ISSN: 0973-7510
E-ISSN: 2581-690X
Because of the strong interest in the use of bio-products as alternatives to chemically derived antibiotics or antimicrobial agents, passion fruits extracts were evaluated for their antibacterial activity and chemical composition. Various solvent extracts of P. edulis were screened for their antibacterial activity by Agar well diffusion technique against an array of pathogenic bacteria (Gram positive and negative). Macro dilution technique was used to determine the Minimum inhibitory concentration of the potent extracts. Bacterial strain showing significant inhibition was further subjected to scanning electron microcopy (SEM) and the morphological changes induced by extracts were noted. Chemical composition of extracts showing strong antibacterial activity was determined by GC-MS and FTIR analysis. Extracts of Passion fruit (pulp with seeds) show significant inhibitory effects against test isolates but in a variable manner. Amongst all the test isolates Bacillus subtilis showed maximum inhibition followed by E. coli and P. aeruginosa. Ethyl acetate extracts had the least activity against the tested microorganisms. Gas chromatography-mass spectrometry of ethanol extracts showed the presence of important chemicals, such as Tetracosamethyl-cyclododecasiloxane; Dodecanoic acid, 10-methyl-, methyl ester cyclosiloxane, hexadecamethyl; 3-isopropoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris (trimethylsiloxy)tetrasil; 9-hexadecenoic acid, 9-octadecenyl ester, (Z,Z)- Fourier transform infrared studies revealed important functional groups which included phenols, esters, flavonoids, aromatic compounds, and alcohols. Significant antibacterial activity of the extracts could be attributed to phenolic compounds, esters and other chemical components identified in ethanolic extracts. Scanning electron micrographs of B. subtilis treated with ethanol extracts showed distorted shapes, rough and corrugated cell margins, and aggregations of cells. Our data depict the significant antimicrobial activity of extracts against Gram positive bacteria while the Gram negative bacteria exhibited weak inhibition by all the extracts. Based on our findings, passion fruits can be used in preparations of antimicrobial formulations against Gram positive microorganisms especially B. subtilis.
Passion fruit, pulp, Rind, scanning electron microscopy, Gas chromatography-mass spectrometry, Fourier transform infrared.
Plants serve as one the largest natural resource reservoirs. These resources have been explored for the presence of potent chemical compounds, applicable in the pharmaceutical, cosmetic, and nutraceutical industries. Yet, from a huge estimate of 250,000–500,000 plant species on Earth, only a very small fraction (1-10%) have been explored for compounds that may serve as antimicrobials1,2. In fact, medicinal plants have always occupied a vital place in our culture since time immemorial. Some Asian populations (80%) still depend on traditional medicines derived from plants 3. Moreover, recent research has discovered chemicals from plants, which possess promising antimicrobial properties that can serve as novel therapeutic compounds. The rise in antimicrobial resistance, has forced mankind to explore new alternatives with fewer irreversible side effects. The best solution to the menace of resistant microbial strains is herbal constituents that are easily available and affordable. Hence, this need has prompted us and various researchers worldwide to investigate plants and their products for their antimicrobial activity against resistant strains of pathogenic bacteria and fungi.
Passion fruit is an exotic tropical and subtropical fruit, belonging to the family Passifloraceae with an estimate of 500 species. Amongst the species, P. edulis, P. ligularis, and P. quadrangularis are chiefly cultivated for their edible fruit, economic importance, ornamental purpose, and medicinal properties4. P. edulis Sims is a perennial vine and growing at higher altitudes. It has two forms; the yellow fruit called the Passiflora edulis f. flavicarpa Deg. and the purple form referred to as Passiflora edulis f. edulis. The purple fruit is a native of Brazil, it is smaller in size with a strong aroma and is more acidic than the yellow type. Passion fruits are rich in vitamin C, A, niacin, and fiber. All the plant parts have medicinal properties and are used in various forms of herbal medicine. Dried flower and fruits are used to treat constipation, gastric ailments, as a digestive stimulant and treating gastric cancer 5 Boiled leaves are used to treat hypertension and chronic dysentery in some parts of India6.
Passion fruit and its peel have shown positive results in treating asthma, high blood pressure, menopausal symptoms, and osteoarthritis, and act as an excellent anti-inflammatory, anti-helminthic, sedative, and diuretic7-11.
For a few years, passion fruit has drawn the attention of many researchers because of its wide chemical composition. The purple fruit is not well studied for its antimicrobial properties and its chemical composition; hence, in the present research, we explore the antimicrobial efficacy of fruit pulp and peel extracts. Further, scanning electron microscopy of severely affected microbial strains will be conducted to understand the potency of extracts on cell morphology.
Plant material: Passion fruits
Fresh, disease- and injury-free fruits of Passiflora edulis f. edulis Sims (purple variety) were collected from a local market in Riyadh (Fig. 1). The fruits were washed with tap water followed by distilled water. Fruits were then cut, and the pulp was separated from the peel. Fruit pulp with the seeds and peels were freeze dried separately, and ground into fine powder with a mixer grinder. The powder was subjected to extraction.
Preparation of extracts
Pulp and powdered peels (20 g) were subjected to extraction with ethanol, methanol, acetone, and ethyl acetate (100 ml). Extracts were placed on a rotator shaker at 180 rpm for 72 h, after which they were filtered using a Whatman filter paper (No. 1). The filtrates were evaporated in a vacuum evaporator. The extracts were reconstituted in mother solvents and used for antibacterial and antifungal assays.
Microbial isolates
Passion fruit extracts were tested against selected human pathogenic microorganisms. Bacterial isolates included Gram-negative and gram-positive bacteria,namely Staphylococcus aureus ATCC 25923, Bacillus subtilis ATCC 6633, Escherichia coli ATCC 25966, Pseudomonas aeruginosa ATCC 27853, Enterococcus faecalis ATCC 29212, and Klebsiella pneumonia (hospital isolate). Fungal isolate screened was Candida albicans ATCC 60193. The tested microorganisms were provided by King Khalid Hospital, Riyadh-Saudi Arabia. Microbial isolates were pre-cultured on nutrient agar.
Antibacterial assay
Antibacterial activities of plant extracts were tested using the agar well diffusion technique12 with slight modifications. The culture plates were prepared by pouring 20 ml of Mueller Hinton (MH) agar medium into sterile Petri dishes. The inoculum suspension of 0.5 McFarland was prepared for all bacterial isolates to be tested and 200µl of this suspension was spread uniformly over the agar medium using sterile cotton swabs. With the help of a sterile cork borer of 6 mm diameter, wells were made equidistantly on the agar surface to be further loaded with 100µl of solvent extracts. Following loading of the extracts, plates were incubated for 24 h at 37°C. The inhibitory activity of these extracts was recorded by measuring the diameter of the inhibition zone (mm) formed around the well. Tetracycline (30µg) and ampicillin (10µg) discs were used for antibiotic sensitivity assays and the largest zone shown by the respective antibiotic was tabulated. Mother solvent served as a negative control, whereas the antibiotic disc was the positive control. Candida albicans was tested for its antifungal activity with Mueller Hinton agar along with bacterial isolates.
Minimum inhibitory concentration of extracts against bacteria
Minimum Inhibitory Concentration (MIC) for bacteria and yeast was determined by Broth tube dilution method with slight modification13,14. A double dilution of extracts (0.125mg/ml – 128 mg/ml) was prepared using Mueller Hinton Broth. Equal volumes of extract and broth were added to a sterile test tube followed by a fixed volume of Microbial cell suspension containing 5 × 105 CFU/ml of cells. This concoction was incubated for 24 h at 37°C . The lowest concentration that did not show any visible growth was regarded as its MIC. The experiment was performed in triplicates and their mean values were noted.
Gas chromatography-mass spectrometry (GC-MS) analysis
Ethanol extract was subjected to GC-MS analysis. GC-MS analysis was carried out on Clarus 500 Mass spectrometer and gas chromatography. Different parameters involved in the operation of the Clarus 500 MS were standardized as follows: mass spectra were taken at 70 eV; acquisition mode – scan 40-550 amu; ion source temperature 230°C; inlet line temperature 200°C; solvent delay time 5 min. Gas chromatography used in the analysis employed a fused silica column [100% dimethyl poly siloxane, 30 nm ׳ 0.25 nm ID ׳ 1µm df]. The column was packed with Elite-1. Helium was used as the carrier gas (1 ml/min). The extract (2µl) was injected into the instrument. The oven temperature program was 2 min at 45°C, 1.5°C/min to 100°C, and 2°C /min to 200°C during the GC extraction process; the split ratio was 25:1. The injector temperature was 250°C. The GC run time was 90 min. The identification of the phytocompounds and interpretation of the mass spectrum were performed with the aid of the standards database of the NIST libraries.
Fourier Transform Infrared (FTIR) Fingerprint Analysis
Fourier transform infrared (FTIR- Perkin Elmer 2000) spectrophotometer was used to identify the functional groups present in ethanol and acetone extracts. The extracts were centrifuged, filtered, diluted (1:10), and subjected to analysis in the scan range 400 to 4000 cm-1.
Bruker OPUS software was used to analyze the spectrum.
Scanning electron microscopy (SEM)
The SEM was performed as previously reported, with slight modification14 B. subtilis cell suspension at its MIC (0.25 mg/ml) treated with ethanol pulp extract was selected for the SEM analysis. The bacterial cell suspension was subjected to centrifugation (8,000 ׳ g for 10 min). Centrifuged cells were fixed by immersing in 2.5% glutaraldehyde and then washed with 0.1 mol/ L tris-acetate buffer (pH 7.2). Dehydrated samples were freeze-dried and observed by SEM (JEOL, Japan). Cells grown in an MH tube with no extract were used as a control.
The anti-microbial results of the extracts in this study are recorded in Tables 1 and 2. Pulp (with seeds) extracts were more effective than that of the peel. All the tested organisms showed variable sensitivity towards the extracts. However, we noted that the highest antibacterial activity was observed with the ethanol extract, particularly against Bacillus subtilis (24 mm), Escherichia coli (19 mm) and Pseudomonas aeruginosa (18 mm), followed by the acetone extract against P. aeruginosa (18 mm) and Bacillus subtilis (17 mm). Ethyl acetate had negligible effects on all the test organisms. On the other hand, Candida albicans was susceptible to both acetone and ethanolic extracts (18 mm and 17 mm) compared to the other organic extracts (Table 1). Peel extracts, however, did not exhibit significant antimicrobial activity, except for C. albicans which showed a maximum zone of inhibition (15 mm) with ethanol extracts.
Table (1):
Antibacterial activity of fruit pulp (with seeds) and zones of inhibition (mm).
Organism |
Acetone |
Methanol |
Ethanol |
Ethyl acetate |
Antibiotic |
|---|---|---|---|---|---|
Staphylococcus aureus |
15.00±0.81 |
14.66±0.94 |
17.00±1.63 |
0.00±0.00 |
29 mm(T) |
Bacillus subtilis |
17.33±0.94 |
16.00±0.00 |
24.66±0.47 |
10.33±0.47 |
30 mm(T) |
Escherichia coli |
14.00±0.81 |
13.00±1.41 |
19.00±0.81 |
0.00±0.00 |
32 mm(T) |
Pseudomonas aeruginosa |
18.66±0.47 |
10.00±0.81 |
18.33±1.24 |
9.00±0.00 |
30 mm(A) |
Klebsiella pneumoniae |
11.00±0.81 |
9.00±0.47 |
10.00±0.00 |
9.00±0.47 |
10 mm(A) |
Candida albicans |
17.00±0.00 |
15.00±0.81 |
17.33±0.94 |
0.00±0.00 |
19 mm(F) |
T-Tetracycline (30 µg), F-Fluconazole (25 µg), A- Ampicillin 40 μg/ml.
Values are means of three replicates and ± SD
Table (2):
Antibacterial activity of fruit peel and zones of inhibition (mm).
Organism |
Acetone |
Methanol |
Ethanol |
Ethyl acetate |
Antibiotic |
|---|---|---|---|---|---|
Staphylococcus aureus |
8.00±0.00 |
10.33±0.94 |
10.00±0.81 |
0.00±0.00 |
29 mm(T) |
Bacillus subtilis |
11.66±0.47 |
0.00±0.00 |
0.00±0.00 |
9.33±0.00 |
30 mm(T) |
Escherichia coli |
0.00±0.00 |
10.00±1.41 |
12.33±0.47 |
0.00±0.00 |
32 mm(T) |
Pseudomonas aeruginosa |
0.00±0.00 |
9.00±0.00 |
9.00±0.81 |
0.00±0.00 |
30 mm(T) |
Klebsiella pneumoniae |
0.00±0.00 |
10.00±1.00 |
0.00±0.00 |
11.33±0.94 |
24 mm(T) |
Candida albicans |
8.33±0.47 |
0.00±0.00 |
15.00±0.81 |
0.00±0.00 |
18 mm(F) |
T-Tetracycline (30 µg), F-Fluconazole (25 µg).
Values are means of three replicates and ±SD
Minimum inhibitory concentration
The MIC required to completely arrest the growth of test isolates with pulp extracts ranged between 0.25 -128 mg/ml-1, while for peel it was between 2 – 128 mg/ml-1 . Amongst all the pulp extracts, ethanol, methanol and acetone extracts inhibited Bacillus subtilis with the least MIC of 0.25 mg/ml-1, and 0.5 mg/ml-1 each . Candida albicans was inhibited at a MIC of 0.5 mg/ml. with ethanol pulp extracts. However, all the peel extracts inhibited the test isolates with a high MIC but in a variable manner. (Table 3, 4)
Table (3):
Minimum inhibitory concentration of passion fruit pulp with seeds (mg/ml-1).
Organism |
Acetone |
Methanol |
Ethanol |
Ethyl acetate |
|---|---|---|---|---|
Staphylococcus aureus |
1 |
4 |
0.5 |
NI |
Bacillus subtilis |
0.5 |
0.5 |
0.25 |
8 |
Escherichia coli |
4 |
32 |
1 |
NI |
Pseudomonas aeruginosa |
0.5 |
16 |
0.5 |
16 |
Klebsiella pneumoniae |
32 |
32 |
32 |
64 |
Candida albicans |
1 |
2 |
0.5 |
NI |
Each value shown in the above table is a mean of three replicates and ±SD. NI-not inhibited.
Table (4):
Minimum inhibitory concentration of passion fruit peel extracts (mg/ml-1).
Organism |
Acetone |
Methanol |
Ethanol |
Ethyl acetate |
|---|---|---|---|---|
Staphylococcus aureus |
128 |
16 |
16 |
NI |
Bacillus subtilis |
8 |
NI |
NI |
64 |
Escherichia coli |
NI |
4 |
4 |
NI |
Pseudomonas aeruginosa |
NI |
8 |
4 |
NI |
Klebsiella pneumoniae |
NI |
16 |
NI |
32 |
Candida albicans |
64 |
NI |
2 |
NI |
Each value shown in the above table is a mean of three replicates and ±SD. NI-not inhibited.
GC-MS
GC-MS of ethanol extracts showed important chemicals, including tetracosamethyl-cyclododecasiloxane, dodecanoic acid, 10-methyl-, methyl ester cyclosiloxane, hexadecamethyl; 3-isopropoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris (trimethylsiloxy)tetrasil; 9-hexadecenoic acid, 9-octadecenyl ester, (Z,Z)-, cyclononasiloxane, and octadecamethyl; 9,12-tetradecadien-1-ol, (Z,E). (Table 5)
Table (5):
Chemical compounds identified from the GC-MS analysis of the ethanol extract of passion fruit pulp with seeds.
S.no |
Name of the compound |
Molecular formula |
Molecular weight |
|---|---|---|---|
1 |
Tetracosamethyl-cyclododecasiloxane |
C24H72O12Si12 |
889 |
2 |
Cyclooctasiloxane, Hexadecamethyl |
C16H48O8 |
592 |
3 |
Dodecanoic acid, 10-methyl-, methyl ester |
C14H28O2 |
228. |
4 |
3-Isopropoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris(trimethylsiloxy)tetrasil |
C18H52O7Si7 |
577 |
5 |
Cyclononasiloxane, Octadecamethyl |
C18H54O9Si9 |
667 |
5 |
9-Hexadecenoic acid, 9-octadecenyl ester, (Z,Z)- |
C34H64O2 |
504. |
6 |
9,12-Tetradecadien-1-ol, (Z,E) |
C14H26O |
210 |
FTIR analyses
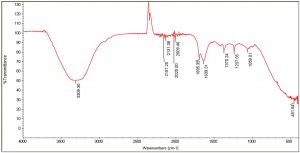
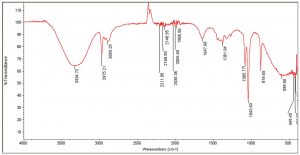
The FTIR spectra of the organic extracts ethanol and acetone, in particular, revealed the presence of the following important functional groups: alcohols, alkanes, esters, aromatic compounds, phenols, carbonyl compounds, and ketones (Tables 6, 7). Peaks at 3309, 3334, and 1370 are caused by the –OH stretch of alcohols and phenols. Similarly, the series of peaks at 2970, 2930, and 2869 were caused by the symmetric and asymmetric stretches of –CH, whereas the peaks between 2006-2211 were attributed to stretches, vibrations, and deformations of CºC and NH. The peaks at 1986, 1695, and 1647 were caused by C=C bending and stretching of aromatic. Peaks at 1043, 1085, and 1237, denoting stretching of C-O (Fig. 2 and 3). Antimicrobial activity could be attributed to the important functional groups, such as alcohols, phenols, aromatic compounds, and esters.
Table (6):
IR Spectrum for passion fruit with pulp and seeds: Acetone extracts.
Peak values (frequency, cm-1) |
Functional group |
|---|---|
3309 |
-OH stretch |
2157 |
C≡C stretching |
2131 |
C≡C stretch |
2025 |
C꞊C stretch |
2009 |
C꞊C stretch |
1695 |
C꞊C stretch |
1639 |
C꞊C stretch |
1370 |
-CH3 |
1237 |
C-O stretches |
1059 |
C-O stretches |
Table (7):
IR Spectrum for passion fruit ethanol extract with pulp and seeds.
Peak values (frequency, cm-1) |
Functional group |
|---|---|
3334 |
-OH stretch |
2975 |
CH2 asymmetry stretching |
2893 |
CH2 symmetry stretching |
2211 |
C꞊C stretch |
2168 |
C꞊C stretch |
2148 |
C꞊C stretch |
2035 |
C꞊C stretch |
2006 |
C꞊C stretch |
1986 |
C꞊C bending |
1647 |
C꞊C symmetric stretching |
1381 |
C-H rocking stretch |
1085 |
C-O stretch |
1043 |
C-O stretch |
SEM
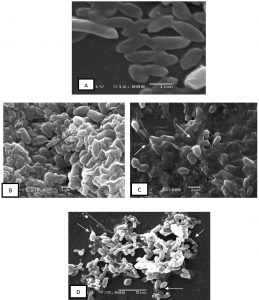
Micrographs of ethanol-treated B. subtilis cells show distorted shape, rough and corrugated cell margins. Blebs, protrusions and the aggregation of cells leading to complete damage of cells was also observed. Control cells, which did not receive treatment exhibited well defined complete and regular morphology. (Fig. 4. A-D).
Fig 4. Microphotographs of Bacillus subtilis cells (treated/control) with ethanol extracts of fruit pulp with seeds
Scanning electron microscopy of B. subtilis A-control cells, non-treated cells with well-defined margins
B and C – Treated cells show clumping and aggregation of cells.
D-Leaked cells with blebs and protrusion, completely distorted and damaged cells can be seen.
The present study showed the significant antibacterial and antifungal activity of passion fruit (pulp) extracts but in a variable manner. Ethanol and acetone extracts were most effective in controlling the growth of test isolates, which could be attributed to the high polarity of solvent, which is excellent in extracting some important phenols and other bioactive compounds. Based on the inhibition zones the antibacterial activity was classified according to Okonko et al., Bacterial isolates were considered sensitive or susceptible if the inhibition zones is >18 mm; intermediate inhibition if the zone is between 13–17 mm; resistant when the zone is <13 mm15. It was interesting to note that Gram positive bacteria were more susceptible to extracts than the Gram negative isolates. The strongest inhibition was shown by ethanol (pulp with seeds) extracts against B. subtilis with a maximum zone of inhibition (24 mm) and low MIC value (0.25 mg/ml -l ).
Similar to our findings, Kanu et al., recently reported the antimicrobial activity of ethanolic extracts of P. edulis var. flavicarpa seeds. Amongst the three microbes screened, C. albicans and S. aureus were more susceptible than E. coli, their inhibition zones ranged between 5 mm-18 mm16. Therefore, as revealed by the antibacterial assay, significant inhibition was observed with gram-positive bacteria in comparison to gram-negative. Our findings are in agreement with Kanu et al.16. The differences in the sensitivity were caused by the chemical composition of their cell envelopes, which differed in permeability. The lipopolysaccharide layer present in the outer membrane of Gram negative bacteria is very tough and impermeable to bioactive antimicrobial compounds, while the peptidoglycan layer of Gram positive bacteria is easily permeable. Hence, the resistance shown by Gram negative bacteria in the present study, could be due to restricted entry of antimicrobial compounds through the complex and rigid outer membrane17. The poor antimicrobial activity shown by other extracts can be due to the weak concentration of certain bioactive antimicrobial compounds extracted by solvents due to their polarity and also the mode of extract preparation18.
In another report Ramaiya et al., screened various extracts from leaves and stems of three species of Passiflora., Passiflora quadrangularis, P. maliformis, and P. edulis. They reported antibacterial activity of various solvent extracts from all species against 10 bacterial strains, amongst them methanolic extracts were the most effective. Furthermore, gram-positive bacteria were more susceptible to extracts than gram-negative bacteria19. Yet, in another study, passion fruit co-products and albedo, were screened for their antibacterial activity The MIC for the co-products ranged between 3.125 mg/mL -50 mg/ml 20. Onuh et al., reported that aqueous extracts of the peel of Passiflora edulis showed strong inhibition of some fungi, especially Rhizopus stolonifer, Aspergillus niger and Penicillium marneffei. Peel extracts were inhibitory towards P. aeruginosa, which is not in agreement with our findings21.
The GC-MS of fruit extracts showed some important oxygenated mono and diterpenes, esters, and phenols. Similar to our findings, compounds like tetracosamethyl-cyclododecasiloxane, dodecanoic acid, 10-methyl-, methyl ester; cyclosiloxane, hexadecamethyl; 3-isopropoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris (trimethylsiloxy) tetrasil; 9-hexadecenoic acid, 9-octadecenyl ester, (Z,Z)-, 9,12-tetradecadien-1-ol, (Z,E), cyclononasiloxane, and octadecamethyl have been reported earlier from Camellia oleifera seed cake and stems of Cola nitida22,23. Compounds, such as tetracosamethyl-cyclododecasiloxane, cyclosiloxane, and hexadecamethyl have shown antibacterial, antifungal, and antioxidant properties24-27 and 9-hexadecenoic acid and 9-octadecenyl ester possess antimicrobial properties28. Yet in another study, the phytochemical screening of P. edulis seeds revealed the presence of terpenes, flavonoids, alkaloids, steroids, tannins, and glycosides. The antimicrobial activity of the extract was linked to the presence of terpenes, flavonoids, alkaloids, and other chemical components found in the extract16.
The significant antimicrobial activity shown by passion fruit pulp extracts (purple variety) in the present study could be attributed to the presence of important bioactive compounds whose presence was indicated in the IR spectrum. Similar to our findings, Wasagu et al., reported the presence of flavonoids, terpenes, phenols, aromatic compounds, and alkaloids from the fruit extracts of P. edulis var flavicarpa (yellow fruits). They further stated that these compounds, which play a crucial role in plant defense against microbes, are also responsible for antibacterial activity29. Earlier studies report high amounts of phenols, polyphenols, alkaloids, terpenes and flavonoids from fruit pulp and rind of passion fruit and related their presence to plant defense against microbes and also for antibacterial activity30. Further, polyphenols, such as isoorientin, orientin, vitexin, isoschaftoside, and luteolin-6-C-fucoside have been identified from yellow passion fruit pulp and seeds20,30,31.
In the present study, microphotographs of treated cells show the damaging effects of ethanolic (pulp with seeds) extracts on cell morphology. B. subtilis cells treated with ethanolic extracts at its MIC concentration show, cell protrusions, deformed morphology resulting in clumping of cells, while the control cells (untreated) regular cell morphology. Similar changes in morphology and cell integrity have been reported previously when bacteria were treated with fruit extracts of Punica granatum and Mesua ferrea32,33.Earlier studies have shown that terpenes, phenols, and flavonoids play an important role in damaging the cytoplasmic membrane, inhibiting cell wall and cell membrane synthesis, causing perforations by reducing membrane fluidity. They also cause inhibition of nucleic acid synthesis34,36. In addition, some flavonoids inhibit ATP synthase, which directly effects energy metabolism37. Therefore, these bioactive compounds basically destabilize cell membrane integrity by altering their permeability, causing cell disruption. However, there are no studies to my knowledge that show the effect of Passion fruit extracts on cell morphology, hence comparative study cannot be presented here.
Our data depict the significant antimicrobial activity of ethanol and acetone extracts of fruit pulp of Passiflora edulis. Our study shows, ethanol and acetone as effective solvents in extracting important bioactive compounds. The IR spectrum and GCMS showed the presence of some key functional groups. Presence of these bioactive compounds could be responsible for distorting effects seen on cell morphology of B. subtilis. Because, the purple variety of the fruit has not been explored thoroughly for its antimicrobial properties and chemical composition, further fractionation and purification will help identify the potential chemical compounds and their mode of action. Because the fruits are easily available, their formulations will be affordable and effective in treating pathogenic infections with fewer side effects.
ACKNOWLEDGMENTS
This Research Project was supported by a grant from the financial support from the Researchers Supporting Project number (RSP-2019/114),King Saud University,Riyadh,Saudi Arabia. The authors would like to extend sincere appreciation for funding this work.
CONFLICT OF INTEREST
The authors declares that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
HR designed the experimental work. NA conducted the experimental work.HR analysed the electron microscopic studies. FA assisted in GCMS and IR analysis. HR wrote the manuscript. FA reviewed the manuscript. All autrs approved the manuscript for publication.
FUNDING
This study received grant-in-aid from the Researchers supporting Project number (RSP2019/114),King Saud University,Riyadh,Saudi Arabia.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
The data that support the findings of this study are available with the corresponding author.
- Borris, R.P. Natural products research: perspectives from a major pharmaceutical company. J. Ethnopharmacol., 1996; 51: 29–38.
Crossref - Osman, K., Evangelopoulos, D. Basavannacharya C et al. An antibacterial from Hypericum acmosepalum inhibits ATP dependent MurE ligase from Mycobacterium tuberculosis. Intern. J. Antimicrob Agents, 2012; 39: 124–129.
Crossref - Tagboto, S., Townson, S. Antiparasitic properties of medicinal plants and other naturally occurring products. Adv. Parasitol., 2001; 50: 199–295.
Crossref - Patil, A.S., Paikrao, H.M. Bioassay guided phytometabolites extraction for screening of potent antimicrobials in Passiflora foetida L. J. App. Pharm. Sci., 2012; 2: 137–142.
Crossref - Patel, S.S., Soni, H., Mishra, K., Singhai, A.K. Recent updates on the genus Passiflora: A review. Int. J. Res. Phytochem. Pharmacol., 2011; 1: 1–16.
- Jamir, T.T., Sharma, H.K., Dolui, A.K. Folklore medicinal plants of Nagaland, India. Fitoterapia, 1999; 70: 395–401.
Crossref - Zibadi, S., Farid, R., Moriguchi, S., et al., Oral administration of purple passion fruit peel extract attenuates blood pressure in female spontaneously hypertensive rats and humans. Nutr. Res., 2007; 27: 408-416.
Crossref - Johnson, M., Maridass, M., Irudayaraj, V. Preliminary phytochemical and anti-bacterial studies on Passiflora edulis. Ethnobotanical Leaflets., 2008; 12: 425-432.
- Watson, R.R., Zibadi, S., Rafatpanah, H. et al., Oral administration of purple passion fruit peel extract reduces wheeze and cough and improves shortness of breath in adults with asthma. Nutr. Res., 2008; 28: 166–171.
Crossref - Farrid, R., Rezaieyazdi, Z., Mirfeizi, Z. et al., Oral intake of purple passion fruit peel extract reduces pain and stiffens and improves physical function in adult patients with knee osteoarthritis. Nutr. Res., 2010; 30: 601–606.
Crossref - Akanbi, B.O., Bondunrin, O.D., Olayanju, S. Phytochemical screening and antibacterial activity of Passiflora edulis. Hygeia. J. Drugs Med., 2011; 3: 46-49.
- Valgas, C., Souza Simone, Mde., Smania, E.F.A., Smania, Jr, Artur. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol., 2007; 38: 369-380.
Crossref - CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. 9th Edn., Clinical and Laboratory Standards Institute,Wayne, PA., USA. 2012.
- Rizwana, H. In vitro antibacterial and antifungal activity of some oils, chemical analysis and their FTIR studies. Int. J. Agric. Biol., 2018; 20: 1488 1496.
- Okonko, O.I., Donbraye-Emmanuel, O.B.,Ljandipe, A., Ogun, A.A.,Adedeji, A.O., Udeze, A.O. Antibiotics sensitivity and resistance patterns of pathogens to nitrofurantoin and nalidixic acid in pregnant women with urinary tract infections in Ibadan, Nigeria. Middle-East. J. Sci. Res., 2009; 4: 105–109.
- Kanu, A.M., Agwu, C.O., Chidiebere, U., Ugochi, N.A. Phytochemical screening and antimicrobial activity of ethanoic extract of Passiflora edulis var. flavicarpa seed on selected pathogens. Uni. J. Microbiol. Res., 2017; 5: 35–39.
- Delcour A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta., Proteins Proteomics, 2009; 1794: 808–816.
Crossref - Nazif, N.M. Phytoconstituents of Zizyphusspina-christi L. fruits and their antimicrobial activity. Food Chem., 2002; 76: 77–81.
Crossref - Ramaiya, S.D., Japar, S.B., Muta, H.Z. Assessment of total phenolic, antioxidant, and antibacterial activities of Passiflora species. Sci. World. J., 2014; 1-10.
Crossref - Lopez-Vargas, J.H., Fernandez-Lopez, J., Perez-Alvarez, J.A., Viuda-Martos, M. Chemical, physico-chemical, technological, antibacterial and antioxidant properties of dietary fiber powder obtained from yellow passion fruit (Passiflora edulis var. flavicarpa) co products. Food Res. Int., 2013; 51: 756–763.
Crossref - Onuh, J.O., Shiriki, D., Ubwa ,S.T., Shambe, T. Isolation of six microorganisms from rotten Dioscorea alata (water yam), and antimicrobial sensitivity test with nine plant extracts. Food Nutr. Sci., 2015; 6: 1381–1394.
Crossref - Olakunle, O.M., Bola, A. GC-MS Analysis of phyto components from the stem bark of Cola nitida Schott & Endl. J. Plant Sci., 2017; 5: 99 –103.
- Li, L., Xuexiang, C., Weiwei, Z., Yunhao, W., Xiang, D., Lili, C., Dangquan, Z., Wanxi, P., Systematic characterization of volatile organic components and pyrolyzates from Camellia oleifera seed cake for developing high value-added products. Arab J. Chem., 2018; 11: 802–814.
Crossref - Lalitharani, S., Ramasamy, V., Mohan, R.G.S. GC-MS analysis of ethanolic extract of Zanthoxylum rhetsa (roxb.) DC spines. J. Herb. Medi. and Toxicol., 2010; 4: 191–192.
- Musthafa, K.S., Sahu, S.K., Ravi, A.V., Kathiresan, K. Anti-quorum sensing potential of the mangrove Rhizophora annamalayana. World J. Microbiol. Biotechnol., 2013; 29: 1851–1858.
Crossref - Ahsan, T., Chen, J., Zhao, X., Irfan, M., Wu, Y. Extraction and identification of bioactive compounds (eicosane and dibutyl phthalate) produced by streptomyces strain kx852460 for the biological control of Rhizoctonia solani ag-3 strain kx852461 to control target spot disease in tobacco leaf. AMB Express, 2017; 7: 54.
Crossref - Hassan, S.R., Zaman, N.Q., Dahlan, I. Influence of seed loads on start up of modified anaerobic hybrid baffled (MAHB) reactor treating recycled paper wastewater. Eng. Heritage J., 2017; 1: 5–9.
Crossref - De Assis Lage, T.C., Montanari, R.M., Fernandes, S.A., De Oliveira Monteiro, C.M., De Oliveira Souza Senra, T., Zeringota, V., Da Silva Matos, R., Daemon, E. Chemical composition and acaricidal activity of the essential oil of Baccharis dracunculifolia De Candole (1836) and its constituents nerolidol and limonene on larvae and engorged females of Rhipicephalus microplus (Acari: Ixodidae). Exp. Parasitol., 2015; 14: 824–29.
Crossref - Wasagu, R.S.U., Lawal, M., Amedu, A.M., Sabir, A.A., Kabir, S., Tukur, U.G., Zaharadeen, A. Comparative chemical analysis, phytochemical screening and antimicrobial activities of the rinds, seeds and juice of (Passiflora edulis var. flavicarpa) passion fruit. J. Nat. Sci. Res., 2016; 6: 138-143.
- Zeraik, M.L., Yariwake, J.H. Quanti cation of isoorientin and total avonoids in Passi ora edulis fruit pulp by HPLC-UV/DAD. Microchem. J., 2010; 96: 86 –91.
Crossref - Dhawan K, Dhawan S, Sharma A. Passiflora: A review update. J. Ethnopharmacol., 2004; 94: 1–23.
Crossref - Anibal, P.C., Peixoto, I.T.A., Foglio, M.A., Hofling, J.F. Antifungal activity of the ethanolic extracts of Punica granatum L. and evaluation of the morphological and structural modifications of its compounds upon the cells of Candida spp. Braz. J. Microbiol., 2013; 44: 839–848.
Crossref - Aruldass, C.A., Marimuthu, M.M., Ramanathan, S., Mansoor, S.M., Murugaiyah, V. Effects of Mesua ferrea leaf and fruit extracts on growth and morphology of Staphylococcus aureus. Microsc. Microanal., 2013; 19: 254–260.
Crossref - Tsuchiya, H., Iinuma, M. Reduction of membrane uidity by antibacterial sophora avanone G isolated from Sophora exigua. Phytomedicine., 2000; 7: 161–165.
Crossref - Plaper, A., Golob, M., Hafner, I., Oblak, M., Solmajer, T., Jerala, R. Characterization of quercetin binding site on DNA gyrase. Biochem. Biophys. Res. Commun., 2003; 306: 530–536.
Crossref - Wu, D., Kong, Y., Han, C. et al. D-Alanine: D-alanine ligases as a new target for the flavonoids quercetin and apigenin. Int. J. Antimicrob. Agents, 2008; 32: 421–426.
Crossref - Chinnam, N., Dadi, P.K., Sabri, S.A., Ahmad, M., Kabir, M.A., Ahmad, Z. Dietary bio-flavonoids inhibit Escherichia coli ATP synthase in a differential manner. Int. J. Biol. Macromol., 2010; 46: 478–486.
Crossref
© The Author(s) 2019. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.