ISSN: 0973-7510
E-ISSN: 2581-690X
Fungi infections are becoming more prevalent and burdensome on a global scale leading to an important concern for immunocompromised patients. Hospitals often become infected with serious, invasive Candida infections. Higher frequency of Non-albicans Candida (NAC) species are found in the hospital setting, and some of these fungi can become opportunistic. Pathogens after a change in the host environment trigger them to move from a commensal to a pathogenic phase. Various clinical symptoms of Candida species, which are common human commensals, range from mucocutaneous overgrowth to bloodstream infections. In many hospitals, phenotypic methods are still considered the gold standard method for identification. Among the 112 isolates, Candida albicans (n=47; 52.64%) was noted as a significant etiology isolated from clinical samples. Further, C. albicans accounted the principal etiology in urine (n=28; 31.36%), and vaginal swab (n=13; 14.56%), followed by C. tropicalis (urine: n=15; 16.8% and vaginal swab: n=5; 5.6%). In blood C. pelliculosa (n=14; 15.68%) was found to be predominant followed by C. tropicalis (n=11; 12.32%). Antifungal susceptibility pattern was performed for (n=51) samples by VITEK AST and 100% susceptibility (voriconazole, and micafungin) was recorded in C. tropicalis and C. albicans. Whereas, fluconazole resistance was observed in C. tropicalis (n=3; 15%), and C. pelliculosa (n=1; 11.11%) and amphotericin B resistance in C. tropicalis (n=1; 5%) and C. albicans (n=1; 9.1%).
Candida, Identification, Conventional, Antifungal
For decades, fungal infection incident has increased frequently, especially the infection due to Candida spp.1 Several varieties of Candida spp. can be found in nature and various settings, such as hospitals, people, domestic animals, and wild animals.2 In addition to the nails, scalp, and oral cavity, this Candida species can take over the mucosal membranes of the respiratory, vaginal, & digestive systems.3 When Candida species overpower commensal species, non-symptomatic colonization may infect. Depending on the host’s surroundings, they are opportunistic and can range from benign to destructive. However, significant systemic Candida infections can develop in people with weakened immune systems.4 Three categories of candidiasis can be distinguished: cutaneous (affecting the skin and its appendages), mucosal (affecting the oropharynx, esophagus, and vulvovaginal area), and systemic (affecting the bloodstream, including candidemia & other types of invasive- candidiasis).5
The species that has the potential to induce both superficial and systemic infections is Candida albicans, C. tropicalis, C. krusei , C. glabrata, and C. parapsilosis are other pathogenic species of Candida that cause, respectively, 25%, 8%, 7%, and 4% of candidiasis.6 NAC species with reduced antifungal resistance have emerged as a major cause of fungemia, despite the fact that C. albicans is the species most frequently isolated in hospitals worldwide.5
Candida-species’ characterisation & susceptibility pattern is essential for managing and guiding appropriate antifungal therapy, optimizing patient outcomes, and halting the development of drug resistance.7–9 It is crucial to recognise each Candida species because they differ from one another exhibit varying levels of virulence and susceptibility to antifungal agents.10 Identification of isolates that may inherently resist some antifungal medications is dependent upon species-level identification.8 Due to widespread and prolonged use of azole, Candida develops resistance to it.9 As a tool for drug development studies and a method to monitor the emergence of antifungal resistance in epidemiological research, in-vitro antifungal susceptibility testing is becoming more and more significant in aiding therapeutic decision-making.11
While several studies investigated prevalence & the distribution of Candida-species in different clinical settings, among rural tertiary care centre, There isn’t enough information on their characterization and susceptibility patterns.9 Rural healthcare facilities often face unique challenges, including limited resources, lack of specialized expertise, and delayed access to diagnostic facilities. These factors can impact the timely identification and management of Candida infections, potentially leading to suboptimal treatment outcomes.9
Thus, the objective of the present research is to identify the Candida species that have been isolated from the various clinical samples taken from patients of a rural tertiary care hospital. Another aim of the investigation is to identify the antifungal susceptibility pattern of these isolates to widely used antifungal drugs.
A prospective investigation examined the identification & susceptibility profile of Candida species isolated from the clinical samples. The specimens have been collected and processed in the Jawaharlal Nehru Medical College, Department of Microbiology, Sawangi (Meghe), Wardha (Maharashtra), India, for two years.
Clinical-isolates of Candida spp. were obtained from the clinical samples (Blood, vaginal swab and urine), and for the species identification, standard conventional method & VITEK ID were done. Conventional isolation of the Candida spp. from urine and the vaginal swab was performed by culturing the sample on a Sabouraud Dextrose Agar (SDA) with chloramphenicol incubating it at 37°C. For the blood sample, BACTEC was used, and after flagging positive, the blood sample from BACTEC bottles was culture on SDA for Candida spp. isolation. Different techniques were used for speciation of Candida spp. by a conventional method, such as the Germ-tube, Dalmau-Plate technique, biochemical methods, CHROMagar, and further, an automated system like VITEK ID also used for confirmation. Antifungal Susceptibility testing for the Candida spp. was performed by the VITEK AST method based on Minimum Inhibitory concentration (MIC) method.
A total of 112 Candida spp. was isolated from various clinical specimens such as urine (n=54; 60.48%), blood (n=32; 35.84%), vaginal swabs (n=19; 21.28%), and others (n=7; 7.84%). The distribution and sources of Candida spp. identified by standard conventional method and confirmed by VITEK ID. Among the isolates, C. albicans (n=47; 52.64%) was the predominant species identified from clinical specimen. C. albicans found to be prevalent species isolated from urine (n=28; 31.36%) and vaginal swab (n=13; 14.56%), followed by C. tropicalis. In blood, the most predominant species was C. pelliculosa accounted for (n=14; 15.68%) followed by C. tropicalis (n=11; 12.32%) (Table 1) (Figure).
Table (1):
Isolation and frequency distribution of Candida spp. from various clinical samples
Specimens |
Candida. albicans |
C. tropicalis |
C. pelliculosa |
C. parapsilosis |
Others* |
Total |
|---|---|---|---|---|---|---|
Urine |
28 (31.36%) |
15 (16.8%) |
– |
5 (4.48%) |
6 (6.72%) |
54 (60.48%) |
Vaginal swab |
13 (14.56%) |
5 (5.6%) |
– |
– |
1 (1.12%) |
19 (21.28%) |
Blood |
2 (2.24%) |
11 (12.32%) |
14 (15.68%) |
– |
5 (4.48%) |
32 (35.84%) |
Others (Sputum, Pus, BAL) |
4 (4.48%) |
3 (3.36%) |
– |
– |
– |
7 (7.84%) |
Total |
47(52.64%) |
34(38.08%) |
14 (15.68%) |
5 (4.48%) |
12(13.44%) |
112 |
* C. glabrata, C. guillermondii, C. krusei, C.lusitaniae
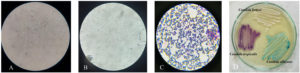
Figure. Pictorial representation of Candida spp.
A- Positive germ tube; B- Negative germ tube; C- Gram staining showing gram positive budding yeast cell ; D- Growth of various Candida species on CHROM Agar
Antifungal susceptibility pattern was performed for (n=51) Candida spp. by VITEK AST. Based on their Minimum Inhibitory Concentrations (MICs), the isolates were categorised as Sensitive (S), Resistant (R), and Susceptible Dose-Dependent (SDD) to each antifungal drug. Out of 51 Candida spp., 11 (21.56%) isolates were C. albicans which showed 100% susceptibility to voriconazole, caspofungin, micafungin and flucytosine. Whereas, 9.1% (n=1) isolates showed resistance to amphotericin B and 18.18% (n=2) isolates were SDD to Fluconazole. Similarly, 100% susceptibility to voriconazole, caspofungin, micafungin and flucytosine were observed in case of C. tropicalis and 15% (n=3) and 5% (n=1) resistance noted in fluconazole and amphotericin B respectively (Table 2).
Table (2):
Antifungal susceptibility testing of Candida species
| Antifungal agent | Fluconazole | Voriconazole | Caspofungin | Micafungin | Amphotericin-B | Flucytosine | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Susceptibility pattern n=51 |
S | SDD | R | S | SDD | R | S | SDD | R | S | SDD | R | S | SDD | R | S | SDD | R |
| Candida albicans (n=11) |
9 (81.81%) |
2 (18.18%) |
0 | 11 (100%) |
– | – | 11 (100%) |
– | – | 11 (100%) |
– | – | 10 (90.90%) |
– | 1 (9.1%) |
11 (100%) |
– | – |
| C. tropicalis(n=20) |
17 (85%) |
– | 3 (15%) |
20 (100%) |
– | – | 19 (95%) |
1 (5%) | – | 20 (100%) | – | – | 19 (95%) |
– | 1 (5%) |
18 (90%) |
1 (5%) | 1 (5%) |
| C. pelliculosa (n=9) |
7 (77.77%) |
1 (11.11%) |
1 (11.11%) |
9 (100%) |
– | – | 8 (88.88%) | 1 (11.11%) |
– | 9 (100%) | – | – | 9 (100%) |
– | – | 9 (100%) |
– | – |
| C. parapsilosis(n=4) |
4 (100%) |
– | – | 4 (100%) |
– | – | 4 (100%) |
– | – | 4 (100%) | – | – | 4 (100%) |
– | – | 4 (100%) |
– | – |
| Others (n=7)* | 3 (42.85%) |
– | 4 (57.14%) | 7 (100%) |
– | – | 5 (71.42%) |
– | 2 (28.57%) | 7 (100%) |
– | – | 6 (85.71%) |
– | 1 (14.28%) | 6 (85.71%) |
– | 1 (14.28%) |
Others (n=7)*- C. glabrata, C. guillermondii,C. krusei,C. Lusitaniae, C. rugosa
S-Sensitive; SDD-Susceptible dose-dependent; R-Resistant
Over the past decades, there has been a rise in cases of candidiasis, the prevalent source of yeast infections that result in a variety of mucocutaneous disorders that can be serious or non-fatal.12 Globally, C. albicans is the most commonly isolated organism from invasive candidiasis cases.5 However, growing recovery of the Non-albicans Candida (NAC) from patients over the past several decades has given rise to a new hazard. The NAC species with the most reports are C. tropicalis, C. parapsilosis, C. krusei, and C. glabrata.13 The clinical manifestations of infections brought on by numerous NAC spp. typically include some NAC developing resistance over time, becoming established against conventional antifungals, or both.14,15 Few Candida spp. like C. glabrata and C. krusei are cross-resistant to triazoles like fluconazole. Different pathogenic Candida species have comparable clinical symptoms that cannot be identified.16 C. auris causes outbreaks of invasive infections in healthcare facilities, resistance to some other antifungals, and also its intrinsic resistance property can cause multi-drug resistance phenomenon.17–23
A study in Karnataka (2014) by Tellapragada et al.24 Vulvovaginal Candidiasis (VVC) was most frequently caused by C. albicans (61%) and C. glabrata (20%), whereas C. tropicalis (46%) was predominant in bloodstream infections (BSI). Another study by Mohandas et al. in Manipal reported 50% C. krusei and 25% C. albicans from the symptomatic UTI cases. Further, they also demonstrated a frequency of 74.19% C. albicans and 25.8% NAC from the salivary sample.25 In all clinical samples, C. krusei isolate was predominant, while the C. albicans isolates (41.37%) were commonly isolated from respiratory tract infection (RTI).25 Also, a study from Nepal (2017) stated 90% of the Candida spp. were reported from sputum and urine, showing a higher prevalence of Candida spp. from UTI and RTI.10 Nowadays, a standard genotypic method for Candida spp. identification is generally used due to being less time-consuming, but conventional techniques are considered the gold standard.26 Numerous studies have recommended using CHROM agar Candida for the primary isolation of Candida spp. and their rapid identification.27 The additional advantage of using CHROMagar for primary isolation is the ability to identify different species of Candida from the clinical sample containing mixed species of Candida.28 In our study, all conventional techniques were used to identify Candida isolates like Germ tube, CHROMagar, biochemical methods and Dalmau plate technique in which, 32.14% were identified as C. albicans, followed by C. tropicalis (12.5%), C. pelliculosa (4.46%), C. parapsilosis (0.89%), C. glabrata (1.78%), C. krusei (1.78%) C. rugosa (0.89%). Rajeevan et al., 29 in Tamil Nadu stated that in blood sample 75% of NAC species were reported. In our study, out of 112 isolates, 12.5% of C. pelliculosa was the principal etiology identified from blood, and afterward, C. tropicalis (9.82%). In contrast to our findings, other studies from India reported C. tropicalis (35-45%) as the major causative agent isolated from blood.30-32 To date, multiple risk factors such as low birth weight, low gestational age, long-term hospitalization, sustained blood alkalosis, surgeries, and inappropriate use of antibiotics are noted for increased cases of C. pelliculosa. Hospital-acquired infections due to C. pelliculosa were documented in various clinical units such as pediatric intensive care units, nurseries, surgical intensive care units, and hematology wards.33,34
Antifungal susceptibility testing (AST) of Candida isolates by MIC method helps the clinician in the treatment of critically ill patients and is further helpful for generating clinical guidelines for the better management of treatment. A study from Karnataka (2014) found that all Candida isolates were susceptible to flucytosine and amphotericin B, but reduced susceptibility was noted in voriconazole for C. parapsilosis (64%) and C. tropicalis (79%). Fluconazole intermediate-susceptible isolate was seen in 6% C. albicans isolated from Vulvovaginal Candidiasis.24 The present study reports C. albicans and C. tropicalis showed 100% susceptibility to voriconazole, and micafungin. In agreement to previous findings,24 we also found intermediate susceptibility to C. albicans (18.18%) whereas, resistance to fluconazole noted for C. tropicalis (15%) and C. pelliculosa (11.11%).
In our study a high prevalence of NAC was noted in blood sample among which the most predominant etiology was C. pelliculosa. As in literature, Most C. pelliculosa infections occur in preterm children with exceptionally low birth weights as the rare fungus dwells mostly in soil, lakes, fermented foods, and industrial pollution. Correct identification for Candida spp. plays significant role in diagnosing the disease and its prevalence. A study by Kaur et al., (2016) (Saudi Arabia) studied the comparison of a yeast identification by conventional techniques and VITEK-2. Out of 172 Candida spp., Vitek 2 appropriately recognized 90.12% of Candida spp., 2.32% were identified correctly with low discrimination, and 7.56% isolated were misidentified. In our study, VITEK identified the isolates to their species level with proper discrimination. A study by Ahmad et al. (2012) (Kuwait)35 showed that molecular methods are less time-consuming and accurate than the conventional and VITEK systems. Our study focused on the identification of isolates through VITEK and conventional methods. The AST of various isolates in our study resistance was shown by C. albicans (9.1%) to amphotericin-B, whereas C. tropicalis isolates (15%) were resistant to fluconazole and (5%) to amphotericin B followed by C. pelliculosa (11.11%) showed resistance to fluconazole. The rest of the isolates were susceptible dose-dependent and susceptible to the tested antifungals as stated in the study, whereas a study from Nepal showed 86% resistance to the isolated Candida species where ketoconazole demonstrated the highest level of resistance. Further sensitive isolates were reported in clotrimazole, miconazole and fluconazole correspondingly.10
The lack of molecular methods for identifying and isolating Candida spp. limits our investigation and may result in the lack of some species that warrant attention. In our study, we performed VITEK ID and VITEK-AST for small number of sample. Thus, for studying the prevalence, there is a need for screening a larger number of samples.
Our study observed C. albicans & C. tropicalis as the predominant organism isolated from urine and vaginal swabs, whereas C. pelliculosa and C. tropicalis were major etiology in blood. Antifungal susceptibility pattern showed 100% susceptibility to voriconazole, and micafungin in C. tropicalis and C. albicans. Resistance to fluconazole noted in C. tropicalis and C. pelliculosa and amphotericin B resistance recorded in C. tropicalis and C. albicans.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Ethics committee at Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences, Sawangi (Meghe), Wardha, India, wide reference number DMIMS(DU)/IEC/May-2020/8843.
- Vallabhaneni S, Mody RK, Walker T, Chiller T. The Global Burden of Fungal Diseases. Infect Dis Clin North Am. 2016;30(1):1-11.
Crossref - Soares LA, Sardi J de CO, Gullo FP, et al. Anti dermatophytic therapy: prospects for the discovery of new drugs from natural products. Braz J Microbiol. 2013;44(4):1035-1041.
Crossref - Katharina K, Anja T, Juergen F, Marcus J, Selim C. Successful use of IFN-y for refractory systemic infection with Candida tropicalis in a child with AML and CARD9 mutation. Arch Pathol Clin Res. 2022;6(1):013-015.
Crossref - Ascioglu S, Rex JH, de Pauw B, et al. Defining Opportunistic Invasive Fungal Infections in Immunocompromised Patients with Cancer and Hematopoietic Stem Cell Transplants: An International Consensus. Clin Infect Dis. 2002;34(1):7-14.
Crossref - Papon N, Courdavault V, Clastre M, Bennett RJ. Emerging and Emerged Pathogenic Candida Species: Beyond the Candida albicans Paradigm. PLoS Pathog. 2013;9(9):e1003550.
Crossref - Marak MB, Dhanashree B. Antifungal susceptibility and biofilm production of Candida spp. isolated from clinical samples. Int J Microbiol, 2018(7495218), 7495218.
- Arendrup MC, Patterson TF. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J Infect Dis. 2017;216(suppl_3):S445-S451.
Crossref - Mohanty S, Xess I, Hasan F, Kapil A, Mittal S, Tolosa JE. Prevalence & susceptibility to fluconazole of Candida species causing vulvovaginitis. Indian J Med Res. 2007;126(3):216-219.
- Sheevani null, Sharma P, Aggarwal A. Nosocomial Candida infection in a rural tertiary care hospital. J Clin Diagn Res. 2013;7(2):405-406.
Crossref - Khadka S, Sherchand JB, Pokhrel BM, et al. Isolation, speciation and antifungal susceptibility testing of Candida isolates from various clinical specimens at a tertiary care hospital, Nepal. BMC Res Notes. 2017;10(1):218.
Crossref - Sanglard D. Emerging Threats in Antifungal-Resistant Fungal Pathogens. Front Med. 2016;3.
Crossref - Kumari KS, Raghunath P, Harshavardhan B, Chaudhury A. Distribution of Candida albicans and the Non-Albicans Candida Species in Different Clinical Specimens from South India. International Journal of Microbiological Research, 2014; 5(1): 01-05.
Crossref - Kunyeit L, Kurrey NK, Anu-Appaiah KA, Rao RP. Probiotic Yeasts Inhibit Virulence of Non-albicans Candida Species. mBio. 2019;10(5):e02307-19.
Crossref - Sullivan DJ, Henman MC, Moran GP, et al. Molecular genetic approaches to identification, epidemiology and taxonomy of non-albicans Candida species. J Med Microbiol. 1996;44(6):399-408.
Crossref - Prasad Singh D, Kumar Verma R, Sarswat S, Saraswat S. Non-Candida albicans Candida Species: Virulence Factors and Species Identification in India. Curr Med Mycol. 2021;7(2):8-13.
Crossref - Magill SS, Shields C, Sears CL, Choti M, Merz WG. Triazole Cross-Resistance among Candida spp.: Case Report, Occurrence among Bloodstream Isolates, and Implications for Antifungal Therapy. J Clin Microbiol. 2006;44(2):529-535.
Crossref - Ahmad S, Alfouzan W. Candida auris: Epidemiology, Diagnosis, Pathogenesis, Antifungal Susceptibility, and Infection Control Measures to Combat the Spread of Infections in Healthcare Facilities. Microorganisms. 2021;9(4):807.
Crossref - Asadzadeh M, Mokaddas E, Ahmad S, et al. Molecular characterisation of Candida auris isolates from immunocompromised patients in a tertiary care hospital in Kuwait reveals a novel mutation in FKS1 conferring reduced susceptibility to echinocandins. Mycoses. 2022;65(3):331-343.
Crossref - Lockhart SR, Lyman MM, Sexton DJ. Tools for Detecting a “Superbug”: Updates on Candida auris Testing. J Clin Microbiol. 2022;60(5):e00808-21.
Crossref - Al-Obaid I, Asadzadeh M, Ahmad S, et al. Fatal Breakthrough Candidemia in an Immunocompromised Patient in Kuwait Due to Candida auris Exhibiting Reduced Susceptibility to Echinocandins and Carrying a Novel Mutation in Hotspot-1 of FKS1. J Fungi. 2022;8(3):267.
Crossref - Welsh RM, Bentz ML, Shams A, et al. Survival, Persistence, and Isolation of the Emerging Multidrug-Resistant Pathogenic Yeast Candida auris on a Plastic Health Care Surface. J Clin Microbiol. 2017;55(10):2996-3005.
Crossref - Abdolrasouli A, Armstrong-James D, Ryan L, Schelenz S. In vitro efficacy of disinfectants utilised for skin decolonisation and environmental decontamination during a hospital outbreak with Candida auris. Mycoses. 2017;60(11):758-763.
Crossref - Singh R, Kaur M, Chakrabarti A, Shankarnarayan SA, Rudramurthy SM. Biofilm formation by Candida auris isolated from colonising sites and candidemia cases. Mycoses. 2019;62(8):706-709.
Crossref - Tellapragada C, Eshwara VK, Johar R, et al. Antifungal Susceptibility Patterns, In Vitro Production of Virulence Factors, and Evaluation of Diagnostic Modalities for the Speciation of Pathogenic Candida from Blood Stream Infections and Vulvovaginal Candidiasis. J Pathog. 2014;1-8.
Crossref - Mohandas V, Ballal M. Distribution of Candida Species in different clinical samples and their virulence: Biofilm formation, proteinase and phospholipase production: A study on hospitalized patients in Southern India. J Glob Infect Dis. 2011;3(1):4-8.
Crossref - Iyampillai T, Michael JS, Mathai E, Mathews MS. Use of CHROMagar medium in the differentiation of Candida species: is it cost-effective in developing countries? Ann Trop Med Parasitol. 2004;98(3):279-282.
Crossref - Horvath LL, Hospenthal DR, Murray CK, Dooley DP. Direct Isolation of Candida spp. from Blood Cultures on the Chromogenic Medium CHROMagar Candida. J Clin Microbiol. 2003;41(6):2629-2632.
Crossref - Yang YL, Chu WL, Lin CC, Tsai SH, Chang TP, Lo HJ. An emerging issue of mixed yeast cultures. J Microbiol Immunol Infect. 2014;47(4):339-344.
Crossref - Rajeevan S, Thomas M, Appalaraju B. Characterisation and Antifungal susceptibility pattern of Candida species isolated from various clinical samples at a tertiary care centre in South India. Indian J Microbiol Res. 2016;3(1):53.
Crossref - Chakrabarti A, Chatterjee SS, Rao KLN, et al. Recent experience with fungaemia: change in species distribution and azole resistance. Scand J Infect Dis. 2009;41(4):275-284.
Crossref - Xess I, Jain N, Hasan F, Mandal P, Banerjee U. Epidemiology of Candidemia in a Tertiary Care Centre of North India: 5-Year Study. Infection. 2007;35(4):256-259.
Crossref - Singh RI, Xess I, Mathur P, Behera B, Gupta B, Misra MC. Epidemiology of candidaemia in critically ill trauma patients: experiences of a level I trauma centre in North India. J Med Microbiol. 2011;60(3):342-348.
Crossref - Jung J, Moon YS, Yoo JA, Lim JH, Jeong J, Jun JB. Investigation of a nosocomial outbreak of fungemia caused by Candida pelliculosa (Pichia anomala) in a Korean tertiary care center. J Microbiol Immunol Infect. 2018;51(6):794-801.
Crossref - Esgin H, Bulut E, Orum C. Candida pelliculosa endophthalmitis after cataract surgery: a case report. BMC Res Notes. 2014;7(1):169.
Crossref - Ahmad S, Khan Z, Asadzadeh M, Theyyathel A, Chandy R. Performance comparison of phenotypic and molecular methods for detection and differentiation of Candida albicans and Candida dubliniensis. BMC Infect Dis. 2012;12(1):230.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.