ISSN: 0973-7510
E-ISSN: 2581-690X
Palm wine, locally called nkwu ellu or ekpo, holds significant cultural and economic importance as it is consumed on a daily basis in many parts of Nigeria. The presence of histamine in palm wine at a level that causes foodborne illnesses and allergic reactions is caused by both native and contaminating bacteria such as members of Enterobacteriaceae. This research determined the prevalence of histamine producing, multidrug-resistant Escherichia species from palm wine during fermentation. Microbiological analysis and histamine production were evaluated on randomly sampled palm wine. Bacterial isolation and phenotypic characterization were done according to standard microbiological procedures. DNA sequencing was employed to verify the specific strain of bacteria isolated. Antibiotic sensitivity testing was performed on the bacteria isolated. Of all the 320 palm wine samples tested, 37 (11.56%) were contaminated with histamine-producing Escherichia species. The strains of Escherichia species cultured include Escherichia coli (E. coli) UFV 251, Escherichia fergusonii APO3, E. coli Z1322PEC0229, E. coli PL-AGW6, E. coli Saman5 and E. coli ZK-1. Antibiotic susceptibility profiles showed that all these strains were multidrug-resistant. In regard to the duration of fermentation, the average histamine content (mg/kg) of the test palm wine tapped from standing life oil palm tree (nkwu ellu) and felled palm tree (ekpo) ranged from 1.00121e-4 to 9.82127e-2 and 1.22120e-2 to 9.96332e-1, respectively. The ability of these multidrug-resistant Escherichia species to produce histamine poses serious public health menace. Therefore, extensive improvement in the hygienic practices during production and handling, and the control of fermentation conditions are necessary to prevent the product contamination and histamine production to ensure the safety of the drink.
Palm Wines, Antibiotic Resistance, Fermentation, Escherichia Species, Histamine Production
Palm wine is a juice rich in alcohol produced from natural fermentation of the fluid tapped from varied types of palms including the raffia palm, African fan palm, wild date palms, Elaeis guineensis (oil palm) and coconut palms.1,2 The juice is a traditional liquid refreshment made as a libation or taken for other purposes in many countries within the tropics.3 In West Africa, the alcoholic drink is normally consumed by about 11,000,000 people drinking it on a daily basis.4 This traditional drink is served in large volumes to people during wedding receptions, birthday parties, burial and life celebrations and other varied gatherings to enjoy and mark important ceremonies. In addition, producing and marketing palm wine by various rural dwellers provide employment opportunities for such people in the area.5 In Nigeria, there are two major categories of these wines according to their tapping protocols. The first category is obtained from the incision made at the base of inflorescent flower of the standing life palm tree and the second category is obtained from the incision created on the terminal bud of the felled palm tree or its trunk.6,7 The local names for the former and latter palm wines in the study area are ‘nkwu ellu’ (meaning that it was tapped from a standing life palm tree) and ‘ekpo’ (meaning that it was tapped from cut down palm tree), respectively. Palm wines are mixtures containing varied constituents such as minerals, vitamins, ethanol, sugar, acetic acid, lactic acid, glycerol, amino acids and proteins.8,9 Despite all the benefits derivable from these wines, they have some qualities that impart negatively on human health. This is due to high nutrient contents of this product that favour the growth and multiplication of fermenting/contaminating bacteria.10 Consequently, palm wines serve as microbiomes of both the constitutive flora and the contaminating microorganisms deposited by flies, myriapods, wine collection equipment and containers as well as the tappers. Microorganisms such as the species of Lactobacillaceae, Leuconostocaceae, Acetobacteriaceae, Enterobacteriaceae, Micrococcus, Streptococcus, Brevibacterium, Serratia, Aerobacter (Klebsiella) and Zymomonas form major part palm wine microbiota.11,12
There are six common species in the genus Escherichia which are E. coli, E. albertii, E. fergusonii, E. hermannii, E. marmotae and E. ruysiae.13 Many strains of E. fergusonii were reported to be present in the clinical samples collected from human wound, blood, urine and stool of various patients.14 E. coli causes frequent passage of watery stool, septicemia in newborns, cystitis and urosepsis.15 E. coli has been investigated to cause 30% and 80% of hospital and non-hospital infections respectively.16 It has been shown that E. coli has a propensity for making histamine under adverse situations as a means to protect itself from environmental factors.17 Histamine is a biogenic amine that affects the functions of the numerous cells of the body organs and systems. Palm wine contains several amino acids including histidine and thus, histamine biosynthesis commences following palm wine production caused by the catalysis of an enzyme-histidine decarboxylase- expressed by invading and constitutive palm wine living microorganisms.18 The determinants of accumulation rate of histamine in palm wine include the availableness of histidine, the bacterial growth and behaviours that generate histidine decarboxylase and nurtures that encourage the multiplication of such organisms.19 The consumers of these nkwu ellu or ekpo cannot perceive the histamine as this histidine derivative is colorless and odorless.20,21 Symptoms associated with histamine invariably manifest as pseudoallergic reactions in human with intolerance to histamine.22 The condition precipitates gastrointestinal accumulation of histamine which subsequently leads to increase in its absorption into the bloodstream.23 Consequently, the build-up of this histidine derivative in the vascular system leads to varied infirmities/ailments such as persistent headaches, menstrual disorder and altered intestinal functions.24 Panja et al.,25 and Benly26 have reported that when histamine is produced exogenously and absorbed in large quantities by the body cells, it triggers a wide array of clinical manifestations such as respiratory allergic diseases, symptoms of the skin and soft tissue allergies and reproductive system disorders in women. Moreover, some of the bacteria that are histamine producers are multidrug-resistant (MDR)27 and their presence in palm wine may be baleful. Globally, the rate at which these MDR bacteria cause infections has been on increase and the danger of treatment failures is ominous.28 The World Health Organization (WHO), in 2019, reported that antimicrobial resistance (AMR) caused the demise of 0.7 million people and estimated that by 2050 the population affected will have increased to 20,000,000, costing more than $ 2.9 trillion.29 Therefore, MDR bacteria found anywhere should be regarded as a serious matter and the danger they pose to public health and our economic lives should not be taken with a pinch of salt.
Currently, many rural areas in Nigeria such as villages in Enugu-Ezike are faced with many health promotion challenges such as lack of maintenance of proper hygiene, unsafe keeping of food/drinks like palm wine, self diagnosis and inappropriate use of antibiotics. In such areas, it is a common practice to dilute palm wine with water which may not be portable and as a consequence, contaminate the drink. Further, the way this natural product is stored, handled and sold in the rural areas encourages microbial contaminations. In the area, it is a common occurrence to observe some consumers of this product manifest immediate or delayed ill-health ranging from nausea/vomiting to severe gastroenteritis after consumption, and the exact cause remains unknown. Based on the foregoing, it is not out of place to ask if contaminating bacteria such as histamine producing Escherichia species can be found in palm wine tapped in Enugu-Ezike. The goal of this study was, therefore, to investigate the incidence of histamine producing, multidrug-resistant Escherichia species in palm wine tapped in Enugu-Ezike.
Study area
Enugu-Ezike, which takes up the whole land mass of Igbo Eze North LGA of Enugu State, Nigeria, in coordinates 6.9449° N, 7.4577° E was chosen as the study area for this research work.
Test sample
Palm wines (nkwu ellu and ekpo). Nkwu ellu is the kind of palm wine tapped from the base of the immature male inflorescence of a standing life oil palm tree while ekpo is the one tapped from the trunk or apical meristem of the chopped down palm tree.
Culture media and antibiotics discs
The culture media and antibiotics discs were bought from Oxoid Chemical (London). They include MacConkey agar, Mueller Hinton agar, Nutrient agar and broth, BHI broth, EMB agar, Brain heart infusion broth, Niven agar, gentamicin (30 µg), ciprofloxacin (10 µg), chloramphenicol (10 µg), ofloxacin (10 µg), pefloxacin (10 µg), cetriaxone (30 µg), amoxicillin (30 µg), ampicillin (30 µg), clindamycin (10 µg), erythromycin (15 µg) meropenem (15 µg) and tetracycline (30 µg).
Collection of test samples
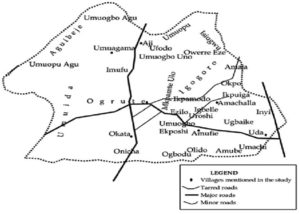
Three hundred and twenty (320) samples of palm wine comprising 160 each of nkwu ellu and ekpo were purchased from the four major markets in Enugu-Ezike. Eighty (80) samples (each in 20 ml sterile universal containers) were collected in turn on a monthly basis from Nkwo market (okpo and Aji), Eke Market (ozzi and Onitcha-Enugu), Afor market (Umuagama and Umuopu), and Orie market (Umuogboagu and Amachala). All these villages serve the entire people of Enugu-Ezike in palm wine trading. The samples were purchased fresh from the wine tappers or their relatives in all the rural communities within the study area as shown in Figures 1 and 2. A 10 ml of each sample used for the baseline histamine studies is kept in the ice bag to halt fermentation. Then, the rest of the test samples were conveyed to the Microbiology unit of Adonai Diagnostic Center and kept in a cool environment until microbial analysis and histamine studies.
Figure 2. Map of Enugu-Ezike, the study area, showing different villages where both nkwu ellu and ekpo are made. Adapted from rural history.30
Screening palm wine for histamine production by HPLC
Extraction of histamine from fermented Palm wine
Sixty (60) palm wine samples, twenty each from a group based on the fermentation duration from the first day to the third day of fermentation, were randomly selected for screening for the presence of histamine. Histamine extraction and analysis were performed by modification of the previously described techniques.31 About 15 mg of the triturated sample was transferred into a volumetric flask (10 ml capacity) and a 5% trichloroacetic acid was added to the volumetric flask mark for dilution, mixed for 1.5 min and sonicated for 6 min. From the solution, 300 ml was collected and diluted to 5 ml in a volumetric flask with methanol:water (50:50). One milliliter of the solution was collected and filtered with membrane filter of size 0.45 µL.
Derivatization
A 100 ml of the agent for derivatization ortho-phthalaldehyde was collected from the reagent vial and injected into the samples. The mixture was vortexed and the reaction was allowed to be completed in 5 minutes. A micro syringe was thoroughly washed and used to pipette 5.0 microliter of the derivatized samples into the HPLC column.
Separation by HPLC
The Agilent 1200 Series, the HPLC system, was set up and allowed to stabilize in 30 min. A 5 µL of the derivatized samples were introduced into the system at a rate of flow of 1.0 mL/min. The conditions for running HPLC were maintained as follows: Column (Chromsep SS C18 measuring 150 mm × 4.6 mm × 5 µm), Mobile phase consisting of Mobile phase (A), Tetrahydrofuran:methanol:phosphate-buffer (100 mmol/L) (1:8:9) and Mobile phase (B), Methanol:phosphate-buffer (100 mmol/L) (80:20). The gradient program was (Min/A%B%): 8/75/25, 12/67/33, 25/50/50, 30/0/100 and 35/67/33. The filtration of mobile phase was done through a membrane filter of pore size 0.4 µm and degassed. Histamine was quantified using a detector (AGILENT 1260) at a wavelength of 254 nm.
Isolation of Escherichia species
Isolation of Escherichia species was performed by modification of the technique described previously.32 One milliliter (1 ml) of each of the test palm wines was introduced into a tube containing peptone water (9 mL of 0.1%) and incubated at 37 °C overnight for homogenization. Then, each of the new samples was mixed with 4 ml per tube of Brain Heart Infusion Broth (BHIB) and incubated overnight at 37 °C. A loopful of the BHIB culture was subcultured in duplicate onto each of MacConkey agar and EMB incubated overnight at 37 °C. On inspection following the incubation, the flat smooth pink colonies cultured on MacConkey which produced yellowish-green metallic sheen with were selected for further characterization. The Escherichia species were identified based on colonial morphology, Gram character, sugar fermentation and confirmed by DNA sequencing.
Isolation of histamine producers
The pure isolates of genetically confirmed Escherichia species were subcultured onto duplicate plates of Niven’s agar and incubated for 48 -72 h at 37 °C under an aerobic condition. Purple colonies were indicative of histamine production.33 The purple colonies were then streaked on EMB agar to obtain pure isolates. The isolates were subcultured onto nutrient agar slant, incubated overnight at 37 °C and then preserved in the refrigerator for future use.
Confirmation of strains of Escherichia species by DNA sequencing
Genomic DNA extraction
The extraction of DNA was carried out using ZR Fungal/Bacterial DNA MiniPrep™50 Preps. Model D6005 (Zymo Research, California, USA). A 2 mL of broth culture of the test bacterial was introduced to a ZR BashingTM Lysis Tube and a 750 µl Lysis Solution was added. It was secured in a bead fitted with 2 ml tube holder assembly and processed at maximum speed for 10 minutes. The lysis tube was spun for 60 seconds at 12,000 x g. A 450 µL of supernatant was transferred to a Zymo-SpinTM IV Spin Filter in a collection tube and centrifuged at 7,000 x g for 60 seconds. Then, a 1,200 µL of binding Buffer was added to the filtrate in the collection tube. The buffer-filtrate mixture (800 µL) was transferred to a Zymo-SpinTM IIC Column in a collection tube and spun at 10,000 x g for 60 seconds. The flow through was discarded from the collection tube and the mixture centrifuged again. A 200 µL DNA Pre-Wash Buffer was added to the Zymo-Spin TM IIC Column in a new collection tube and centrifuge at 10,000 x g for 60 seconds. Then, a 500 µL of Fungal/Bacterial DNA wash buffer was transferred to the column and centrifuge at 10,000 x g for 60 seconds. The Zymo-SpinTM IIC Column was added to a clean 1.5 ml microcentrifuge tube and a 100 µl DNA elution buffer added directly to the column matrix. It was centrifuged at 10,000 x g for 30 seconds to elute the DNA.
The ultra-pure resulting filtrate (DNA) collected was used as a template during the assay.
16S rRNA gene amplification and Sequencing
The polymerase chain reaction mix was composed of 12.5 µL of Taq 2X Master Mix from New England Biolabs (M0270); 1 µL each of 10 µM forward (27F: AGAGTTTGATCMTGGCTCAG) and reverse (1525R: AAGGAGGTGWTCCARCCGCA) primer; 2 µL of DNA template and then made it up with 8.5 µL nuclease-free water. The cycling conditions for the amplification of the 16S rRNA gene involve initial denaturation at 94 °C for 5 minutes, followed by 36 cycles of denaturation at 94 °C for 30 seconds, annealing at 56 °C for 30 seconds and elongation at 72 °C for 45 seconds. This is followed by final elongation step at 72 °C for 7 minutes and hold temperature at 10 °C forever. The amplified fragments were sequenced using a Genetic Analyzer 3130xl sequencer from Applied Biosystems using manufacturers’ manual while the sequencing kit used was that of BigDye Terminator v3.1 cycle sequencing kit. Bio-Edit software and MEGA X were used for all genetic analysis
Test for antibiotic sensitivity
Genetically confirmed strains of the Escherichia species were subcultured on nutrient agar for 12-16 h at 37 °C to obtain fresh cultures. After the incubation, the colonies were standardized by matching the turbidity of each isolate with that of 0.5 McFarland Opacity Standard. The standardized colonies were subjected to disc agar diffusion test using some selected antibiotic discs (Oxoid UK), comprising gentamicin (30 µg), ciprofloxacin (10 µg), chloramphenicol (10 µg), ofloxacin (10 µg), pefloxacin (10 µg), ceftriaxone (30 µg), amoxicillin (30 µg), ampicillin (30 µg), clindamycin (10 µg), erythromycin (15 µg) meropenem (15 µg) and tetracycline (30 µg). The plates were incubated overnight and zones of inhibition diameter were measured. The sensitivities of the isolates were determined using the guidelines of Clinical Laboratory Standard Institute (CLSI) version 2023.34 Wild type E. coli ATCC 25922 was used as a quality control strain.
Histamine content of the test palm wine
The test wines subjected to HPLC analysis revealed the presence of histamine in all the sixty samples tested. Table 1 shows the average quantity of histamine in the test palm wine as obtained by HPLC enhanced integrator analysis. The average histamine content per group (mg/kg) of nkwu ellu and ekpo ranged from 1.00121e-4 to 9.82127e-2 and 1.22120e-2 to 9.96332e-1, respectively.
Table (1):
Histamine content of the test palm wine obtained by HPLC enhanced integrator analysis
Group of test samples according to the time of analysis after tapping |
No. of samples per group |
Average quantity per group in mg/kg of nkwu ellu |
Average quantity per group in mg/kg of ekpo |
|---|---|---|---|
Group 1: (Baseline; 1 h) |
10 |
1.00121 e-4 |
1.22120 e-2 |
Group 2: (24 h) |
10 |
1.43320 e-4 |
1.86151 e-1 |
Group 3: (48 h) |
10 |
2.15312 e-3 |
6.96221 e-1 |
Group 4: (72 h) |
10 |
9.82127 e-2 |
9.96332 e-1 |
Characterization of histamine-producing Escherichia species in the test palm wines
Out of three hundred and twenty samples of palm wine screened, 37(11.56%) were investigated to have been contaminated with histamine-producing Escherichia species. nkwu ellu and ekpo yielded 8 and 29 strains of Escherichia species, respectively. The strains of Escherichia species cultured include Escherichia coli UFV 251, Escherichia fergusonii APO3, E. coli Z1322PEC0229, E. coli PL-AGW6, E. coli Saman5 and E. coli ZK-1 with respective accession numbers as PQ220163, PQ220164, PQ220166, PQ220169, PQ220299 and PQ220301 (Table 2). DNA sequencing results confirmed that more of the strains of Escherichia species were isolated from ekpo palm wine when compared with nkwu ellu.
Table (2):
Molecular characterization of the test isolates
Sample number |
Type of Palm wine |
Scientific Name |
Strain |
Max Score |
Total Score |
Query Cover |
E value |
Accession Number |
|---|---|---|---|---|---|---|---|---|
1 |
nkwu ellu |
E. coli |
UFV 251 |
1724 |
1724 |
99% |
0 |
PQ220163 |
2 |
ekpo |
E. fergusonii |
APO3 |
1953 |
1953 |
99% |
0 |
PQ220164 |
3 |
ekpo |
E. coli |
Z1322PEC0229 |
1829 |
12718 |
96% |
0 |
PQ220166 |
4 |
ekpo |
E. coli |
PL-AGW6 |
1435 |
9948 |
99% |
0 |
PQ220169 |
5 |
ekpo |
E. coli |
Saman5 |
1243 |
1243 |
99% |
0 |
PQ220299 |
6 |
ekpo |
E. coli |
ZK-1 |
1735 |
1735 |
99% |
0 |
PQ220301 |
E. fergusonii, Escherichia fergusonii, Max, maximum, E value, expectation value in blast
Antibiotic sensitivity pattern of the histamine producing Escherichia species
The susceptibility test results of the isolates are shown in Table 3. All the test isolates (100%) showed resistance to ampicillin and erythromycin. More than 50% of the isolates exhibited resistance to amoxicillin, pefloxacin, chloramphenicol, tetracycline, ofloxacin, clindamycin, and ceftriaxone. Many of the isolates were resistant to the antibiotics evaluated except for 54.1 % and 67.6% of all the isolates that were sensitive to ciprofloxacin and gentamicin, and meropenem, respectively.
Table (3):
Antibiotics susceptibility pattern of Escherichia species. (N = 37)
No. |
Test antibiotics |
Susceptibility n (%) |
Intermediately sensitivity n (%) |
Resistance n (%) |
|---|---|---|---|---|
1 |
Amoxicillin |
3 (8.1) |
0 (0) |
34 (91.9) |
2 |
Chloramphenicol |
6 (16.2) |
3 (8.1 |
28 (75.7) |
3 |
Pefloxacin |
18 (48.6) |
0 (0) |
19 (51.4) |
4 |
Tetracycline |
6 (16.2) |
0 (0) |
28 (75.7) |
5 |
Ciprofloxacin |
20 (54.1) |
2 (5.4) |
15 (40.5) |
6 |
Gentamycin |
20 (54.1) |
0 (0) |
17 (45.9) |
7 |
Ofloxacin |
18 (48.6) |
0 (0) |
19 (51.4) |
8 |
Clindamycin |
1 (2.7) |
3 (8.1) |
33 (89.2) |
9 |
Ampicillin |
0 (0) |
0 (0) |
37 (100) |
10 |
Meropenem |
25 (67.6) |
1 (2.7) |
11 (29.7) |
11 |
Erythromycin |
0 (0) |
0 (0) |
37 (100) |
12 |
Ceftriaxone |
18 (48.6) |
0 (0) |
19 (51.4) |
Table 4 shows the profile of antibiotic sensitivity-resistance and MARI of the isolates studied. All the test isolates were resistant to ciprofloxacin, amoxicillin, erythromycin. ampicillin, chloramphenicol and tetracycline, showing that they are multidrug resistant organisms. Extensively drug resistant Escherichia fergusonii APO3 and Escherichia coli Z1322PEC0229 were part of the superbugs isolated. The values of MARI in this study range from 0.58 to 1.0.
Table (4):
Evaluating multi-antibiotic resistance (MAR) index of the isolates
Test isolate |
Sensitive to |
Resistant to |
MAR Index |
|---|---|---|---|
Escherichia coli UFV 251 |
MP, CTO, GN, |
AMX, PEF, CLN, AMP OFX, ERY, CH, TET, CPX |
0.75 |
Escherichia fergusonii APO3 |
MP, CTO |
AMX, PEF, CLN, AMP OFX, ERY, CH, TET, GN, CPX |
0.83 |
Escherichia coli Z1322 PEC0229 |
OFX |
AMX, PEF, CLN, AMP, ERY, CH, TET, CPX, MP, CTO, GN |
0.92 |
Escherichia coli PL-AGW6 |
MPN, PEF GN, PEF, OFX |
AMX, CLN, AMP, ERY, CH, TET, CTO |
0.58 |
Escherichia coli Saman5 |
MP, CTO, GN |
AMX, PEF, CLN, AMP OFX, ERY, CH, TET, CPX |
0.75 |
Escherichia coli ZK-1 |
AMX, PEF, CLN, AMP OFX, ERY, CH, TET, CPX, MP, CTO, GN |
1.0 |
CPX = Ciprofloxacin, LEV = Levofloxacin, OFX = Ofloxacin, PEF = Pefloxacin, GN = Gentamicin, CTO = Ceftriaxone, AMX = Amoxicillin, ERY = Erythromycin, AMP = Ampicillin, MP = Meropenem, CH = Chlorampenicol, TET = Tetracycline
Histamine poisoning (HIPo) and histamine intolerance (HITo) caused by the intake of fermented foods or drinks containing exogenous histamine are serious public health problems.35 HIPo manifests as emesis, abdominal pain, diarrhea, itchy rash, and dyspnea36 and it occurs as a result of diminished activity of diamine oxidase (DAO), causing the build-up of blood histamine after ingestion. The harmful effects related to the consumption of histamine in food and drinks are multiplexed because its receptors are found in every part of the human body. Though, our study focused on the determination of the incidence of histamine-producing Escherichia species that form part of the contaminants of palm wine, it is important to note that there are many works which reported the presence of Escherichia coli and other bacteria.37-39
This study reported 11.46% as the prevalence of Escherichia species that are histamine producers. This capability of Escherichia species and some other enterobacteria to produce exogenous histamine has been supported by the results of other researchers.40-42 Considering the large microbiome that exists in this particular palm juice, the high isolation rate investigated in this work showcases these organisms as one of the most significant occurring species of fermenters, considering the potential presence of other fermenters in nkwu ellu and ekpo microbiota, which is in agreement with the previous studies.43 The existence of histamine in all the palm wines analyzed is a strong indication of microbial decarboxylation of the histidine content of the drinks.18 Our study revealed the remarkable role the fermentation duration plays in histamine accumulation as its concentration increased with increase in time (Table 1), which is consistent with the results of the work done elsewhere.27 The presence of measurable concentration of histamine, though at very low levels, one hour after tapping showed that histamine production by both native and contaminating histamine producers commences immediately after palm wine tapping. Though the concentration of histidine in this drink may be relatively low, its decarboxylation is of great significance to the health of the consumers especially in individuals who exhibit histamine pseudo-allergy in which a low concentration of histamine in the body can trigger a whole lot of observable adverse reactions affecting dermatological system (erythema and urticarial rash), gastrointestinal system (diarrhea with or without emesis, indigestion and abdominal discomfort) and cardiovascular system (hypotension and altered pulse rate.44 It has been reported that histamine accumulation of more than 40 mg/meal or 0.75 mg/kg body weight is considered hazardous.45 Pertaining to the duration of fermentation, our study reports average histamine content (mg/kg) of palm wine tapped from standing life palm tree (nkwu ellu) and chopped down palm tree (ekpo) to be values ranging from 1.00121e-4 to 9.82127e-2 and 1.22120e-2 to 9.96332e-1, respectively. Significantly, the average quantity of histamine in ekpo is greater than that of nkwu ellu. The HPLC analyses of varied palm wine groups conducted at 1, 24, 48 and 72 h post tapping demonstrated that the respective average quantity of histamine in ekpo was about 120, 1299, 323 and 10 times greater than that of nkwu ellu (Table 1). The variation in the mean concentration of histamine made per unit time by the fermenters may be determined by the rate of fermentation and metabolism by these organisms. A freshly made ekpo and nkwu ellu, as evident in this work, contain little histamine because of the short time the fermentation has occurred. The proportional increase in the histamine constituent of the test wines up to 24 h during storage may be accounted for by the increase in the rate of fermentation resulting from the proliferating bacteria. However, we discovered that at 48-72 h, both the stored ekpo and nkwu ellu showed a declining level of histamine in inverse proportion with time. The cause of the reduction in the level of histamine is not clear, but may be connected with bacterial involution and cell lysis due to accumulation of waste products and exhaustion of histidine content of the wines. Compared with the amount of histamine produced in ekpo, the amount produced in nkwu ellu is significantly lesser (P <0.05). The reason for higher amount of histamine in ekpo than in nkwu ellu may be due to the differences in production methods, microbial contamination level, histidine content and rate of fermentation. The ekpo is tapped from chopped down oil palm tree which is lying horizontally on the ground. The method is highly prone to contamination because the tapping site is always exposed to insects (mainly flies, ants and cockroaches), myriapods, rodents and reptiles. These animals (especially flies), after perching on the human or animal excreta or other reservoirs of infectious agents, would perch on the tapping site thereby contaminating the source of the ekpo palm wine. The nkwu ellu which is tapped from a standing life tree does not suffer animal-associated contamination like ekpo palm wine. Another source of contamination of both drinks is green leaves used by the wine traders to cover the mouth of the containers as stoppers and also, to cover the jars of drinks to protect them from direct sunlight which hastens the activity of fermenters. By doing so, the rate of fermentation is retarded and the taste of the wines is preserved. However, the leaves are not sterile and contain some microorganisms which contaminate the drinks as both come in direct contact with each other. These sunlight-proof leaves are used more often in ekpo than nkwu ellu. Therefore, the more contaminated ekpo always favours histamine production because of the high microbial load and this might have contributed to higher amount of histamine in ekpo than in nkwu ellu, hence, the activity of the invaders (Escherichia spp.) and the native producers. Moreover, stemming from the histamine content of the two categories of palm wines, our findings support the work which reported that the methods of palm wine production affect the microbial quality of the sap and hence the histamine concentration.46 The average concentrations of histamine produced in both palm wines at 48-72 h are high and can be hazardous when the palm wine is consumed in large quantities. The portentous danger associated with the intake of these wines especially the ekpo is that many of the tappers or makers of palm wine secretly mix old wines (48-72 h post tapping) with a newly made one, all in a bid to make more money. In such a situation, the high level of histamine in the old wines may expose the consumers to minatory risks especially in individuals that suffer histamine intolerance. HIPo or HITo may be suspected if a set of unexpected manifestations occurs in various ways following the ingestion of fermented palm wine. Such conditions/manifestations call for serious public health concerns and possible etiologic agents should be investigated. This is because some of the invading organisms such as the bacteria under study have the capability of producing exogenous histamine and more importantly, their leading position in the cause of different infections.47,48
In the current investigation, we genetically confirmed the presence of E. coli UFV 251, E. coli PL-AGW6, E. coli Saman5, E. fergusonii APO3 and E. coli Z1322PEC0229 and E. coli ZK-1 in the test palm wines. Our findings that the strains of Escherichia species are in the drinks might be connected with the contamination of the wines through the tapping site, equipment, collecting gallons and/or calabash. More so, the detection of E. coli in this study underscores the point that contamination of the wine can occur at any stage of its production, which agrees with the report of investigation carried out elsewhere.49,50 Again, the presence of E. fergusonii APO3 might be related to the application of impure water to the wine to increase its volume or due to its contamination by the tappers. According to Maheux et al.,51 E. fergusonii was cultured from water consumed by humans and animals and this added a new insight to the possible habitat of the non E. coli species. Furthermore, we investigated that the test isolates exhibited non-susceptibility to ciprofloxacin, amoxicillin, erythromycin, ampicillin, chloramphenicol and tetracycline, showing that they are multidrug-resistant organisms. Our findings are in agreement with other researchers who reported that strains of E. coli are becoming increasingly insensitive to several antibiotics.52,53
We reported high multi-antibiotic resistance indices possibly because of several exposures to various antibiotics during treatments (Table 4). Regular exposure of bacteria to drugs gives room for antibiotic selection pressure. It is therefore, not surprising to note that the test isolates were totally resistant to most of the antibiotics under study. Escherichia species, especially E. coli known to be a leading human pathogen, inhabits the gut and has been exposed to antibiotics. This may have accounted for its high resistance index.
This study observed high prevalence of multidrug-resistant Escherichia species with the capability to produce histamine in palm wines sold in Enugu-Ezike, Nigeria. Their capacity to synthesize histamine in palm wine poses a serious health threat and their non-susceptibility to antibiotics constitutes a serious therapeutic challenge. In light of the foregoing, there is an ominous public health risk associated with the presence and involvement of antibiotic resistant Escherichia species in histamine production in stored palm wine, as the wines are rendered unsafe for human consumption. Antibiotic treatment of histamine toxicity or poisoning associated with these strains may be defeated because there are no treatment options available or the number of such options is reduced to minimum due to antibiotic resistance. Therefore, proper hygiene and adequate control of palm wine fermentation conditions must be improved to ensure their safety.
ACKNOWLEDGMENTS
The authors appreciate all the laboratory personnel and assistants of the laboratory division of the Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, Enugu State University of Science and Technology, Enugu State, for supporting this work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
CA, PO, TU, RO, US, OH, VE, EJ, IA and FA performed sample collection, data collection and experimental analysis. CA and PO wrote the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Dayo-Owoyemi I, Boboye B, Akinyosoye FA. Organoleptic analysis of doughs fermented with yeasts from a Nigerian palm wine (Elaeis guineensis) and certain commercial yeasts. Open Microbiol. J. 2008; 2: 115-120.
Crossref - Amoa-Awua WK, Sampson E, Tano-Debrah K. Growth of yeasts, lactic and acetic acid bacteria in palm wine during tapping and fermentation from felled oil palm (Elaeis guineensis) in Ghana. J App Microbiol. 2007;102(2):599-606.
Crossref - Wijaya L, Sumerta IN, Napitupulu TP, Kanti A, Keim AP, Howell K, Sudiana IM, Cultural, nutritional and microbial perspectives of tuak, a traditional Balinese beverage. J Ethn Food. 2024;11(1):4. doi: 10.1186/s42779-024-00221-x
Crossref - Mbuagbaw L, Noorduyn S.G. The palm wine trade: occupational and health hazards. Int J Occup Env Med. 2012;3(4):157-164.
- Yameogo J, Belem-Ouedraogo M, Bayala J, Ouedraogo MB, Guinko S. Uses and commercialization of Borassus akeassii Bayton, Ouedraogo, Guinko non-wood timber products in South-Western Burkina Faso, West Africa. Biotechnol Agron Soc Env. 2008;12(1):47-55.
- Onuche P, Shomkegh SA, Tee TN. Palm wine tapping methods among Idoma and Tiv ethnic groups of Benue state, Nigeria: implications on conservation of palm trees (Elaeis guineensis) J. E Issues Agric. Dev. Count. 2012; 4(1):86-91.
- Ouoba LII, Kando C, Parkouda C, Sawadogo-Lingani H, Diawara B, Sutherland JP. The microbiology of Bandji, palm wine of Borassus akeassii from Burkina Faso: identification and genotypic diversity of yeasts, lactic acid and acetic acid bacteria. J App Microbiol. 2012;113(6):1428-1441.
Crossref - Oluwole O, Kosoko S, Familola O, Ibironke O, Cheikyoussef A, Raheem D, Saraiva A, Raposo A. Fermented traditional wine from palm trees: Microbial, nutritional attributes and health impacts. Front Food Sci Technol. 2023;3:
Crossref - Lucky GB, Cookey GA, Ideriah TJK. Physicochemical and Nutritional Parameters in Palm Wine from Oil palm Tree (Elaeis guineensis) and Raffia Palm (Raphia hookeri) in South-South Nigeria. Chem Res J. 2017;2(6):146-152.
- Jaaskelaine E, Jakobsen LMA, Hultman J, Eggers N, Bertram HC, Bjorkroth J. Metabolomics and bacterial diversity of packaged yellowfin tuna (Thunnus albacares) and salmon (Salmo salar) show fish species-specific spoilage development during chilled storage. Int J Food Microbiol. 2019;293:44-52.
Crossref - Poirel L, Madec J, Lupo A, et al. Antimicrobial Resistance in Escherichia coli. Microbiol Spectr. 2018;6(4):10.1128.
Crossref - Djeni TN, Kouame KH, Ake FDM, Amoikon LST, Dje MK, Jeyaram K. Microbial Diversity and Metabolite Profiles of Palm Wine Produced From Three Different Palm Tree Species in Cote d’Ivoire. Sci Rep. 2020 .3;10(1):1715.
Crossref - Parte AC, Carbasse JS, Meier-Kolthoff JP, Reimer LC, Goker M. List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ. Int J Syst Evol Microbiol. 2020;70(11):5607-5612.
Crossref - Gaastra W, Kusters JG, van Duijkeren E, Lipman LJA. Escherichia fergusonii. Vet Microbiol. 2014;172(1-2):7-12.
Crossref - Camins BC, Marschall J, DeVader SR, Maker DE, Hoffman MW, Fraser VJ. The clinical impact of fluoroquinolone resistance in patients with E. coli bacteremia. J Hosp Med. 2011;6(6):344-349.
Crossref - Ferry SA, Holm SE, Stenlund H, Lundholm R, Monsen TJ. The natural course of uncomplicated lower urinary tract infection in women illustrated by a randomized placebo controlled study. Scand J Infect Dis. 2004;36(4):296-301.
Crossref - Barcik W, Pugin B, Westermann P, et al. Histamine-secreting microbes are increased in the gut of adult asthma patients. J Allergy Clin Immunol. 2016;138(5):1491-1494.
Crossref - Ibegbulem CO, Igwe CU, Okwu GN, Ayalogu E.O. Total amino acid profiles of heat-processed fresh Elaeis guineensis and Raphia hookeri wines. Food Chem. 2013 ;138(2-3):1616-1620.
Crossref - Visciano P, Schirone M, Paparella A. An overview of histamine and other biogenic amines in fish and fish products. Foods. 2020;9(12):1795.
Crossref - Garcia-Tapia G, Barba-Quintero G, Gallegos-Infante JA, Aguilar JA, Ruiz-Cortes JA, Ramirez. Influence of physical damage and freezing on histamine concentration and microbiological quality of yellowfin tuna during processing. Food Sci Technol. 2013;33(3):463-467.
Crossref - Lin CM, Kung HF, Huang YL, Huang C-Y, Su YC, Tsai YH. Histamine production by Raoultella ornithinolytica in canned tuna meat at various storage temperatures. Food Control. 2012;25(2):723-727.
Crossref - Lee YC, Chen YF, Huang YL, Kung HF, Chen TY, Tsai YH. Hygienic quality, adulteration of pork and histamine production by Raoultella ornithinolytica in milkfish dumpling. J. Food Drug Anal. 2016;24(4):762-770.
Crossref - Kovacova-Hanuskova E, Buday T, Gavliakova S, Plevkova J. Histamine, histamine intoxication and intolerance. Allergol Immunopathol. (Madr.). 2015;43(5):498-506.
Crossref - Visciano P, Schirone M, Tofalo R, Suzzi G. Histamine poisoning and control measures in fish and fishery products. Front Microbiol. 2014;5:500.
Crossref - Panja SK, Bhattacharya B, Lahiri SC. Role of histamine as a toxic mediator in the pathogenesis of vitiligo. Indian J Dermatol. 2013; 58(6):421-8.
Crossref - Benly P. Role of histamine in acute inflammation. J Pharm Sci Res. 2015;7(6):373-376.
- Ezema JN, Adonu CC, Agbo EC, et al. Isolation and characterization of histamine-producing, multi-drug-resistant Enterococcus species in fermented oil bean seeds in Nsukka, Nigeria. Malaysian J Micro. 2024;20(1):67-74.
Crossref - Gajdacs M, Albericio F. Antibiotic Resistance: From the Bench to Patients. Antibiotics (Basel). 2019;8(3):129.
Crossref - Watkins RR, Bonomo RA. Overview: global and local impact of antibiotic resistance. Infect Dis Clin North Am. 2016;30(2):313-322.
Crossref - Apeh AA, Opata CC. The oil palm wine economy of rural farmers in Nigeria: evidence from Enugu Ezike, south-eastern Nigeria. Rural History. 2019;30(2):111-128.
Crossref - Bueno-Solano C, Lopez-Cervantes J, Sanchez-Machado DI. Campas-Baypoli ON. HPLC determination of histamine, tyramine and amino acids in shrimp by-products. J Braz Chem Soc. 2012;23(1):96-102.
Crossref - Cheesbrough M. District Laboratory Practice in Tropical Countries Part 2 Cambridge University Press, Dock House, cape town 8001, 2000 S/ Africa. 157-159
- Niven CF, Jeffery MB, Corlett DA. Differential plating medium for quantitative detection of histamine-producing bacteria. Appl Environ Microbiol. 1981;41(1):321-322.
Crossref - CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 33rd ed. CLSI supplement M100. Clinical and Laboratory Standards Institute; 2023. https://webstore.ansi.org/standards/clsi/clsim100ed33. Accessed on December 22, 2024.
- Colombo FM, Cattaneo P, Confalonieri E, Bernardi C. Histamine food poisonings: A systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2018;58(7):1131-1151.
Crossref - Yves AK, Honore OG, Germain KT, Zeze GK. Molecular identification and species diversity of the microbiota associated with soumbara, a traditional fermented food commonly consumed in Cote d’Ivoire. Res J Food Sci Nutr. 2019;4:48-57.
Crossref - Adeiza ZD, Joshua I, Israel OA, Danjuma SY, Armstrong AA, Samuel A. Microbiological Examination of Palm Wine Sold within Selected Places in Anyigba, Kogi State. J Food Technol Pres. 2023; 5(3): 147
Crossref - Odafe OJ, Olorode OA. The Assessment of Palm Wine for its Composition of Probiotic Lactic Acid Bacteria and their Therapeutic Implication on Diabetic Management. Asian Journal of Medical Principles and Clinical Practice. 2023;6(2):366-375.
- Ikeh IM, Anele BC, Ukanwa, CC, Njoku SO. Analysis of the Microbial Quality of Locally Consumed Palm Wine Sold in Elele Community of Rivers State Nigeria. Eur J Nutr Food Saf. 2021;13(7):62-69.
Crossref - Shalom C, Prabha C, Ragunathan R, Johney J. Isolation of Histamine Producing Bacteria (Escherichia coli) from Tiger Prawns (Penaeus monodon) and its Control Measures Using Plant Extracts. Asian J Res Biochem. 2025;15(2):136-151.
Crossref - Okunye OL, Idowu PA, Okanlawon BM, Adejumo OE, Lawal AS, Ademola SR. Detection of Extended Spectrum Beta-Lactamase Escherichia coli and Histamine Contents in Raw Mackerel Sold in Open Markets in shagamu, Nigeria. University of Lagos Journal of Basic Medical Sciences. 2022;10(1 & 20).
Crossref - Adonu CC, Ezema JN, Onyi P, Onwusoba R. Phenotypic and Genotypic Characterization of Histamine-Producing Bacillus Species and Enterobacter cloaca Isolated from Fermented African Locust Beans in Enugu State, Nigeria. Trop J Nat Prod Res. 2025;9(6);2852-2857.
Crossref - Yu Y, Wang P, Bian L, Hong S. Rare death via histamine poisoning following crab consumption: A case report. J Forensic Sci. 2017;63(3):980-982.
Crossref - Maintz L, Novak N. Histamine and histamine intolerance. Am J Clin Nutr. 2007;85(5):1185-1196.
Crossref - Douen D, Davaatseren M, Chung MS. Biogenic amines in foods. Food Sci Biotechnol. 2017;26(6):1463-1474.
Crossref - Ogbulie TE, Ogbulie JN, Njoku MO. Comparative study on the shelf life stability of palm wine from Elaeis guineensis and Raphia hookeri obtained from Okigure, Nigeria. Afr J Biotech. 2007;6(7):914-922.
- Abernethy J, Guy R, Sheridan EA, et al. Epidemiology of E. coli Bacteraemia Sentinel surveillance Group. Epidemiology of Escherichia coli bacteraemia in England: results of an enhanced sentinel surveillance programme. J Hosp Infect. 2017;95(4):365-375.
Crossref - Kourtis AP, Sheriff EA, Weiner-Lastinger LM, Elmore K, Preston LE, Dudeck M, McDonald LC. Antibiotic Multidrug Resistance of Escherichia coli Causing Device- and Procedure-related Infections in the United States Reported to the National Healthcare Safety Network, 2013-2017. Clin Infect Dis. 2021; 6;73(11): 4552-4559.
Crossref - Karamoko D, Djeni NT, N’guessan KF, Bouatenin KMJP, Dje KM. The biochemical and microbiological quality of palm wine samples produced at different periods during tapping and changes which occurred during their storage.Food Control. 2012;26(2):504-511.
Crossref - Obi CN, Ogbulie JN, Nkwo A. Assessment of microbial growth and survival in fresh rafia palmwine from Umuariaga community, Ikwuano L.G.A. Abia State, Nigeria. Int J Curr Microbiol App Sci. 2005;4(1):484-494.
- Maheux AF, Boudreau DK, Bergeron MG, Rodriguez MJ. Characterization of Escherichia fergusonii and Escherichia albertii isolated from water. J Appl Microbiol. 2014;117:597-609.
Crossref - Banerjee R, Johnson R. A new clone sweeps clean: the enigmatic emergence of Escherichia coli sequence type 131. Antimicrob Agents Chemother. 2014;58(9):4997-5004.
Crossref - Ghafourian S, Sadeghifard N, Soheili S, Sekawi Z. Extended spectrum beta-lactamases: definition, classification and epidemiology. Curr Issues Mol Biol. 2015;17:11-21.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.