ISSN: 0973-7510
E-ISSN: 2581-690X
Recent cases of monkeypox (MPX), a zoonotic illness caused by monkeypox virus (MPXV), outside of Africa have prompted international public health concerns. The emergence, re-emergence, and global dispersion of zoonoses are profoundly impacted by a wide variety of causes, including but not limited to climate change, urbanization, animal migration, quick means of travel and tourism, vector biology, anthropogenic influences, and natural factors. Human MPX was first identified in the Democratic Republic of the Congo (DRC) in 1970, and since then it has spread throughout Africa, particularly to West and Central Africa, with some instances even emerging outside of Africa. Since the 1970s, there has been an increasing trend in the occurrence of human MPX, with the DRC seeing the largest increase. The median age at first presentation has increased from 4 years in the 1970s to 21 years in the current time. The total fatality rate was 8.7%, although there was a significant variation between clades: Central African (10.6%) and West African (3.6%). Since 2003, sporadic outbreaks have occurred outside of Africa due to imports and travel-related dissemination. Risky practices that could lead to contracting MPX include having contact with infected animals or people. There is still much to learn about MPXV, such as the reason for the sudden increase in cases while travel links from endemic countries have not yet been established profoundly, identity the natural reservoir animal(s), make advances in diagnostics, increase surveillance and monitoring, carry out in-depth epidemiological investigations, genome sequencing and phylogenetic analysis, explore the reasons for the changing epidemiology and evolving nature of the virus, its ecological niche, and the discovery of effective treatment and management of MPX. This l mini-review aims to reveal an increase in the number of reported cases of MPX worldwide, with the highest concentration in the DRC, as well as its spread to other countries and a shift in the median age of patients from infants to teenagers and young adults highlighting from older years to current 2022 MPX outbreaks. Some cross-protection against MPX was provided by smallpox vaccination, suggesting that its discontinuation may have contributed to an increase in human-to-human transmission. The disease’s worldwide significance is underscored by the fact that it has spread beyond Africa. As the epidemiology of this resurging disease is constantly shifting, surveillance and detection programs are crucial to keeping up with it.
Monkeypox, Monkeypoxvirus, Epidemiology, Outbreak, Zoonoses, Case Fatality Rate
The viral zoonosis known as monkeypox (MPX), caused by monkeypox virus (MPXV), has been related to negative effects on the health of both humans and animals. Symptomatically, it is not as bad as smallpox, but it is nonetheless unpleasant.1,2 Despite the fact that smallpox was eradicated more than 40 years ago and smallpox immunization was discontinued, MPX has become the most important orthopoxvirus for public health concerns.3,4 MPXV is a member of the Poxviridae family, the Chordopoxvirinae subfamily, and the Orthopoxvirus genus. MPX is similar to that caused by the variola virus (smallpox virus), which is to which it is genetically linked. Vaccination against smallpox using vaccinia virus (another orthopoxvirus) has shown to be about 85% effective against MPX in the past. However, routine immunization against smallpox was no longer suggested after its eradication in 1980, and no orthopoxvirus vaccination campaign has been conducted in almost past four decades.5-10
In 1958, a Danish research team found the virus in captive monkeys, leading to the name “monkeypox.” A 9-month-old kid in Zaire (now the Democratic Republic of the Congo, DRC) was the first human to get the disease in 1970. Since then, MPX has spread from the DRC throughout the rest of Africa, primarily to Central and West Africa.11,12 The virus is relatively large with a size of 200-250nm having linear double-stranded DNA genome.13 The genetic makeup of MPX can be broken down into two distinct groups: the Congo Basin clade (also known as the Central African clade) and the West African clade. Aside from the Congo Basin, several nations in Central and West Africa have also reported cases of MPX in humans and wildlife. However, surveillance is difficult in endemic areas due to a lack of the infrastructure and resources required for epidemiological and ecological investigation.14,15 When comparing the West African clade to the Congo basin clade, only 3.6% of cases in West Africa are fatal. It is possible that the mortality rate is higher for patients with immunodeficiencies. The incubation period typically lasts between 6 and 13 days, though it can go on for up to 21 days in extreme cases.16,17 Viruses like MPXV presumably originate in rodents. Humans are typically infected by direct contact with an infected person or animal, or indirectly through contact with contaminated objects or surfaces. Droplets, body fluids, and contaminated surfaces are all potential vectors for human-to-human transmission. However, it is important to note that MPX is not always detected with a proper diagnosis. Chickenpox, which is caused by the varicella zoster virus (VZV), is the most common misdiagnosis for this illness. Parallel infections with VZV and MPXV are rare but likely.18,19
Multiple MPXV isolates have been made from wild animals such as rope squirrels found in the DRC, and the sooty mangabey which is native to Ivory Coast. Squirrels, African dormice, monkeys, primates, prairie dogs, hedgehogs, pigs, and mice found in the African regions7 and giant pouched rats are thought to be reservoirs of MPXV; however, the virus can also infect non-human primates. Non-human primates, on the other hand, serve only as accidental hosts, and not as reservoirs, for the virus. High viral loads have been detected in body fluids viz urine, saliva, semen, faeces and the swabs from oropharynx and rectum, suggesting that sexual transmission being greatly responsible for the transmission.20,21 It is still unclear where in nature MPXV actually resides.22 The current mini-review article presents a timeline of MPXV’s epidemiology from its first identification until its present outbreaks in 2022.
Pathophysiology
The virus, which can enter through the mouth (oropharynx), nose (nasopharynx), or skin (intradermal), multiplies at the inoculation site, spreads to nearby lymph nodes (where it causes vireamia), and eventually seeds elsewhere in the body. Through blocking T-cell receptor-mediated T-cell activation, the virus reduces the generation of inflammatory cytokines in human cells. Having a low viral load has been shown to significantly reduce T-cell driven cytokine responses by about 80%.23,24 Incubation period of the disease being 7-14 days with the maximum of 21 days. Lesions first appear in oropharynx then skin and serum antibodies can be detected at time lesions appear.25,26
Earliest detection of MPXV
The vesiculo-pustular rash sickness, known as MPX, was first identified as a human pathogen in 1970, thanks to the efforts of the Commission to Certify Smallpox Eradication in Western Africa and the Congo Basin, which was supported by the World Health Organization (WHO).7,8 Only 182 of 1177 specimens sent to the WHO Collaborating Centers in Atlanta or Moscow between 1969 and 1971 were positive for variola, while 9 tested positive for monkeypox virus. The MPXV-positive samples were from Zaire, Nigeria, Cote d’Ivoire, Sierra Leone, and Liberia, and all were either thought to be single instances or co-primaries (from a common non-human animal source).27 In 1958, MPXV was discovered to be a member of the Orthopoxvirus genus, which coincided with an expansion in the use of monkeys and monkey tissues in the development, testing, and eventual commercialization of inactivated and live attenuated poliovirus vaccines. The State Serum Institute in Copenhagen investigated an epidemic of generalized vesiculo-pustular rash sickness in a captive cynomologous monkey colony, and a virus was found within the rash lesions.28 Evidence from the lab confirmed that this virus is a distinct species within the Orthopoxvirus genus, including cross-neutralization with sera from convalescent animals, biologically distinct virus growth on chorioallantoic membrane (white pocks at 2 days, hemorrhagic at 3 days), genome restriction mapping, and later sequence analysis of single genes, and whole genome. Between 1958 and 1968, researchers defined eight epidemics in monkey colonies and one in a European zoo.29 In the second epidemic, numerous animal species were ill, including a huge anteater. There was no link found between any of the primate outbreaks in Europe and North America that were contained in zoos or primate colonies. Between 1970 and 1979, researchers across Western and Central Africa’s forested ecosystems documented 54 occurrences of human sickness, of which 44 (or 80%) occurred in DRC. Five possible secondary occurrences of human-to-human transfer were reported in the DRC, but no cases involving transmission to a third generation were found. In contrast to the 8/38 (21%) of initial cases reported from DRC that resulted in mortality during acute illness, no deaths were documented in cases from West Africa (Liberia, Nigeria, Ivory Coast and Sierra Leone).7,8,30
Epidemic of MPXV in humans for the first time (1970-1986)
Although smallpox was declared eradicated in the DRC (previously Zaire) in 1968, human MPXV was first identified in August 1970. Since the late nineteenth century in DRC, human cases of MPX have also been discovered in Gabon, Cote d’Ivoire, Liberia, Nigeria, Sierra Leone, and South Sudan. A little boy from Bokenda, a remote community in the DRC province, was diagnosed with smallpox in August 1970 and admitted to Basankusu Hospital. The sample was transferred to the Smallpox Reference Center in Moscow for further examination due to concerns that it might be a smallpox strain. That led to the detection of MPXV in humans for the very first time. Members of the household have said that monkey was a common dinner choice. However, they were unable to confirm whether or not monkey meat was eaten in the household prior to the newborn’s MPX positivity or if the baby had any interaction with monkeys. The infant, however, was the only member of the household who had not had the smallpox vaccine. Infectious human MPXV was initially noted to be spreading during the years of 1970 and 1986. There were 59 new cases reported to the World Health Organization (WHO) between 1970 and 1980.31,32
The WHO and the Global Commission on the Certification of Smallpox Eradication both agree that since 1980, when smallpox was finally eradicated, MPX has become the most significant male-specific Orthopoxvirus. They argue that MPXV’s ecology and epidemiology should be tracked constantly.33 From 1981 until 1986, MPXV was under constant watch by the WHO. There were 338 confirmed cases of MPX and 33 deaths (CFR 9.8%) reported. Among the findings of this surveillance was the finding that the number of cases was lowest in regions not subject to WHO surveillance. According to the WHO, 404 instances were reported between 1980 and the end of 1997.11,12,34
Second MPX epidemic (1987-1998) and its intensity
Several major developments concerning MPX illness occurred between 1986 and 1998. Four instances of MPX were reported in Cameroon, only one of which was confirmed. DRC experienced a protracted outbreak in 1996 and 1997. Midway through February 1997, the community of Akungula in the DRC reported the first case, although the outbreak did not really pick up steam until July of that year. The number of affected communities was estimated at around 13 by the end of August 1997. There were 71 possible cases in these communities, with 11 of them being verified. At least 511 instances were documented between February 1998 and October 1998. Evidence suggests that MPXV and VZV co-circulated at the time.35,36 Of note, MPXV transmission at the human level has recently shown elevated levels in comparison to the past. This increase in MPX instances may be attributable, in part, to a decline in antibody protection following the removal of the original smallpox vaccine.28,36 Additionally, local people were more likely to come in contact with animals that carry MPXV because social turmoil, economic instability, and a severe food shortage drove them deeper into the rainforest.37,38
Third wave of MPX illness (2001-2010)
While no new cases were documented between 1998 and January 2001, 31 suspected cases were reported in the Equatorial province of the DRC between February and August 2001. About six of the first suspected 14 cases were ultimately verified at that time. Four human cases of MPX were reported to the Central African Republic (CAR) in the same year. Up until 2002, the DRC Ministry of Health has documented 1265 possible cases of MPX; of these, 215 samples were tested, and 88 were confirmed as MPX. As was the case in the past, men were more severely impacted by the smallpox outbreak than women, and the majority of the population had not been immunized.35,39,40
Between 2001 and 2004, the National Disease Surveillance Program of the DRC Ministry of Health identified 2,734 instances of possible human MPX. As a result of the ongoing civil war at the time, the surveillance operation had to be temporarily shut down. A case of MPXV was reported in the Republic of the Congo (ROC) in April 2003.18 The national confirmation rate that year was three out of 12 suspected cases. Human-to-human transmission was documented during this outbreak, and symptoms were more severe than in previous cases. Approximately seven generations of the virus have been passed on, with six of those generations having been passed on in a chain.41
In 2003, 72 people in the United States were reported to have suspected cases of MPX due to close contact with pets, as reported by the Centers for Disease Control and Prevention (CDC).22 However, no reports of secondary transmission (human-to-human) occurred, and no patients succumbed to their illness. Small mammals brought from Ghana to Texas were likely the original carriers of the virus. As 2005 came to a close, 49 possible cases were recorded in South Sudan, of which 10 were confirmed. To our knowledge, this was the first report of MPXV outside of its natural habitat.42
Because of the ethnic conflict in the northwest of the DRC, many people fled to the Ubangi River region in 2010. Simultaneously, ten cases in the Likouala region of the ROC were deemed very suspicious, with only two instances ultimately being confirmed.43 After 2003, the ROC epidemic is thought to have been reintroduced due to refugees from the DRC. CAR confirmed two cases of murine prion disease (MPX) in June of that same year, both of which were caused by eating a certain species of wild rat (bemba).44,45
Further spread of MPXV (2014-2021)
In 2014, almost 40 years after the first incidence of MPXV, there was a single case recorded in Sierra Leone. From December 2015 to February 2016, a total of 12 persons were reported in Bangassou, Mbomou province, CAR; three of them passed afterwards. There were 6 suspicious cases reported in Likouala province, ROC, in 2016, with 8 confirmed cases.43,46 The outbreak of MPX was first reported by the government of Congo on March 13 of the same year. Due to a lack of effective monitoring and preventative measures, MPX is continuing to expand across Africa. A further risk concern for the surrounding countries was the influx of refugees from CAR, DRC and Chad.5,27,40,43,47
In 2017, Sierra Leone once again confirmed an instance of MPX. Also, in 2017–18, Nigeria dealt with an MPX epidemic. Between September 2017 and April 2018, authorities received reports of around 244 suspicious instances, of which 101 cases were confirmed. MPXV was first detected in Israel and the United Kingdom in September 2018.17,34,48 The current cases are on rise in developed countries of Europe and America.47 Cases of MPX have been documented in the UK across a variety of years and months (2018-2022). Singapore confirmed a case of MPX in May 2019.27,31,40,42,49 Human cases of MPXV from 1970 through 2021, including those with only a suspicion of the virus and those with confirmed diagnosis is presented in Table 1.
Table (1):
Human instances of monkeypox from 1970 through 2021, including both suspected and confirmed cases (adopted, modified and updated from: WHO 2022a9; WHO 2022b51; WHO 2022c52; WHO 2022d53).
| Year | Country | Suspected Cases | Confirmed Cases | Region Type |
|---|---|---|---|---|
| 1970-1986 | The Democratic Republic of the Congo | 386 | 386 | Endemic |
| The Central African Republic | 6 | 6 | Endemic | |
| Cameroon | 2 | 1 | Endemic | |
| Nigeria | 2 | 2 | Endemic | |
| Ivory Coast | 2 | 2 | Endemic | |
| Liberia | 4 | 4 | Endemic | |
| Benin | 1 | 1 | Endemic | |
| Sierra Leone | 1 | 1 | Endemic | |
| 1987 | Gabon | 4 | 1 | Endemic |
| 1989 | Cameroon | 1 | 1 | |
| 1997 | The Democratic Republic of the Congo | 511 | 511 | Endemic |
| 2001 | The Central African Republic | 4 | 4 | Endemic |
| 2003 | Republic of Congo | 12 | 3 | Endemic |
| USA | 72 | 37 | Non-endemic | |
| 2005 | South Sudan | 49 | 10 | Endemic |
| 2009 | Republic of Congo | 10 | 2 | Endemic |
| 2010 | The Central African Republic | 2 | 2 | Endemic |
| 2014 | Sierra Leone | 1 | 1 | Endemic |
| 2015-2019 | The Central African Republic | 78 | 41 | Endemic |
| Cameroon | 7 | 1 | Endemic | |
| Nigeria | 276 | 122 | Endemic | |
| Liberia | 16 | 2 | Liberia | |
| Sierra Leone | 1 | 1 | Endemic | |
| Republic of Congo | 97 | 9 | Endemic | |
| UK | 3 | 2 | Non-endemic | |
| Israel | 1 | 1 | Non-endemic | |
| Singapore | 1 | 1 | Non-endemic | |
| 2021 | UK | 3 | 3 | Non-endemic |
| USA | 1 | 1 | Non-endemic |
MPX outbreak in the year 2022
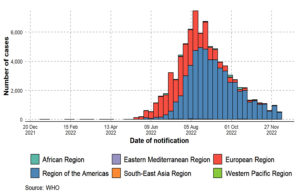
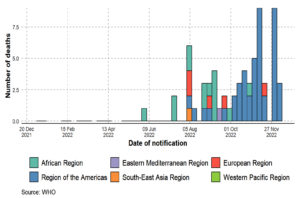
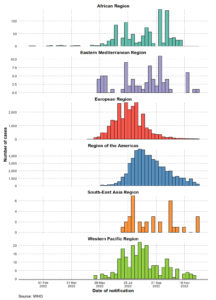
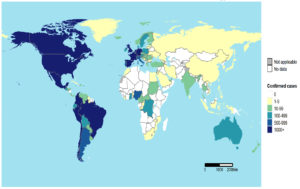
There have been 110 Member States reporting MPX cases to WHO from all 6 WHO regions since 1 January 2022. A total of 82,768 laboratory-confirmed cases of MPX and 65 fatalities were reported to WHO from 110 countries throughout all six WHO regions between 1 January 2022 and 15 December 2022 (Figure 1, Figure 2, Figure 3, Figure 4, Table 2). An alarming number of these cases have been reported from nations with no history of MPXV transmission since 13 May 2022.4 It is the first-time cases and ongoing chains of transmission have been recorded outside of West and Central Africa. Men who have recently reported having sex with one or more partners continue to be disproportionately affected by the continuing MPX outbreak, with the exception of countries in West and Central Africa.7,8,40 No evidence points to continued transmission beyond these networks at this time. When there is even a single confirmed case of MPX in a country, an outbreak is declared. Since MPX suddenly appeared in multiple countries with no apparent epidemiological ties to West or Central Africa, it is possible that the disease had been spreading undetected for quite some time. Worldwide, WHO sees a moderate danger. In terms of geographic regions, the WHO considers the risk to be “High” in the Americas and “Moderate” in Africa, the Eastern Mediterranean, Europe, and South and Southeast Asia. Western Pacific Region risk is rated as low. On October 20, 2022, the International Health Regulations (IHR) Emergency Committee met for the third time to discuss the ongoing multi-country epidemic of MPX. After hearing from committee members and advisors and taking into account other factors consistent with the IHR (2005), the WHO Director-General has reaffirmed that this outbreak remains a public health emergency of international concern and issued updated temporary recommendations in response to the situation.9,50,51
Figure 1. Monkeypox confirmed case rates by WHO regions from 1 January 2022 to 15 December 2022, as shown by an epidemiological curve (adopted, modified and updated from: WHO 2022a9 and WHO 2022c52)
Figure 2. Monkeypox confirmed deaths by WHO region, from 1 January 2022 to 15 December 2022, as shown by an epidemiological curve (adopted, modified and updated from: WHO 2022a9; WHO 2022c52)
Figure 3. Monkeypox confirmed case rates in individual WHO regions from 1 January 2022 to 15 December 2022, as shown by an epidemiological curve (adopted, modified and updated from: WHO 2022a9; WHO 2022b51; WHO 2022c52)
Figure 4. Cumulative cases of monkeypox from 1 January 2022 to 15 December 2022 (adopted, modified and updated from: WHO 2022a9; WHO 2022b51; WHO 2022c52; WHO 2022d53)
The United States (n = 29,513), Brazil (10,264), Spain (7,416), France (4,110), Colombia (3,908), the United Kingdom (3,730), Germany (3,675), Peru (3,566), Mexico (3,509), and Canada (1,459) round out the top 10 countries with the most impacted citizens. Approximately 85.9% of the world’s total cases come from these nine countries. Twelve nations have reported an increase in the weekly number of cases in the past week, with Mexico reporting the largest increase. In the past 21 days, 73 countries have reported zero new cases.9,50,51-53
Table (2):
Monkeypox cases and fatalities reported to World Health Organization from the from January 1, 2022, through December 15, 2022 (adopted, modified and updated from: WHO 2022a9; WHO 2022b51; WHO 2022c52; WHO 2022d53).
WHO Region |
Confirmed cases |
Deaths |
|---|---|---|
Region of the Americas |
55695 |
44 |
African Region |
982 |
14 |
Eastern Mediterranean Region |
80 |
1 |
European Region |
25600 |
5 |
South-East Asia Region |
35 |
1 |
Western Pacific Region |
228 |
0 |
Cumulative |
82768 |
65 |
Nextstrain routinely performs sequence alignment and visualization of sequences accessible in NCBI Genbank, employing both historical sequences and sequences related to the 2022 multi-country MPXV outbreak. Scientists from various disciplines and countries came to an agreement on 12 August 2022, leading WHO to call the Congo Basin MPX clade as Clade one (I) and the West African mpox clade as Clade two (II). And it was settled that there are two branches of Clade II, designated as IIa and IIb. The following visualizations were constructed using Nextstrain alignments of Genbank data annotated as MPXV. Visit the Nextclade website or GitHub for more information on the methodology used and the interactive visualisation.40 The R programs ggtree and treeio were used to generate images of the phylogenetic trees. As of the 21st of November, 2022, 586 sequences had been visualized. It should be pointed out that GISAID data submissions, the second primary venue for disseminating MPXV genomic data, are not included in these results. Clade IIb sequences account for 100% of the current outbreak of monkeypox in 2022. Most of these have been linked to the B.1 branch of Clade IIb. Despite this, many sequences have been linked to the closely related A.2 branch.27 The similarities between the sequences uploaded from different parts of the world suggest that the continuing outbreak does not entail several zoonotic spillover events, and that transmission is now being perpetuated by human-to-human transmission. Analyzing the diversity of sequences from the time period preceding to the present outbreak in areas that witnessed continuous circulation of MPXV is crucial for understanding when sustained human-to-human transmission began.40,47,50-53
Reduced herd immunity due to the end of smallpox vaccination, increased human contact with potential MPXV animal reservoir hosts as a result of climate change and deforestation, increased consumption of bush meat, and a lack of adequate health and research infrastructure could all contribute to the re-emergence of MPXV in countries located in the tropical rain forest belt. In recent years, visitors have brought MPX from Africa to United States, UK, Israel, and Singapore, expanding its range beyond the endemic regions. So, MPX is a highly hazardous re-emerging disease that affects people all over the world. In order to prevent MPXV from taking over the ecological niche previously occupied by VARV and potentially evolving into a more lethal pathogen than it is now, researchers on a national and international scale should step up their efforts to identify virulence markers of disease, host and viral factors that modulate MPXV evolution, human behaviors that support zoonotic spillover events, surrogates for asymptomatic infection, and virus and host determinants of immunity. In endemic regions, routine epidemiological surveillance of MPXV in humans and potential host species is warranted rather than waiting for an outbreak to conduct such monitoring.
An unexpectedly high number of cases in the men who have sexual intercourse with men (MSM) or gay community have been linked to anal and vaginal lesions, indicating that the monkeypox virus may be spreading through sexual contact in this 2022 outbreak. During the human monkeypox epidemic that swept Nigeria in 2017, this novel mode of transmission was hypothesized but never confirmed. On the other hand, the MSM population is more likely to contract sexually transmitted diseases like HIV. Therefore, more studies are required to comprehend the efficacy of anti-viral treatment among people who are co-infected with HIV and human MPXV. Public health initiatives aimed at lowering MPX transmission in MSM communities must also address barriers such as homophobia, stigma, and prejudice. The factors that influence illness transmission, virus virulence, and the acquired or host genetic traits that predispose to disease susceptibility/virulence need to be better understood, and research in these areas is warranted. It is important to analyze human actions that “induce” disease in addition to identifying reservoir, amplifying, and transmitting hosts. This insight can then be used to implement effective strategies for disease prevention and control, including but not limited to educational programs, alterations in lifestyle, and the use of vaccines and other treatments. Especially in the wake of the COVID-19 pandemic, the recent spread of MPX to over 27 nonendemic nations has caused widespread alarm. This mini-review article shows that adults, and not just children, have been diagnosed with MPX in the recent past. Rash, fever, headache, respiratory symptoms, and lymphadenopathy were the most prevalent, as predicted. Just like with COVID-19, the disease can spread by direct contact with infected wounds or scabs, as well as through the exchange of bodily fluids or respiratory droplets. In addition, recent cases in men who have sex with men may point to sexual transmission of the virus, which necessitates additional in-depth investigation to rule out.
The epidemic of COVID-19 over the past two years has been a harsh challenge for global public health. The worldwide and notably the American increase in confirmed cases of MPX may have a severe effect on the global economy. In light of the WHO’s declaration that monkeypox constitutes an international public health emergency of public health significance,11 it is imperative that efforts be made to increase diagnostic capacity and public knowledge of the monkeypox pandemic. There is a need to better educate and train public health workers in the clinical care of monkeypox, as well as in infection prevention and control. Equally important is ensuring that all members of the MSM community have equal access to healthcare, including preventative measures like immunizations. Finally, we need to launch a worldwide partnership to perform clinical trials on MPX vaccines and antiviral medications to determine their efficacy and safety. Studies of outbreaks would benefit from providing details on the required response. This would make it possible to analyze outbreak morbidity measurements by intervention, which in turn could help direct suggestions to local teams going forward. In a similar vein, there is a lack of information about treatment interventions that might be helpful in less accessible endemic settings. Prophylactic antibiotic treatment for secondary cutaneous infections was mostly based on anecdotal evidence, which was insufficient for drawing firm judgments regarding antibiotic efficacy or standard practice. A broader study effort can be undertaken, and the results can be presented in the wider literature, if the current local research efforts into MPX are recognized and supported. However, the risks to populations in endemic places are clear, even if MPXV has not established and propagated itself in the human population since the end of smallpox vaccination. To better enhance case management and public health response, the MPX research portfolio urgently requires significant improvements in the quality and quantity of outbreak data gathering.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Chakraborty S, Mohapatra RK, Chandran D, et al. Monkeypox vaccines and vaccination strategies: Current knowledge and advances. an update – Correspondence. Int J Surg. 2022a;105:106869.
Crossref - Chakraborty S, Chandran D, Mohapatra RK, et al. Clinical management, antiviral drugs and immunotherapeutics for treating monkeypox. An update on current knowledge and futuristic prospects. Int J Surg. 2022b;105:106847.
Crossref - Xiang Y, White A. Monkeypox virus emerges from the shadow of its more infamous cousin: family biology matters. Emerg Microbes Infect. 2022;11(1):1768-1777.
Crossref - WHO. 2022a. World Health Organization. Disease Outbreak News; Multi-country monkeypox outbreak in non-endemic countries. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON396. Accessed on December 16, 2022.
- Adler H, Gould S, Hine P, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22(8):1153-1162.
Crossref - Ahmed M, Naseer H, Arshad M, Ahmad A. Monkeypox in 2022: A new threat in developing. Ann Med Surg. 2022;78:103975.
Crossref - Chandran D, Dhama K, Chakraborty S, et al. Monkeypox: An update on current knowledge and research advances. J Exp Biol Agr Sci. 2022; 10(4):679-688.
https://doi.org/10.18006/2022.10(4).679.688 - Damon IK. Status of human monkeypox: clinical disease, epidemiology and research. Vaccine. 2011;29:54-59.
Crossref - CDC. 2022a. 2022 Monkeypox Outbreak Global Map. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html. Accessed on December 16, 2022.
- Mahase E. Seven monkeypox cases are confirmed in England. BMJ. 2022;377:o1239.
Crossref - Guarner J. Monkeypox in 2022. Am J Clin Pathol. 2022;158(2):160-161.
Crossref - Guarner J, Del Rio C, Malani PN. Monkeypox in 2022-What Clinicians Need to Know. JAMA. 2022;328(2):139-140.
Crossref - Alakunle E, Moens U, Nchinda G, Okeke MI. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses. 2020;12(11).
Crossref - Kumar N, Acharya A, Gendelman HE, Byrareddy SN. The 2022 outbreak and the pathobiology of the monkeypox virus. J Autoimmun. 2022;131:102855.
Crossref - Mohapatra RK, Tuli HS, Sarangi AK, et al. Unexpected sudden rise of human monkeypox cases in multiple non-endemic countries amid COVID-19 pandemic and salient counteracting strategies: Another potential global threat? Int J Surg. 2022;103:106705.
Crossref - Hraib M, Jouni S, Albitar MM, Alaidi S, Alshehabi Z. The outbreak of monkeypox 2022: An overview. Ann Med Surg. 2022;79:104069.
Crossref - Peter OJ, Kumar S, Kumari N, Oguntolu FA, Oshinubi K, Musa R. Transmission dynamics of Monkeypox virus: a mathematical modelling approach. Model Earth Syst Environ. 2022;8(3):3423-3434.
Crossref - Adalja A, Inglesby T. A novel international monkeypox outbreak. Ann Intern Med. 2022;175(8):1175-1176.
Crossref - Hasso M, Perusini S, Eshaghi A, et al. Monkeypox Virus Detection in Different Clinical Specimen Types. Emerg Infect Dis. 2022;28(12).
Crossref - Jaiswal V, Nain P, Mukherjee D, et al. Symptomatology, prognosis, and clinical findings of Monkeypox infected patients during COVID-19 era: A systematic-review. Immun Inflamm Dis. 2022;10(11):e722.
Crossref - Peiro-Mestres A, Fuertes I, Camprubi-Ferrer D, et al., Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Euro Surveill. 2022; 27(28).
Crossref - Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox Virus Infection in Humans across 16 Countries – April-June 2022. N Engl J Med. 2022;387(8):679-691.
Crossref - McCollum AM, Damon IK. Human monkeypox. Clin Infect Dis. 2014;58:260-267.
Crossref - Sah R, Mohanty A, Siddiq A, et al. Monkeypox reported in India – South East Asia Region: Health and economic challenges. Lancet Reg Health Southeast Asia. 2022a;4:100063.
Crossref - Hutson CL, Carroll DS, Gallardo-Romero N, et al. Comparison of Monkeypox Virus Clade Kinetics and Pathology within the Prairie Dog Animal Model Using a Serial Sacrifice Study Design. Biomed Res Int. 2015;2015:965710.
Crossref - Harapan H, Ophinni Y, Megawati D, et al. Monkeypox: A Comprehensive Review. Viruses. 2022;14(10):2155.
Crossref - Bunge EM, Hoet B, Chen L, eta l. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16(2):e0010141.
Crossref - Ranganath N, Tosh PK, O’Horo J, Sampathkumar P, Binnicker MJ, Shah AS. Monkeypox 2022: Gearing Up for Another Potential Public Health Crisis. Mayo Clin Proc. 2022;97(9):1694-1699.
Crossref - Saxena SK, Ansari S, Maurya VK, et al. Re-emerging human monkeypox: A major public-health debacle. J Med Virol. 2022:e27902.
Crossref - Velavan TP, Meyer CG. Monkeypox 2022 outbreak: An update. Trop Med Int Health. 2022;27(7):604-605.
Crossref - Sah R, Humayun M, Baig E, et al. FDA’s authorized “JYNNEOS” vaccine for counteracting monkeypox global public health emergency; an update – Correspondence. Int J Surg. 2022b;107:106971.
Crossref - Jezek Z, Gromyko AI, Szczeniowski MV. Human monkeypox. J Hyg Epidemiol Microbiol Immunol. 1983;27(1):13-28.
- Sklenovska N, Van Ranst M. Emergence of monkeypox as the most important orthopoxvirus infection in humans. Front Public Health. 2018;6:241.
Crossref - Durski KN, McCollum AM, Nakazawa Y, et al. Emergence of monkeypox – West and Central Africa, 1970-2017. MMWR Morb Mortal Wkly Rep. 2018;67(10):306-310.
Crossref - Fine PE, Jezek Z, Grab B, Dixon H. The transmission potential of monkeypox virus in human populations. Int J Epidemiol. 1988;17(3):643-50.
Crossref - Khaity A, Hasan H, Albakri K, et al. Monkeypox from Congo 1970 to Europe 2022 is there a difference? Int J Surg. 2022;104:106827.
Crossref - Bragazzi NL, Kong JD, Mahroum N, et al. Epidemiological trends and clinical features of the ongoing monkeypox epidemic: A preliminary pooled data analysis and literature review. J Med Virol. 2022.
Crossref - McCarthy MW. Recent advances in the diagnosis monkeypox: implications for public health. Expert Rev Mol Diagn. 2022;22(7):739-744.
Crossref - Sale TA, Melski JW, Stratman EJ. Monkeypox: an epidemiologic and clinical comparison of African and US disease. J Am Acad Dermatol. 2006;55(3):478-81.
Crossref - Beer EM, Rao VB. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis. 2019;13(10):e0007791.
Crossref - Kaler J, Hussain A, Flores G, Kheiri S, Desrosiers D. Monkeypox: A comprehensive review of transmission, pathogenesis, and manifestation. Cureus. 2022;14(7):e26531.
Crossref - Kraemer MUG, Tegally H, Pigott DM, et al. Tracking the 2022 monkeypox outbreak with epidemiological data in real-time. Lancet Infect Dis. 2022;22(7):941-942.
Crossref - Petersen E, Abubakar I, Ihekweazu C, et al. Monkeypox – Enhancing public health preparedness for an emerging lethal human zoonotic epidemic threat in the wake of the smallpox post-eradication era. Int J Infect Dis. 2019;78:78-84.
Crossref - Brown K, Leggat PA. Human monkeypox: Current state of knowledge and implications for the future. Trop Med Infect Dis. 2016;1(1):8.
Crossref - Kantele A, Chickering K, Vapalahti O, Rimoin AW. Emerging diseases-the monkeypox epidemic in the Democratic Republic of the Congo. Clin Microbiol Infect. 2016;22(8):658-9.
Crossref - Adnan N, Haq ZU, Malik A, et al. Human monkeypox virus: An updated review. Medicine. 2022;101(35):e30406.
Crossref - Berthet N, Descorps-Declere S, Besombes C, et al. Genomic history of human monkey pox infections in the Central African Republic between 2001 and 2018. Sci Rep. 2021;11(1):13085.
Crossref - Cabanillas B, Valdelvira R, Akdis CA. Monkeypox outbreak in Europe, UK, North America, and Australia: A changing trend of a zoonotic disease. Allergy. 2022dur;77(8):2284-2286.
Crossref - Diaz JH. The disease ecology, epidemiology, clinical manifestations, management, prevention, and control of increasing human infections with animal orthopoxviruses. Wilderness. Environ Med. 2021;32(4):528-536.
Crossref - CDC. 2022b. CDC and Health Partners Responding to Monkeypox Case in the U.S., May 18, 2022. https://www.cdc.gov/media/releases/2022/s0518-monkeypox-case.html. Accessed on October 4, 2022.
- WHO 2022b. Monkeypox. 15 December 2022. https://www.who.int/news-room/fact-sheets/detail/monkeypox. Accessed on December 16, 2022.
- WHO. 2022c. Multi-country monkeypox outbreak: situation update. Published on October 2, 2022. Accessed on December 16, 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON393
- WHO. 2022d. WHO situation update: Multi-country Monkeypox outbreak: situation update https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON393 Accessed on December 16, 2022.
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.