan for prevention strategies.
- Kashinkunti M D, Gundikeri S K, Dhananjaya M. Acute undifferentiated febrile illness- clinical spectrum and outcome from a tertiary care teaching hospital of north Karnataka. Int J Biol Med Res. 2013; 4(3): 3399- 3402.
- Abhilash KP, Jeevan JA, Mitra S, Paul N, Murugan TP, Rangaraj A, David S, Hansdak SG, Prakash JA, Abraham AM, Ramasami P, Sathyendra S, Sudarsanam TD, Varghese GM. Acute undifferentiated febrile illness in patients presenting to a Tertiary Care Hospital in South India: clinical spectrum and outcome. J Global Infect Dis 2016; 8:147-54.
- Tadesse H and Tadesse.K. The etiology of febrile illnesses among febrile patients attending Felegeselam Health Center, Northwest Ethiopia. Amer J of Biomed Life Sci 2013; 1(3): 58-63.
- Matthew R K , Patrick J B, Sok T, Buth S, Yasuda C Y , Maya Williams et al. Infectious Etiologies of Acute Febrile Illness among Patients Seeking Health Care in South-Central Cambodia. Am J Trop Med Hyg 2012; 86(2): 246–253
- Andrews M A, Aleena E, Praveenlal K . Clinical profile of acute undifferentiated febrile illness in patients admitted to a teaching hospital in Kerala. Health Science, 2014; 1(3):1-8
- Garima Mittal , Sohaib Ahmad, RK Agarwal, Minakshi Dhar, Manish Mittal, Shiwani Sharma. Aetiologies of Acute Undifferentiated Febrile illness in Adult Patients – an Experience from a Tertiary Care Hospital in Northern India. J Clin Diag Res. 2015 Dec, Vol-9(12): DC22-DC24
- Joshi R, Colford JM, Reingold A. Nonmalarial acute undifferentiated fever in a rural hospital in central India – Diagnostic uncertainity and overtreatment with anti malarial agents. Am J Trop Med Hyg. 2008; 78(3):393-99.
- Thangarasu S, Natarajan P. A protocol for emergency department management of acute undifferentiated febrile illness in India. Int Journal of Emer Med. 2011; 4:57.
- Laudari S, Patowary B S, Nanda K, Dewan K, Subedi K, Toyana R et al. A study of pattern of acute febrile illnesses at COMS-TH, Bharatpur,Nepal.Asian Pac J Trop Dis 2014; 4(4): 297-300
- Chrispal A, Boorugu H, Gopinath KG, Chandy S, Prakash JA, Thomas EM, et al. Acute undifferentiated febrile illness in adult hospitalized patients: the disease spectrum and diagnostic predictors – an experience from a tertiary care hospital in South India. Trop Doctor.2010; 40(4):230-34.
- Mueller TC, Siv S, Khim N, Kim S, Fleischmann E, Ariey F, et al.Acute undifferentiated febrile illness in rural Cambodia: A 3-year prospective observational study. PLoS One 2014; 9:e95868
- Suttinont C, Losuwanaluk K, Niwatayakul K, Hoontrakul S, Intaranongpai W, Silpasakorn S, et al.Causes of acute, undifferentiated, febrile illness in rural Thailand: Results of a prospective observational study. Ann Trop Med Parasitol 2006; 100:363-70.
- Manock SR, Jacobsen KH, de Bravo NB, Russell KL, Negrete M, Olson JG, et al.Etiology of acute undifferentiated febrile illness in the Amazon basin of Ecuador. Am J Trop Med Hyg 2009; 81:146-51.
- Malavige GN, Fernando S, Fernando DJ, Seneviratne SL (2004) Dengue viral infections. Postgrad Med J80: 588–601.
- Jena B, Prasad MNV, Murthy S. Demand pattern of medical emergency services for infectious diseases in Andhra Pradesh – A geo-spatial temporal analysis of fever cases. Indian Emergency Journal. 2010; 1(5):821.
- Pappachan MJ, Sheela M, Aravindan KP. Relation of rainfall pattern and epidemic leptospirosis in the Indian state of Kerala. J Epidemiol Community Health 2004; 58:1054.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


