ISSN: 0973-7510
E-ISSN: 2581-690X
The present study reports isolation of Candida tropicalis RA1 from oil contaminated refinery soil. The study depicted production of 31 g sophorolipid (SLs) l-1 by Candida tropicalis RA1 until 240 h, using soya oil. Semi-purified SLs had critical micelle concentration of 0.5%, minimum surface tension of 30 mN m-1, oil spreading activity of 32.2 mm2, and emulsification index of 85% against crude oil. The major SLs forms observed were non-acetylated lactonic and di-acetylated acidic type, with 35.5% and 32.2% relative abundance. Semi–purified SLs inhibited human pathogens; Escherichia coli, Listeria monocytogenes and Staphylococcus aureus at 1000, 500, and 250 µg ml-1 minimum inhibitory concentrations (MIC) respectively. In addition, C. tropicalis RA1 represented 74% mercury biosorption at 0.5 mg l-1 concentration, by day 15. The present study reports establishment of equilibrium phase of lead, cadmium, and mercury at concentrations higher than maximum contaminant levels (MCL) standards. The observations reveal the future potential applications of C. tropicalis RA1 in the industrial, biomedical, and bioremediation fields respectively.
Antimicrobial properties, Biosurfactant, Biosorption, Candida tropicalis, Heavy metals, Sophorolipid.
Sophorolipids (SLs) are natural surfactants of microbial origin. Covalent bonding between hydrophilic and hydrophobic segments of glycolipid demonstrates low toxicity along with solubilization and emulsification properties. Biosurfactant have sculptured a promising position due to uncommon environmental friendliness. They overcome major drawbacks associated with chemical surfactants. Biosurfactants can be produced from renewable materials. They possess high specificity and remain functional under extreme conditions. In recent times, more attention is being paid to investigate biomedical functionalities of biosurfactant including; antimicrobial, antiviral, and anticancer properties1. Also, their ionic nature effectively removes the heavy metals from polluted land sites or aquatic bodies2.
Nonetheless, advantages of these bio-molecules contradict with huge costs of manufacturing and recovery. It demands significant enhancement and innovation in order to escalate the commercialization rate of biosurfactant. Also, novel microbial strains and biosurfactants are equally significant to fulfill new challenges.
Sophorolipids are commercial biosurfactant that demonstrates excellent surface activity, and antimicrobial, anticancer, antiviral, and biodegradation properties. Sophorolipid biosurfactant are produced by Candida bombicola, Candida apicola, Candida batistae, Wickerhamiella domercqiae, and Rhodotorula bogoriensis. The most famous, Saccharomyces bombicola ATCC 22214 is reported for high SLs yields using vegetable oils and saccharide substrates. In addition, Rispoli et al.3 reported 177 g SLs l-1 by S. bombicola ATCC 22214 using simplex–centroid design studies. The SLs production using feed stocks and microbial strains was also reported by Shao et al.4 and Cavalero and Cooper5. However, few researches by Chandran and Das6, Almeida et al.7, and Daylin et al.8 state SLs production using C. tropicalis, where diesel oil, oleic acid have been reported substrates. The current research reports SLs production by C. tropicalis RA1, isolated from oil contaminated refinery soil. The study investigates SLs characteristics using FT-IR and LC-MS analysis. In addition, antimicrobial and biosorption properties of C. tropicalis RA1 are reported.
Isolation of yeast
Soil samples from oil contaminated refinery area, Pune, Maharashtra, India were used for the isolation of biosurfactant producing microorganisms. Samples were collected in sterile glass tubes. Isolation of yeast was followed according to Gumel et al.9 with some modifications. The soil sample (1 g) was enriched in 100 ml Yeast extract–Peptone–Dextrose broth medium (YPD) containing; Yeast extract, 10 g l-1; Peptone, 20 g l-1; Dextrose, 20 g l-1, and 100 mg l-1 Azithromycin used as antibacterial agent. It was incubated at 30 ± 2°C for 48 h. The grown biomass (0.1 ml) was spread inoculated on YPD agar medium. It was incubated at 30 ± 2°C for 48 h. Isolated colonies were purified on YPD agar medium.
The isolated colonies were evaluated for biosurfactant production using surface tension (ST) reduction, oil displacement, drop collapse, and emulsification index (E24) analysis. The ST reduction was determined using Minimum salt medium (MSM), containing NaNO3, 15 g l-1; KCl, 1.1 g l-1; NaCl, 1.1 g l-1; FeSO4, 0.00028 g l-1 KH2PO4 3H2O, 3.4 g l-1; K2HPO4, 4.4 g l-1; MgSO4, 0.5 g l-1; Yeast Extract, 0.5 g l-1; and 2% soya oil. Digital tensiometer (Kruss Tensiometer) was used for ST analysis.
Gene
18S rRNA gene sequencing and phylogenetic analysis
Yeast strain RA1 was selected for 18S rRNA gene sequencing. Extraction and purification of DNA was performed using Cetyl trimetyl ammonium bromide (CTAB) and Chloroform/Isoamyl alcohol (24:1, v v-1)10. The polymerase chain reaction (PCR) was performed using a pair of 18S F (GTCAGAGGTGAAATTCTTGG-ATTTA) and 18S R (AGGGCAGGGACGTAA TCAACG) primers. The PCR reaction was performed in 25 µl volume using 1 µl of each primer, deoxynucleotide triphosphates (dNTPs, 10 mM each), 0.5 µl Taq DNA polymerase (Takara) supplied with 10x PCR buffer (5 µl), and water. The PCR was performed using program : 95°C for 5 min, 95°C, 1 min; 50°C, 1 min; 72°C, 2 min, and 72°C for 5 min followed by 30 cycles. The PCR products were purified by QIAquick gel extraction kit (Qiagen, Cat no-28704) and directly sequenced with Big dye terminator V3.1 Cycle sequencing Kit (Applied Biosystems, Cat no-4337455). Result of cycle sequencing reaction was observed on Genetic Analyzer computer using sequencing analysis software 5.2. The similarity of the 18S rRNA sequence to the GenBank genomic sequences was investigated using BLASTN software (http://www.blastn.ncbi.nlm.nih.gov). The isolated yeast strain was identified after bioinformatics analysis.
Nucleotide sequence accession number
Partial 18S rRNA gene sequence of 634 bp obtained in the present study was deposited in the GenBank database (accession number MH161378).
Culture preservation, inoculum preparation, and fermentation
Yeast strain RA1 was preserved in YPD broth medium supplemented with 15% (v v-1) glycerol. The strain was archived at -80°C. Viability testing was performed after a monthly interval.
For inoculum preparation, one vial of glycerol stock was inoculated in 100 ml YPD broth in 250 ml capacity Erlenmeyer flask and incubated at 30 ± 2°C for 24 h. Inoculum was observed microscopically. Microbial cell count of the inoculum was determined using Neubar Chamber Haemocytometer (Rohem, India). Fermentation studies were performed using 1 liter MSM supplemented with 10% inoculum and 2% soya oil, at reaction conditions of 30 ± 2°C until 240 h. The sample was analysed for biomass concentration (g/l), SLs concentration (g/l), pH, and ST after every 24 h.
Biomass determination and biosurfactant extraction
In the process of biomass determination, 10 ml of whole cell broth was sampled from fermenter at the interval of 24 h. It was centrifuged at 23008 x g for 15 min. The wet biomass was rinsed three times using distilled water. It was dried in a hot air over at 105°C until 24 h, followed by dry weight estimation 11. SLs were solvent extracted using ethyl acetate as described by Smyth et al.12. For this, whole cell broth was treated with ethyl acetate (1:1, v v-1) three times, and pellet containing SLs was removed, washed properly with distilled water.
Purification of biosurfactant
Sophorolipid purification was performed using silica gel column chromatography12. The method was modified in which the column (24 ׳ 3.0 cm2) was packed with silica gel slurry (mesh size 60–120 mm) mixed with chloroform. Before loading to the column, crude SLs (1g) extract was dissolved in 10 ml chloroform. Gradient system of chloroform: methanol (0%–50%) was used for the elution of samples. Elutes were collected and vacuum dried at 50°C. The samples were stored at -20°C till further usage.
Biosurfactant characterisation
Surface tension and critical micelle concentration were determined to characterise the biosurfactant produced by strain RA1. Fermented broth of strain RA1 was used for evaluation; every 24 h, until 240 h. The surface tension was measured using tensiometer at 25°C with pendant drop method. Tensiometer was calibrated using distilled water (ST 72.5 mN m-1) to have definite analysis. Critical micelle concentration was obtained using Rufino et al.13 method. For this, semi–purified SLs was used in the range of 10 mg l-1 to 300 mg l-1.
Emulsification index analysis
Emulsification index was determined using Cooper and Goldenberg14 method, It was modified wherein; oil substrate and cell–free broth were mixed in the test tube (1:1, v v-1); vortexed for 120 s. The mixture was left until 24h. Emulsification index was calculated using following equation.
Where, E24= Emulsification index, He= Height of the emulsion layer and Ht= Height of total mixture.
Stability determination
The cell free broth was used to determine the stability of SLs biosurfactant. The ST reduction was examined for pH, temperature, and salinity parameters. The pH stability was examined using pH range of 2.0–10.0. The values were adjusted using 50% NaOH or 50% HCl. The thermal stability was analysed at temperature range of 20°C to 121°C. The salinity was determined using 5%–20% NaCl (w v-1).
Compositional analysis of biosurfactant
Thin layer chromatography
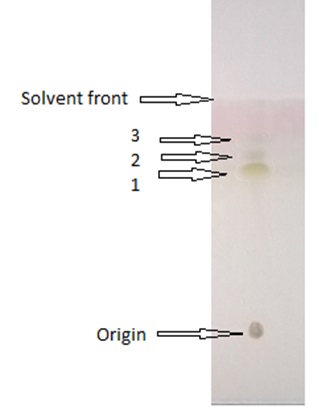
Thin layer chromatography (TLC) was performed using solvent extracted SLs. For this, 10 µl of the phase separated semi-purified SLs was applied over thin layer chromatography on pre-coated silica gel of standard 20 x 20 Kiesel-gel 60 F 254 Merck plates using solvent system of Chloroform / Methanol / Water (6.5:1.5:0.2, v v-1)12. P–anisaldehyde was used for visualization purpose.
Fourier transformation infrared spectroscopy
Semi–purified SLs was used for Fourier transformation infrared spectroscopy (FT-IR) analysis. The FT–IR spectra were reported using Perkin-Elmer 31725 X FT–IR spectrophotometer in a spectral region of 4000–400 cm-1, where potassium bromide (KBr) solid cells were used. Spectra were recorded and analysed using standard method15.
Liquid chromatography-mass spectrometry analysis
Mass spectra of SLs were reported on MS system. It consists of an HPLC (Agilent 1200 Series, Agilent Technologies) and 6210 Time–of–Flight LC–MS (Agilent Technologies). Zorbax Eclipse Plus C18 column and a DAD detector were used for recording purpose. The mobile phase contained solvent A (0.2% formic acid in water) and B (acetonitrile) in a gradient mode: 0–1.5 min 95% A, 1.5–12 min 95–5% A, 12–15 min 5% A, 15–16 min 5–95% A16.
Antibacterial activity
The antibacterial activity of SLs was studied against human pathogens; Escherichia coli (ATCC 35218), Listeria monocytogenes (ATCC 19215) and Staphylococcus aureus (NCIM 5345, NCIM 2079) using micro-broth dilution technique. It was performed using two fold serial dilutions in a 96–well flat bottom microtiter plate10. The assay was interpreted using minimum inhibitory concentration (MIC). It is described as the lowest concentration at which; no growth is recorded.
Heavy metal ions biosorption
Yeast biomass preparation for biosorption
The yeast strain, C. tropicalis RA1 was inoculated in 100 ml YPD broth (pH 7.0) and incubated at 30°C ± 2°C for 24 h. The cell biomass was harvested by centrifugation (Eppendorf, 5810 R) at 6603 x g for 30 min. The biomass was washed thrice with sterile water followed by freeze-drying using lyophilizer (Heto Powder Dry LL 3000).
Biosorption experiment
Freeze–dried cells (0.5 g) were inoculated to a series of 500 ml Erlenmeyer flasks (Three flasks for each metal) containing 200 ml diluted solution of 0.1 mg l-1, 0.5 mg l-1, and 1.0 mg l-1 arsenic, lead, cadmium, mercury, chromium, and nickel. It was added independently in three sets for each different concentration studied. The concentrations of heavy metals were finalized on the basis of maximum contaminant levels (MCL) standards17. Initial pH of the broth was adjusted to 7.0. It was incubated at 30°C for 16 d. Cell broth (5 ml) was collected on d0, d5, d10, and d15 intervals. It was filtered using 0.2 µm membranes. The supernatant was analysed for metal ions, using Inductively Coupled Plasma Mass Spectrometry (ICP–MS, Perkin Elmer Optima 2100 DV). The biosorption experiments were run in triplicates. Also, blank experiments were conducted to ensure that no adsorption had taken place on the experimental apparatus used (data not shown). The heavy metal removal was calculated according to Ibrahim et al.18.
Where, Co is the initial metal ions concentration (mg l-1) and Ce is the equilibrium concentration of metal ions (mg l-1).
Statistical analysis
The experiments were performed in triplicates. The standard deviation (SD) was represented by error bars in the graphs. The significant differences between fermentation time (h) and SLs yield (g l-1) was calculated using simple linear regression where response of SLs yield (g l-1) was determined against independent variable of fermentation time (h). Differences between means were considered significant at a P value of 0.05. Whereas, significant differences between yeast biomass and heavy metal biosorption was analysed using multivariate analysis of variance (MANOVA) at CI of 95%. Statistical analysis was performed using the Minitab@ 17.1.0.
Isolation of yeast
Morphologically twelve different yeasts were isolated from oil contaminated refinery soil. Among all, strain RA1 exhibited promising activities in primary screening. The strain displayed positive results in drop collapse with 85% E24 against crude oil. Oil displacement denoted an area of 32.2 mm2, along with decrease in ST (from 68.8 mN m-1 to 29.9 mN m-1) was observed. The 18S rRNA gene sequencing of strain RA1 generated a contig sequence of 634 bp. NCBI BLAST analysis of strain RA1 revealed 99% similarity with C. tropicalis KY118179.1 and KU341838.1 respectively. Based on the results, strain RA1 was identified as C. tropicalis (Fig. 1a–1b).
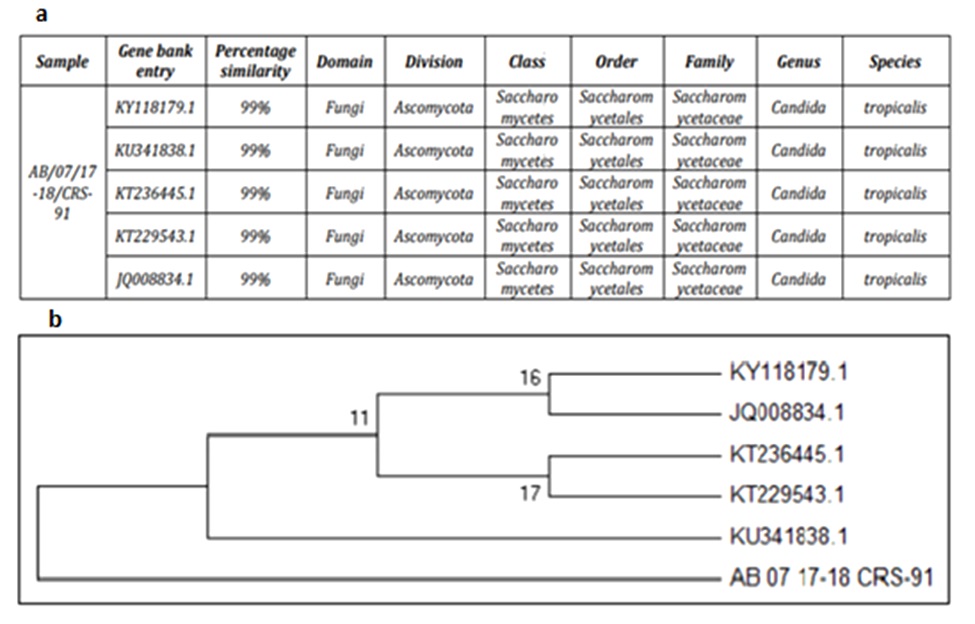 Fig. 1a–1b.Phylogenetic tree of Candida tropicalis RA1 and its related sequences retrieved from NCBI
Fig. 1a–1b.Phylogenetic tree of Candida tropicalis RA1 and its related sequences retrieved from NCBI
 Fig. 2. . Growth kinetics, pH, biomass production, and sophorolipid yield by Candida tropicalis RA1 using minimal salt medium + 2% soya oil (w v-1), plotted as a function of time (240 h)
Fig. 2. . Growth kinetics, pH, biomass production, and sophorolipid yield by Candida tropicalis RA1 using minimal salt medium + 2% soya oil (w v-1), plotted as a function of time (240 h)Biosurfactant production and kinetics
The growth characteristic feature and SLs production by C. tropicalis RA1 are presented in Fig. 2. Current research reports a short lag phase while yeast growth. The strain displayed exponential phase until 144 h. During this period, 35 g l-1 biomass and 1.7×1010 colony forming units (CFU) ml-1 were observed. The stationary phase represented 36 g l-1 cell biomass, after 216 h of growth. Reduction in biosurfactant yield from 32 g l-1 to 22 g l-1 after 240 h, was statistically significant (P < 0.01). The present study reports 3.21×1010 CFU ml-1 after 72 h wherein ST reduction observed from 68 mN m-1 to 29.9 mN m-1 remained stable until 240 h. No significant change in the pH was observed during the experiment. The present research exemplifies 35 g SLs l-1 yield by C. tropicalis RA1 using 2% soya oil.
Surface tension measurement and critical micelle concentration
The sophorolipid biosurfactant of up to 0.5% concentration decreased the ST from 72.2 mN m-1 to 30 mN m-1 (Fig. 3).
 Fig. 3. Critical micelle concentration and minimum surface tension analysis of biosurfactant by C. tropicalis RA1
Fig. 3. Critical micelle concentration and minimum surface tension analysis of biosurfactant by C. tropicalis RA1Emulsification index (E24)
The sophorolipid biosurfactant generated stable emulsion with crude oil. It has showed E24 of 85%, stable for 24 h (Fig. 4).
 Fig. 4. Emulsification index analysis of 240 h fermented broth of Candida tropicalis RA1. The study includes 0.1% SDS and water used as a positive and negative control respectively
Fig. 4. Emulsification index analysis of 240 h fermented broth of Candida tropicalis RA1. The study includes 0.1% SDS and water used as a positive and negative control respectivelyStability study
The stability evaluation of SLs is represented in Fig. 5a–5c. SLs demonstrated stable ST reduction while increase in NaCl concentration from 5–20%, pH range of 2.0–10.0 and temperature range of 20°C to 121°C.
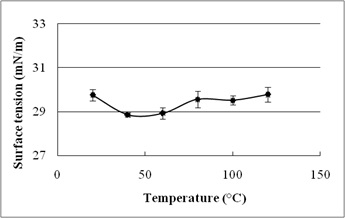
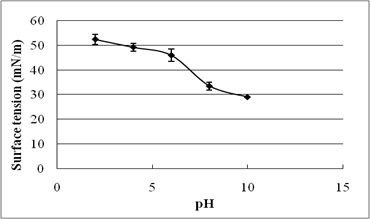
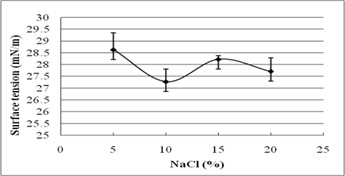
Fig. 5. Effect of (a) temperature (°C) (b) pH and (c) salinity (%) on surface tension reduction. Error bars illustrate standard error of mean (SEM), calculated from two independent experiments run in triplicates
Compositional analysis of biosurfactant
Thin layer chromatography
Thin layer chromatography analysis of the SLs biosurfactant is represented in Fig. 6.
Thin layer chromatogram revealed ‘sophorolipid’ nature of the biosurfactant. In addition, it was determined to be composed of lactonic form SLs (LS). The Rf values of the lactonic SLs were 0.48, 0.58, and 0.65 respectively.
Fourier transform infrared spectroscopy
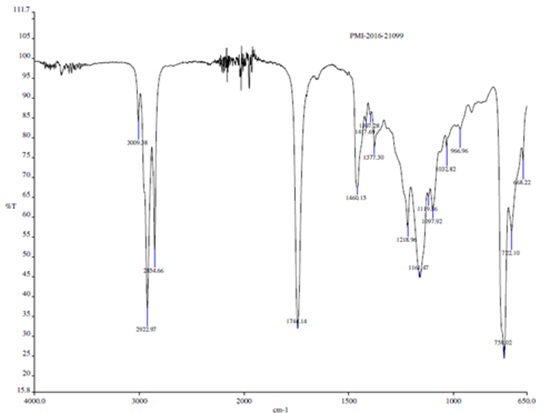
The functional groups of biosurfactant were confirmed by FT–IR spectra of C. tropicalis RA1 as represented in Fig. 7.
FT–IR analysis revealed that, heterogeneity evidenced by different characteristic peaks was in agreement with the possible presence of amino, carboxylic, hydroxyl and carbonyl groups. FT–IR spectrum peaks at 3009 cm-1, 2922 cm-1, 2854 cm-1 and 1744 cm-1 confirmed the presence of glycolipid biosurfactant.
The FT–IR range of C. tropicalis RA1 had specific stretching vibration band of –OH, throughout 3009.38 cm-1. The potent peaks at 2922.97 cm-1 and 2854.66 cm-1 were originated by the bending and stretching of –CH groups. Immersion around 2922.97 cm-1 and 2854.66 cm-1 is specified to the asymmetric C–H stretch of CH2 and CH3 groups of aliphatic chains. Also, a weak symmetric stretching peak at 1741.14 cm-1 demonstrated the existence of ester carbonyl group (C=O in COOH) in the biosurfactant. The band at 1417.69 cm-1 and 1460.15 cm-1 be compatible to C–O–H in the plane binding of carboxylic acid (–COOH). The ester carbonyl group was demonstrated by the band at 1377.30 cm-1 corresponding to C–O deformation vibrations, although different groups were absorbed in this region. Absorption bands at 1218.96 cm-1 represents O–H deformation. The peak at 1161.47 cm-1, 1097.92 cm-1,1019.36 cm-1, 1032.82 cm-1 and 966.96 cm-1 represent C–O stretching and CH3 rocking.
Liquid chromatography–mass spectrometry
The LC–MS analysis of SLs biosurfactant was acquired in the positive mode (Fig. 8a.–8i.).
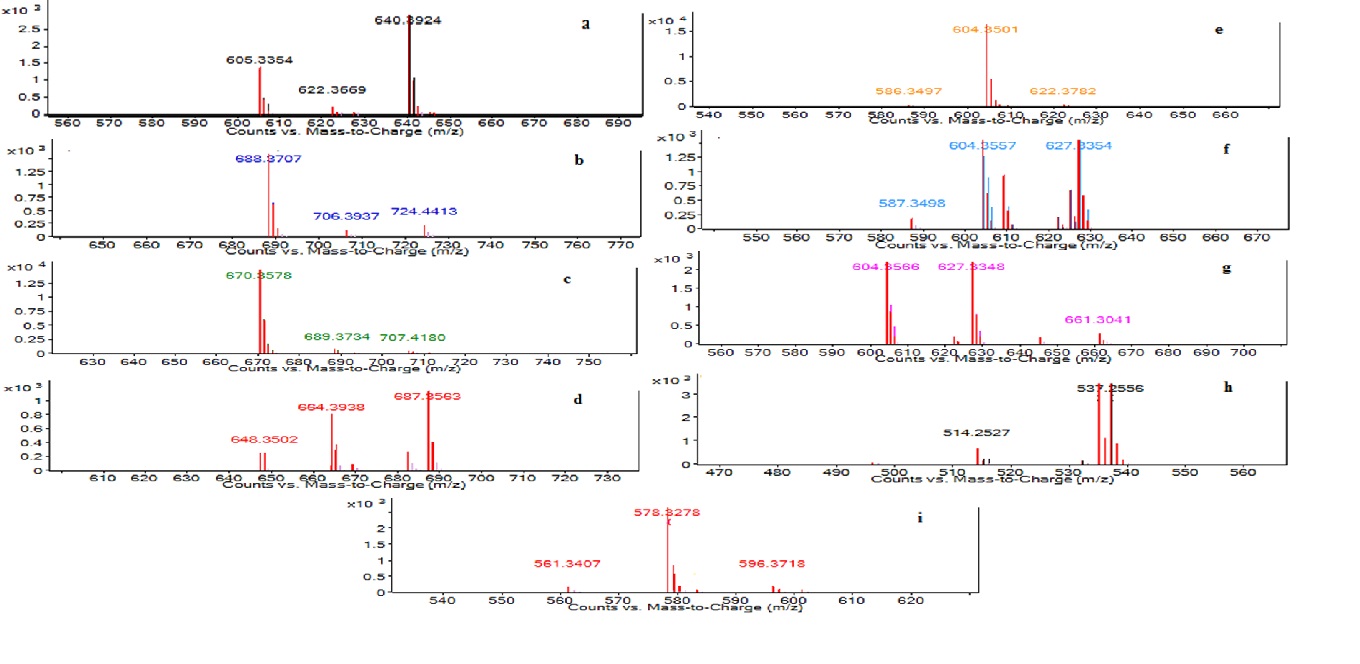 Fig. 8a.–8i. LC–MS Characterization of sophorolipid by Candida tropicalis RA1 [LC–MS run in positive electrospray ionization mode (+ESI)]
Fig. 8a.–8i. LC–MS Characterization of sophorolipid by Candida tropicalis RA1 [LC–MS run in positive electrospray ionization mode (+ESI)]The present study reports nine forms of semi-purified SLs. Each eluting fragment of SLs had distinct MS chromatogram (8a–8i). The semi-purified SLs had four AS and three LS homologues with C 18:1 unsaturated fatty acid chain. Non-acetylated AS was detected like [M+NH4]+, [M+Na]+[-H2O] at m/z 640.39, 627.33 respectively. Additionally, [M+K]+[-H2O] unique non-acetylated AS was detected at m/z 537.25. The peak at m/z 687.35 correspond to [M+Na]+of mono-acetylated AS. M+[-H2O] and M+[-H2O] ions for di-acetylated AS were observed at m/z 688.37 and 670.35 respectively. Non-acetylated LS were detected with sum of three ions such as [M+Na]+[-H2O] at m/z 609.32 and two M+ ions at m/z 604.35 and 578.32 respectively. Table 1 represents complete list of SLs homologues detected in C. tropicalis RA1.
Table (1):
The chemical formula, molecular mass, and structural features of sophorolipid homologues by Candida tropicalis RA1 determined using LC–MS
Structural Features |
Formula |
Molecular Mass |
Sophorolipid Type |
|---|---|---|---|
Non-acetylated acidic SL fatty acid C18:1 |
C30H54O13 |
622.35 |
Acidic |
Di-acetylated acidic SL fatty acid C18:1 |
C34H58O15 |
706.38 |
Acidic |
Di-acetylated acidic SL fatty acid C18:1 |
C34H56O14 |
688.36 |
Acidic |
Mono-acetylated acidic SL fatty acid C18:1 |
C32H56O14 |
664.36 |
Acidic |
Non-acetylated lactonic SL fatty acid C18:1 |
C30H52O12 |
604.35 |
Lactonic |
Non-acetylated lactonic SL fatty acid C18:1 |
C30H52O12 |
604.35 |
Lactonic |
Non-acetylated acidic SL fatty acid C18:1 |
C30H54O13 |
622.36 |
Acidic |
Non-acetylated acidic SL fatty acid C11 |
C22H42O13 |
514.26 |
Acidic |
Non-acetylated lactonic SL fatty acid C16 |
C28H50O12 |
578.33 |
Lactonic |
Antibacterial activity
The antibacterial activity of semi–purified SLs evaluated against human pathogens; Escherichia coli, Listeria monocytogenes, and Staphylococcus aureus is presented in Table 2. The minimum inhibitory concentration (MIC) was determined therein.
Table (2):
Antibacterial activity of sophorolipid by Candida tropicalis RA1 against human pathogens
Human Pathogens* |
Minimum Inhibitory Concentration (µg ml-1) |
|---|---|
Escherichia coli |
1000 |
Listeria monocytogenes |
500 |
Staphylococcus aureus |
250 |
Staphylococcus aureus |
250 |
Human pathogens collection resource: Escherichia coli: ATCC 35218, Listeria monocytogenes: ATCC 19115, Staphylococcus aureus: NCIM 5345 and Staphylococcus aureus: NCIM 2079
The SLs was highly effective against S. aureus (NCIM 5345, 2079) followed by E. coli (ATCC 35218), and L. monocytogenes (ATCC 19115) respectively.
Heavy metal ions biosorption
The biosorption efficacy of C. tropicalis RA1 against 0.10 mg l-1, 0.5 mg l-1, and 1.0 mg l-1 concentration of arsenic, lead, cadmium, mercury, chromium, and nickel is represented in Fig. 9a–9c.
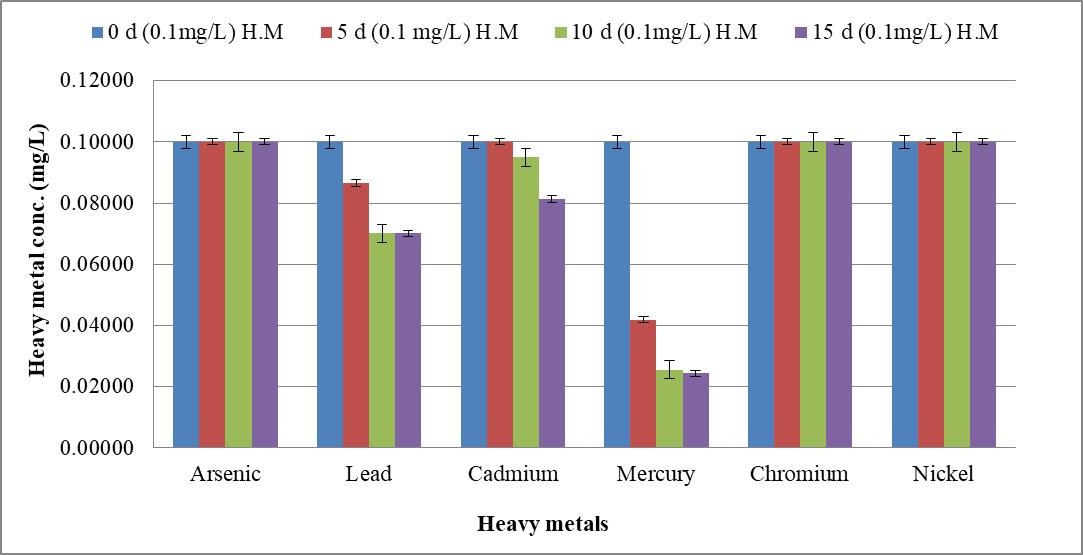
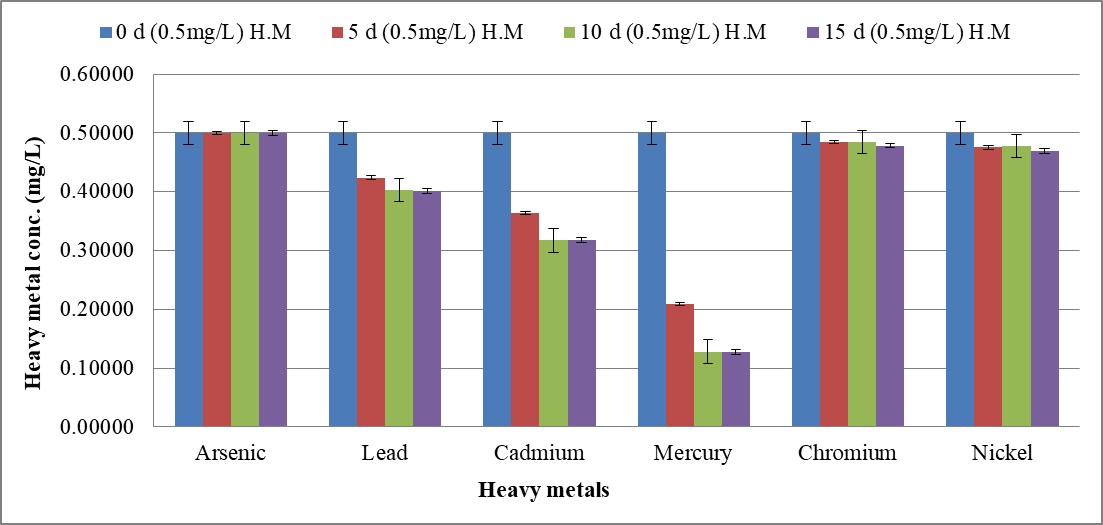
 Fig. 9. Heavy metal ion biosorption by Candida tropicalis RA1 using (a) 0.1 mg l-1 (b) 0.5 mg l-1 and (c) 1.0 mg l-1 concentration of heavy metal ions
Fig. 9. Heavy metal ion biosorption by Candida tropicalis RA1 using (a) 0.1 mg l-1 (b) 0.5 mg l-1 and (c) 1.0 mg l-1 concentration of heavy metal ionsHighest heavy metal reduction was observed for mercury from 58 to 74% until d15, at 0.1 mg l-1 and 0.5 mg l-1 concentrations respectively. The mercury with 1.0 mg l-1 concentration was reduced up to 67% on d15.
Beside this, lead in 0.1 mg l-1 concentration had 13% reduction in d5, was noted with 29% decrease until d15. The reduction trend was slowed down at 0.5 mg l-1, to 19%. Further, no lead reduction was observed at 1.0 mg l-1.
The cadmium in 0.5 mg l-1 was reduced up to 36% until d15. Its further reduction was slowed down to 8% at 1.0 mg l-1 concentration. There was no change in percent reduction of cadmium and mercury at 0.1 mg l-1, 0.5 mg l-1, and 1.0 mg l-1 concentrations. The similar observation was noted for lead at 0.1 mg l-1 and 0.5 mg l-1 concentrations respectively. Nonetheless; 0.1 mg l-1 chromium and nickel were reduced by 4% and 6% therein.
The present research is aimed at screening of novel SLs biosurfactant producing microorganisms. It was followed by physico–chemical characterisation, biomedical, and bioremediation application studies. The microbial cultivation using water immiscible hydrocarbons supports biosurfactant production19. In this context, some of the highlighted references are of Dhail et al. 20 and Sneha et al. 21. They have preferred oil reserviors; oil spilled marine water, and marine sediments for isolation of biosurfactant producing microorganisms. In fulfillment of our research objectives, oil contaminated refinery soil has been selected as a sampling source. Since oil is crucial element for the growth of biosurfactant producing microorganisms, most efficient strains can be isolated using oil contaminated refinery soil. The microbes acclimatize well to the high concentration of oil being used as a sole carbon source. In this context, yeast strain RA1 isolated from oil contaminated refinery soil exhibited promising activities in primary screening towards SLs biosurfactant production.
Since E24 determination and drop collapse test belong to selective methods22, promising activity in drop collapse test and 85% E24 against crude oil marks unique properties of the SLs. Morikawa et al.23 reported direct proportion between zone of clearance and the concentration of surface active agent. In this view, oil displacement of 32.2 mm2 stands out efficacy of the SLs. According to Banat et al.24, microbes can decrease the ST to 35 mN m-1. The present study reports ST reduction from 68.8 mN m-1 to 29.9 mN m-1 suggesting SLs efficacy.
The strains displaying short lag phase can be considered for SLs biosurfactant production. Inversely, some strains represent long lag phase and biosurfactant production at late log phase or stationary phase25. The current study reports a short lag phase while yeast growth and exhibited an exponential phase until 144 h. However, maximal cell biomass production occurred during the stationary phase. The statistically significant difference between SLs yield and yeast growth phase, suggests about growth associated kinetics. Since yeast strains have different mechanisms behind SLs production, the interrelationship varies.
It suggests that, SLs is produced as primary metabolite accompanying cellular biomass increase26 with effective production under thermostat conditions27. Also, decrease in ST from 68 mN m-1 to 29.9 mN m-1 represents 3.21×1010 CFU ml-1 that remained stable until 240 h. This is in agreement with Accorsini et al.28 where, exponentially growing cells of Candida antarctica exhibited greatest ST reduction. Similarly, Rufino et al.29 and Sobrinho et al.30 reported highest ST reduction during exponential growth phase of Candida lipolytica and Candida sphaerica. However, Rufino et al.13 stated direct proportion between biosurfactant and biomass, having inverse proportion to ST. Previous research conducted by Amezcua-Veja et al.31 had similar observations for biosurfactant production and ST reduction by Candida ingens. This indicates that; biosurfactant production and ST reduction are not cell growth associated. No significant change in pH was observed during experiment which is in accordance with Luna et al.32.
Previous researches report that; SLs yield differs according to the type of substrate used. Accorsini et al.28 reported highest decrease in ST, using 6% soya oil and glutamic acid substrates. Rufino et al.29 and Sobrinho et al.30 reported 4.5 g SLs l-1 using secondary carbon source. The present study represents 35 g SLs l-1 using 2% soya oil, exemplyfying the efficacy.
Critical micelle concentration is one of the integral properties of the SLs. The current research reports decrease in ST from 72.2 mN m-1 to 30 mN m-1, by increasing the SLs concentration up to 0.5%. A lower CMC represents high efficiency of the surfactant33. Accorsini et al.28 reported 130 mg l-1 (CMC) and 39 mN m-1 (ST) for a mixture of SLs produced by C. bombicola. Sen et al.10 reported similar CMC for SLs biosurfactant by Rhodotorula babjevae YS3. Daylin et al.8 reported decrease in ST from 70 mN m-1 to 28.8 mN m-1; at CMC concentration of 1.5% in C. tropicalis UCP 1613. The CMC varies in different strains due to nature of the acyl chain and open-chain or lactone form. Price et al.34 reported CMCs of 5.6 mg l-1 and 6.9 mg l-1 for S. bombicola sophorolipids; 60, 600-diO-acetyl-b-D-Glcp-21-O-b-D-Glcp-17-hydroxystearate 1,40 –lactone and 60, 600-di-O-acetyl-b-D-Glcp-21-O-b-D-Glcp-17-hydroxyoleate 1,40–lactone. Also, the novel anionic, open-chain sophorolipid 60, 600-di-O-acetyl-b-D-Glcp-21 -O-b-D-Glcp-18-hydroxyoleic from Candida sp. NRRL Y-27208 had CMC of 46.4 mg l-1. The current study reports three lactonic and six open chain SLs forms (Table 1), may have association with 0.5% CMC.
Sen et al.10 reported stable emulsion of R. babjevae YS3 SLs until 168 h, with E24 of 98%. The current study reports stable emulsion until 24 h with E24 of 85%.
In addition, biosurfactant stability under extreme environmental conditions is an important parameter of evaluation10. The present study reports excellent stability of SLs for pH, temperature, and salinity. It indicates their efficacy in bioremediation and oil recovery applications. Biosurfactant stability plays vital role in commercial and field applications. The current research reports ST reducing ability of SLs at 5-20% NaCl, indicating their application in marine environments. Luna et al.32 reported stable ST reduction and emulsification ability of biosurfactant by Candida sphaeria UCP0995 under extreme pH, temperature, and salinity. We report excellent ST reduction by SLs under extreme pH and temperature range of 2-10 and 20°C to 121°C.
Moreover, TLC analysis revealed the lactonic composition SLs. Sen et al.10 reported AS and LS forms representing Rf values of 0.49, 0.56, and 0.68. They do correspond with Rf values of the present study (0.48, 0.58, and 0.65). Fig. 6 chromatogram revealed ‘sophorolipid’ nature of the biosurfactant based on35,36.
The FT–IR range reveals the heterogeneity as evidenced by different characteristic peaks, representing the presence of amino, carboxylic, hydroxyl, and carbonyl group; the spectral observations were in alignment with research reports37,38,39. Similarly, the mass spectral ions from LC–MS analysis were determined using previous research35.
The biosurfactants demonstrate the antimicrobial properties; thus can replace the antibiotics that are plaguing the world today. Biosurfactants may exhibits antagonism through cellular membrane destabilization. It leads to cell rupture. Previous research demonstrated antibacterial activity of biosurfactant against Candida sp.40 and Pseudomonas sp.41. Morya et al.42 and Shah et al.43 reported that, antimicrobial activity of SLs biosurfactant varies with production media composition. We report the antimicrobial activity of SLs produced using 2% soya oil against E. coli, S. aureus and L. monocytogenes.
Fundamentally, SLs homologues may vary in different media, resulting into varying effects against pathogens44. The microbes including; Bacillus subtilis, Magnetospirillum gryphiswaldense, Rhizopus arrhizus, Saccharomyces cerevisiae, and Chaeto-morphalinum have been reported as potential biosorbents45, 46. Candida sp. is one of the most widely studied yeast for biosorption performance47. The current study reports 74% mercury reduction until d15 at 0.1 mg l-1 and 0.5 mg l-1. Further, 67% mercury reduction was reported at 1.0 mg l-1 concentration therein. The MCL guidelines17 reported 0.00003 mg l-1 to be an acceptable limit for mercury. This remarkable mercury reduction demands comprehensive research.
Beside this, decreasing trend of lead reduction was observed with increase in concentration from 0.1 mg l-1 to 0.5 mg l-1. Lead reduction was not observed at 1.0 mg l-1 concentration describing the MIC therein. Nonetheless, cadmium reduction was slowed down with increase in concentration from 0.5 mg l-1 to 1.0 mg l-1.
The present study reports uniform % reduction of lead, cadmium, and mercury at 0.1 mg l-1, 0.5 mg l-1 and 1.0 mg l-1 concentrations, on d10 and d15. The similar observation was noted for lead at 0.1 mg l-1 and 0.5 mg l-1, suggesting the establishment of equilibrium phase. The current research remarks heavy metal biosorption efficacy of C. tropicalis RA1 in polluted water or land sites.
In conclusion; the present research states that, oil refinery area can be one of the dynamic sources for isolation of most efficient biosurfactant producing microorganisms. To our knowledge, this is the first report wherein, C. tropicalis RA1 is recorded with nine distinct forms of SLs. The batch fermentation yields of 31 g SLs l-1 with short lag phase state its efficacy. The growth associated kinetics report the SLs production as primary metabolites. In addition, excellent antibacterial activity of SLs highlights its distinct advantage in the biomedical field. The biosorption efficacy of C. tropicalis RA1 against heavy metals, beyond MCL standards recommend the application in polluted sites.
Acknowledgements
R A is grateful to the Praj Matrix R & D Center, Division of Praj Industries Ltd, Pune; Maharashtra, India for supporting the research work conducted at Praj Matrix.
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
None.
Author Contributions
RA collected the samples, conducted the research, analysed data and prepared the manuscript. MC guided the entire research and manuscript preparation.
Data Availability
All datasets generated or analyzed during this study are included in the manuscript and can also be obtained from the corresponding author on reasonable request.
Ethics Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
- Wu, Y.S., Ngai, S.C., Goh, B.H., Chan K.G., Lee L.H., Chuah, L.H. Anticancer activities of surfactin and potential application of nanotechnology assisted surfactin delivery. Front. Pharmacol., 2017; 8: 761.
Crossref - Sarubbo, L.A., Junior, R.B., Luna, J.M., Rufino, R.D., Santos, V.A., Banat, I.M. Some aspects of heavy metals contamination remediation and role of biosurfactants. Chem. Ecol., 2015; 31: 707–723.
Crossref - Rispoli, F.J., Badia, D., Shah, V. Optimization of the fermentation media for SLs production from Candida bombicola ATCC 22214 using a simplex centroid design. Biotechnol. Prog., 2010; 26: 938–944.
Crossref - Shao, L., Song, X., Ma, X. Bioactivities of SLs with different structures against human esophageal cancer cells. J. Surg. Res., 2012; 173: 286–291.
Crossref - Cavalero, D.A., Cooper, D.G. The effect of medium composition on the structure and physical state of SLs by Candida bombicola ATCC 22214. J. Biotechnol., 2003; 103: 31–41.
Crossref - Chandran, P. and Das, N. Biosurfactant production and diesel oil degradation by yeast species Trichosporon asahiiisolated from petroleum hydrocarbon contaminated. Int. J. Eng. Sci. Technol., 2010; 2: 6942-6953.
- Almeida, D.G., Silva, S.F., Luna, J.M. Rufino, R.D., Santos V.A., Sarubbo, L.A. Response surface methodology for optimizing the production of biosurfactant by Candida tropicalis on industrial waste substrates. Front. Microbiol., 2017; 8: 1–13.
Crossref - Daylin, R.R., Rosieide, F. da. S. A., Goretti, S. Da. S., Rodrigo, A. De. H., Milagre, A.P., Paricia, N., Jose, C. V. J., Maria, A. De. R.S., Campos, T.G.M. Promising biosurfactant produced by a new Candida tropicalis UCP 1613 strain using substrates from renewable-resources. African J. Microbiol. Res., 2017; 11(23): 981–991.
Crossref - Gumel, A.M., Annuar, M.S., Chisti, Y. Recent advances in the production, recovery and applications of polyhydroxyalkonates. J. Polym. Environ., 2013; 21: 580–605.
Crossref - Sen, S., Borah, S.N., Bora, A., Deka, S. Production, characterization, and antifungal activity of biosurfactant produced by Rhodotorula babjevae YS3. Microbial Cell Factories, 2017; 16: 1–14.
Crossref - Nunez, A., Ashby, R., Foglia, T.A., Solaiman D. K.Y. Analysis and characterization of sophorolipids by liquid chromatography with atmospheric pressure chemical ionization. Chromatographia, 2001; 53: 673–677.
Crossref - Smyth, T.P., Perfumo, A., Marchant, R., Banat, I.M. Isolation and analysis of low molecular weight microbial glycolipids. Handbook of Hydrocarbon and Lipid Microbiology, 2010; 3705–3723.
Crossref - Rufino, R.D., Luna, J. M., Campos Takaki, G.M., Sarubbo L.A. Characterization and properties of the biosurfactant produced by Candida lipolytica UCP 0988. Electron J. Biotechnol., 2014; 17: 1–5.
Crossref - Cooper, D.G., Goldenberg, B.G. Surface-active agents from two Bacillus Species. Appl. Environ. Microbiol., 1987; 53: 224–229.
- Imura, T., Kawamura, D., Morita, T., Sato, S., Fukuoka, T. Yosuke, Y., Takahashi, M., Wada, K., Kitamoto, D. Production of sophorolipids from non-edible Jatropha oil by Stamerella bombicola NBRC 10243 and evaluation of their interfacial properties. J. Oleo. Sci., 2013; 62: 857–864.
Crossref - Samad, A., Zhang, J., Chen, D., Liang, Y. Sophorolipid production from bio-mass hydrolysates. Appl. Biochem. Biotechnol., 2015; 175: 2246–57.
Crossref - Babel, S., Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard Mater, 2003; 97: 219–243.
Crossref - Ibrahim, W.M., Hassan, A.F., Azab, Y.A. Biosorption of toxic heavy metals from aqueous solution by ulva lactuca activated carbon. Egypt J. Basic. Appl. Sci., 2016; 3: 241–249.
Crossref - Almeida, D.G., Silva, S.F., Luna, J.M., Rufino, R.D., Santos, V.A., Banat, I.M., Sarubbo, L.A. Biosurfactants: promising molecules for petroleum biotechnology advances. Front. Microbiol., 2016; 7: 1718.
Crossref - Dhail, S. Isolation of potent biosurfactant producing bacteria from oil spilled marine water and marine sediments. Afr. J. Biotechnol, 2012; 11:16751–16757.
- Sneha, K.S., Padmapriya, B., Rajeshwari, T. Isolation and Screening of Biosurfactants produced by Pseudomonas aeruginosa from oil spilled soils. Int. J. Pharm. Biol. Sci. Arch., 2012; 3:321–325.
- Nayarisseri, A., Singh, P., Singh, S.K. Screening, isolation and characterization of biosurfactant producing Bacillus subtilis strain ANSKLAB03. Bioinformation, 2018; 14(6): 304–314.
Crossref - Morikawa, M., Hiratr, Y., Imanaka, T.A. Study on the structure- function relationship of lipopeptide biosurfactants. Biochem. Biophys. Acta., 2000; 1488 (3): 211–218.
Crossref - Banat, I.M. Biosurfactants production and possible uses in microbial enhanced oil recovery and oil pollution remediation/ : A Review. Bioresour. Technol., 2014; 51: 1–12.
Crossref - Sobrinho, H.B. de. S., Luna, J.M., Rufino, R.D., Porto, A.L.F., Sarubbo L.A. Assessment of toxicity of a biosurfactant from Candida sphaerica UCP 0995 cultivated with industrial residues in a bioreactor. Electron J. Biotechnol., 2013; 16(4).
Crossref - Abouseoud, M., Maachi, R., Amrane, A. Biosurfactant production from olive oil by Pseudomonas fluorescens. Trends Microbiol., 2007; 340–347.
- Klein, J., and Wagner, F. Different Strategies to optimize the production phase of immobilized cells. Ann. N.Y. Acad. Sci., 1987; 501: 306–316.
Crossref - Accorsini, F.R., Mutton, M.J.R., Lemos, E.G., Benincasa, M. Biosurfactants production by yeasts using soybean oil and glycerol as low cost substrate. Braz. J. Microbiol., 2012; 43: 116–125.
Crossref - Rufino, R.D., Sarubbo, L.A., Campos-Takaki, G.M. Enhancement of stability of biosurfactant produced by Candida lipolytica using industrial residue as substrate. World J. Microbiol. Biotechnol., 2007; 23: 729–734.
Crossref - Sobrinho, H.B.S., Rufino, R.D., Luna, J.M., Salgueiro, A.A., Campos-Takaki, G.M., Leite L.F.C., Sarubbo, L.L. Utilization of two agro industrial by-products for the production of a surfactant by Candida sphaerica UCP0995. Process Biochem., 2008; 43: 912–917.
Crossref - Amezcua, V.C., Poggi, V.H.M., Esparza, G.F., Rodriguez, V.R. Effect of culture condition on fatty acids composition of a biosurfactant produced by Candida ingens and changes of surface tension of culture media. Bioresour. Technol., 2007; 98: 237–240.
Crossref - Luna, J.M., Rufino, R.D., Sarubbo, L.A., Campos-Takaki, G.M. Characterization, surface properties and biological activity of a biosurfactant produced from industrial waste by Candida sphaerica UCP0995 for application in the petroleum industry. Colloids Surf. B. Biointerfaces, 2013; 102: 202–209.
Crossref - Pacwa-P³ociniczak, M., P³aza, G.A., Piotrowska-Seget, Z., Cameotra, S.S. Environmental applications of biosurfactants: Recent advances. Int. J. Mol. Sci., 2011; 12: 633–654.
Crossref - Price, N.P.J., Ray, K.J., Vermillion, K.E., Dunlap, C.A., Kurtzman, C.P. Structural characterization of novel sophorolipid biosurfactants from a newly identified species of Candida yeast. Carbohydr. Res., 2012; 348: 33–41.
Crossref - Asmer, H.J., Lang, S., Wagner, F., Victory, W. Microbial production, structure elucidation and bio-conversion of sophorose lipids. J. Am. Oil Chem., 1988; 65: 1460–1466.
Crossref - Ribeiro, I.A., Bronze, M.R., Castro, M.F., Ribeiro, M.H. Optimization and correlation of HPLC-ELSD and HPLC-MS/MS methods for identification and characterization of sophorolipids. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci., 2012 899: 72–80.
Crossref - Bajaj, V., Tilay, A., Annapure, U. Enhanced production of bioactive sophorolipids by Starmerella bombicola NRRL Y-17069 by design of experiment approach with successive purification and characterization. J. Oleo. Sci., 2012; 61: 377–386.
Crossref - Hu, Y., and Ju, L. Sophorolipid production from different lipid precursors. Enzyme Microb. Technol., 2001; 29: 593–601.
Crossref - Ilori, M.O., Amobi, C.J., Odocha, A.C. Factor’s affecting biosurfactant production by oil degrading Aeromonas spp. isolated from a tropical environment. Chemosphere, 2005; 61: 985–992.
Crossref - Arutchelvi, J.I., Bhaduri, S., Uppara, P.V., Doble, M. Mannosylerythritol lipids: A review. J. Ind. Microbiol. Biotechnol., 2008; 35: 1559–1570.
Crossref - Benincasa, M., Abalos, A., Oliveira, I., Manresa, A. Chemical structure, surface properties and biological activities of the biosurfactant produced by Pseudomonas aeruginosa LBI from soapstock. Antonie van Leeuwenhoek, 2004; 85: 1–8.
Crossref - Morya, V.K., Park, J. ho., Kim, T.J., Jeon, S., Kim, E.K. Production and characterization of low molecular weight sophorolipid under fed-batch culture. Bioresour. Technol., 2013; 143: 282–288.
Crossref - Shah, V., Badia, D., Ratsep, P. Sophorolipids having enhanced antibacterial activity. Antimicrob. Agents Chemother., 2007; 51: 397–400.
Crossref - Joutey, N.T., Sayel, H., Bahafid, W., Ghachtouli N. Mechanisms of hexavalent chromium resistance and removal by microorganisms. Rev. Environ. Contam. Toxicol., 2015; 233: 45–69.
Crossref - Romera, E., Gonzalez, F., Ballester, A., Blazquez, M.L., Munoz, J.A. Biosorption with algae: A statistical review. Crit. Rev. Biotechnol., 2006; 26: 223–235.
Crossref - Vijayaraghavan, K., Yan, Y.S. Bacterial Biosorbents and Biosorption. Biotchnol. Adv., 2008; 26(3): 266-91.
Crossref
© The Author(s) 2019. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.