ISSN: 0973-7510
E-ISSN: 2581-690X
Biosurfactants are surface active compounds, which may be of microbial, animal or plant origin. They are typically less toxic and less persistent than the synthetically derived surfactants. The current study intended to analyze the biosurfactants production and its antagonistic activity against Candida albicans biofilm formation. Isolation of biosurfactant producing organism was carried out using swab sample of human vagina and from oil contaminated soil samples. Isolates were screened for biosurfactant production by using oil spread assay and the organisms showing higher activity were selected. The Emulsification assay was done and the E24 was found to be 20.83% for cell free extract of growth medium of isolate B1.The selected isolates were further studied for yield of biosurfactant produced by cultivation in MRS broth and extraction by chloroform and methanol (3:1) extraction. The yield of biosurfactant for isolate B1was found to be4.55gl-1.Theextracted biosurfactant was separated by TLC and identified to be a lipopeptide by FTIR spectroscopy. The isolate with maximum yield of biosurfactant was identified as Lactobacillus fermentum using VITEK II Compact System for microbial identification system. The percentage biofilm inhibition activity of the biosurfactant was studied by CFU assay followed by adhesion assay and by pre-coating experiment. On the basis of above studies, it concludes that use of biosurfactant producing organism can be effective weapon against colonizing opportunistic C. albicans and can be applied in medical devices for inhibition of biofilms formation. Microbial adhesion also decreased from 85% to 11% with78.125 to 2500 µg/ml of biosurfactant. The lipopeptide extracted from isolated isolate B1 also showed powerful penetration capacity in the biofilm and killed 91% C. albicans as seen by CFU assay and a highest inhibition at 2500µg/ml and 1250µg/ml concentration as studied by pre-coating experiment.
Biosurfactant, Candida albicans, anti-biofilm activity, lipopeptide
Surfactant is a compound word formed from ‘surface active agent’. They are categorized by their hydrophilic group: anionic, cationic, non-ionic and amphoteric1. Biosurfactants are commonly used in agriculture, pharmaceutics and petroleum industry2. The vaginal normal flora, including Lactobacillus species,is protective barrier to infection by competing with pathogens for adhesion and colonizing uterine wall and by synthesizing biosurfactants3. Biofilm is a matter of concern in both medical and industrial fields. Combating the problem of biofilms is difficult because of its ability to strongly adhere to the surface of any material. Biofilms that form on the medical devices such as catheters, serve as a source of around 60% of the nosocomial infections4,5.
The microbial biosurfactant production has been observed primarily in Bacillus and Pseudomonas genera. Biosurfactants are of diverse chemical nature and hence possess properties like solubility of non- polar compounds, heavy metals sequestration, antimicrobial and anti-adhesive properties to prevent biofilm formation. Also, the biodegradability of biosurfactants makes their use advantageous over chemical surfactants6. There has been a keen interest in the biosurfactant producing bacteria of human microbiota due to their probiotic effects7, and the ability to inhibit pathogenic bacteria and fungi8. Hence the current study was designed to study the production of biosurfactants and its impact on the C. albicans biofilm formation.
Biosurfactant producing organisms were isolated from the lateral vaginal wall, using cotton swab and oil contaminated soil samples. The swab samples were isolated on Rogosa agar plate and incubated in desiccator under micro- aerophilic condition for 7 days at 28°C. Whereas 0.5g of oil contaminated soil sample was mixed in 50ml of Minimal Synthetic Medium (MSM)[KH2PO4-0.1%; MgSO4– 0.05%; FeSO4-0.001%; NaNO3-0.15%; CaCl2-0.0002%; (NH4)2SO4– 0.15%; Hi Media Pvt. Ltd., Mumbai, Maharashtra, India]and kept at 30°C for 3 days. Serially diluted sample from dilutions was used for spread plating on sterilized Nutrient agar plates with 0.08% Tween 80 and incubated at 37°C for 24h9.
The highest biosurfactant producer bacterium was selected by cultivation of isolates in DeMan, Rogosa and Sharpe (MRS- HiMedia M1164) broth and in Nutrient broth with 0.08% tween 80, in static conditions, at 28°C,for 20h. The biomass was separated from culture media by centrifugation at 10000 rpm, for 10 min. The supernatant was used to determine the surface tension reducing activity by the oil spreading assay10. A thin oil layer was made using 10 μl of crude oil on 40ml of distilled water. Then, 10 μl of culture supernatant was added gently in the centre of the oil layer. The biosurfactant, if present in the supernatant, displaced oil to form a clearing zone. The diameter of this clearing zone on the oil surface was indicative of relative oil displacement activity.
Biosurfactants possess hemolytic activity, which was studied for the supernatant by incubating 0.5ml of 5% RBC suspension with 0.5 ml of supernatant,incubate at 28°C for 10min11.
Emulsification index (E24) has been used to characterize the biosurfactant emulsifying activity. In a test tube 2ml cell free supernatant and 2ml oil were vortexed for 2min and height of emulsion layer was measured at the end of 24h. Emulsification index was derived using the following formula12.
E24= (Height of emulsion layer)/(Height of total solution)×100
As a biochemical detection test for identification of lactic acid bacteria13, the CO2 production by glucose fermentation was evaluated by hot loop test. Cultures were grown in APT broth ( Casein hydrolysate- 1.25%; Yeast Extract- 0.75%; dextrose-1%; Sodium citrate- 0.5%; Sodium chloride-0.5%; Dipotassium phosphate-0.5%; Magnesium sulphate- 0.08%; Manganese chloride-0.01%; Ferrous sulphate- 0.0045; Polysorbate 80-0.02%; Thiamine hydrochloride- 0.0001%, pH- 6.7±0.2[HiMedia M227]), at 28°C. After 24h of growth, a heated red-hot inoculating loop was plunged into the culture to observe stream of bubbles13.
The isolate showing the highest activity in Oil spread assay was selected for the biosurfactant production. Sterile MRS broth was inoculated with 108cells/ml of selected isolate culture and incubated at 37°C, for 96h. To separate the biomass from supernatant the broth culture was centrifuged at 10000rpm for 30 min. For extraction of biosurfactant, pH of supernatant was adjusted to pH 2.0with 6N HCl and kept at 4°C, for 24h. Organic solvent extraction was done with ethyl acetate and methanol(4:1), using separating funnel. The extracted biosurfactant was kept for drying at 55°C, for 72h14. The crude biosurfactant was spotted on two TLC plates (Merck, India) along with the solvent extract purified biosurfactant. They were separated using the solvent chloroform: water: methanol in ratio 65: 24: 415. Ninhydrin reagent and Anthrone reagent was sprayed on the two chromatograms respectively. Sudan black B staining for lipid identification was done. The extracted biosurfactant was further investigated by Fourier Transform Infrared Spectroscopy (FTIR) to defineits functional groups, chemical bonds as structural characteristics. The analytic sample was prepared by homogeneously dispersing 1 mg biosurfactant in pellet of potassium bromide16.
The Candidal adhesion assay was performed by addition of 500μL of sterile Brain Heart Infusion (BHI) broth to three Eppendorf tubes and 100 μL of a saline suspension of C. albicans adjusted at 0.6 OD at 600nm. 100μL of biosurfactant was added in the tubes and kept for 1.5h adhesion phase. Each tube was washed twice with 1000μL of sterile saline to remove loose cells. 500μL of Sterile BHI medium was pipetted into each of the washed tubes and incubated at 37°C, for 96h. The growth medium was replenished after every 24h. After 96h of incubation, all tubes were washed off with sterile saline. In empty tubes, 100μL of sterile saline was added forcefully and it was used for further serial tenfold dilutions. Tenfold dilution was performed by sterile saline and 100μL of diluted sample from last three dilutions viz., 4, 5 and 6 were used for the colony forming unit (CFU) assay and incubated at 37°C, for 48h17.
The anti- biofilm assay of the biosurfactant was studied using pre-coating experiment. 200μL of biosurfactant concentration ranging from 2.500μg/ml to 78μg/ml was added in polystyrene 96-well microtiter plate. The negative control well containing sterile saline was also prepared. The plate was then incubated at 37°C, 130rpm, for 24h. The wells were emptied and gently washed twice with Phosphate Buffer Saline (PBS) pH 7.2. 150μL C. albicans suspension (1.0×107 CFU/ml) in Sabouraud dextrose broth (HiMedia M033) was added to each well and incubated at 37°C, 75rpm for 3h. The un-attached cells were removed by gently washing the wells twice with PBS and then 150μl of sterile Sabouraud dextrose broth was added to wells. Plates were incubated again at 37°C, 75 rpm for 48h.C. albicans biofilm production was quantified by the crystal violet method. 100μl of 2% crystal violet (CV) solution was added to the wells and kept for 20 min; the additional CV was washed with distilled water and air-dried. 150μl of 33% acetic acid was used to release the bound CV, the extinction of which was measured at 590nm18. The percentages microbial adhesion was calculated using the formula:
Percentage Microbial Adhesion= [ Absorbance of the well with biosurfactant concentration /Absorbance of Control well ] × 100
The samples were collected from oil contaminated soil and from healthy human vagina. The samples were enriched in MSM medium and isolated on Rogosa agar and Nutrient agar plate. Isolate obtained from urogenital tract was labeled asisolate B1 and the isolates obtained from soil sample were labeled as isolate B2 and isolate B3 respectively. The screening of isolated bacteria for the biosurfactant production was done by addition of culture supernatant to the center of diesel oil layer. The supernatants of isolates B1 and B2 showed a clear zone of diameter 4.5cm and 2.5cm respectively. The bacterial isolates B1 supernatant showed higher oil displacement activity. The Emulsification assay with 2ml of diesel oil and supernatant each revealed the E24 to be 20.83%. The supernatant of the isolate B1 growth medium showed large amount of foaming activity in the flasks after incubation at 30°C, 200rpm for 72h. The isolated isolate B1 when streaked on blood agar plate showed the γ-hemolytic activity. Only small stream of bubbles of CO2was observed on insertion of hot loop into the culture grown in APT broth with 1% dextrose and 0.5% sodium acetate at 27°C. These observations were comparable with the biosurfactant derived from Lactobacillus fermentum SHU6343 studied by Ghasemi et al19.
From the oil spreading assay, it was found that the isolate B1 showed highest oil displacement activity, hence it was isolated and identified using different bio-chemical tests. And further confirmation was done by VITEK II Compact system for microbial identification system and the isolate was identified as L. fermentum. The 4.55gl1 of biosurfactant was extracted from isolate B1 growth in MRS broth. The extracted biosurfactant was separated using TLC. The solvent used in chromatographic process was chloroform: water: methanol in the ratio 65:24:4. The chromatograms were developed using ninhydrin reagent or anthrone reagent. Red spots developed on the TLC plate which was sprayed by ninhydrin reagent and no spots were developed on another plate which is sprayed by anthrone reagent. Thus,indicating that the extracted biosurfactant had a peptide component.
The nature of biosurfactant was confirmed by FTIR analysis to be lipopeptide. The FTIR spectrum showed strong adsorption bands of peptide at 3281 cm-1, 1645 cm-1 and 1531 cm-1. It also indicates the C-H stretching bond which had a band of 2980 cm-1. The band 2980 cm-1 and 1447 cm-1 reflect aliphatic compound. And the band 743 cm-1 and 507 cm-1 bands indicate CH out of plane deformation mode. 1124 cm-1 bands indicates C=S stretching mode.1215 cm-1 band showed C-O-C asymmetric stretching mode (Fig. 1). According to Ghasemi et al19. the lipoprotein biosurfactant produced by P. dextrinicus SHU1593 also depicted most prominent bands at the range of 3000-3600 cm-1 and 1640-1700 cm-1, as well as 1500-1620 cm-1, correspond to NH group, C=O stretching in proteins (AmI band) and NH bending in proteins (AmII band), respectively. Abdalsadiq et al20 identified the structure of Lactobacillus acidophilus -based biosurfactants by MS to be a lipopeptide compound containing nine amino-acids and C12–C17 β-hydroxy fatty acids. Comparatively few lipopeptide biosurfactants produced by lactobacilli have been studied by FTIR and X-ray photoelectron spectroscopy21,22. In another study by Ramani et al23, it was ascertained that absorption in 1500-1620 cm-1 is not normally observed in the FTIR spectra of glycolipid biosurfactants.
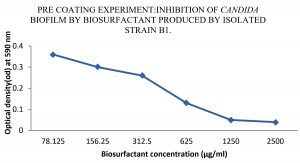
Anti-biofilm activity of extracted bio-surfactants was observed against C. albicans by viability assay. Extracted bio-surfactant exhibited 91% of efficiency. It was demonstrated that, incidence of anti- biofilm activity among biosurfactant should impact the expression of bacteria virulence factors, which are essential during infection because they disturb cell to cell signaling. Therefore, preliminary bacterial adhesion and subsequent biofilm formation can be prevented by lipopeptide. Pre-coating of the micro-titer wells with extracted biosurfactants affected biofilm formation by C. albicans strains. When C. albicans exposed to extracted biosurfactants, there was a decrease in OD at 590nm from 0.36 to 0.05, with surfactant concentrations ranging from 78.125 to 2500 µg/ml. Microbial adhesion also decreased from 85 to 11 percent with respective concentration. Hence, we can conclude that pre-coating with extracted surfactants inhibits the formation of C. albicans biofilm. Biosurfactant showed biofilm inhibition activity at concentration 2500µg/ml and 1250µg/ml. The study of Frachia et al.16 has discerned the percentage cell adhesion of various strains of C. albicans reduced significantly up to 86% at pre-coating of at 2,500 μg/mL of crude CV8LAC biosurfactant. In another study, it was observed that biosurfactants, significantly reduced C. albicans adhesion and biofilm growth between 39.0% and 46.2% and between 74.1% and 80.4%, respectively24.
The lipopeptide surfactant when analyzed for its anti-biofilm activity revealed a directly proportional effect with increase in concentration. The lipopeptide extracted from isolate B1 also showed powerful penetration capacity in the biofilm and killed 91% C. albicans as seen by CFU assay and a highest inhibition at 2500µg/ml and 1250µg/ml concentration as studied by pre-coating experiment (Fig. 2).
Fig. 2. Effect of various concentrations of biosurfactant on C. albicans biofilms in the presence of biosurfactant (precoated on the wells of a microtiter plate)
A large number of lactobacilli have been studied for biosurfactant production and the corresponding impact on biofilm disruption24-26.Maldonado et al.27 found that whole cells of Lactobacillus and its supernatant were successfully employed to treat catheter- associated infections (CAI) caused by Klebsiella. Variety of lactobacilli produced biosurfactants have been checked for their anti-microbial and anti-biofilm activity28,-31. Therefore, Lactobacillus was included into the potential protective microorganisms to be used for bacteriotherapy and prevent CAI and urinary tract infections32.
Various indwelling medical devices like catheters in critical patients are prone to colonization by biofilm producing bacteria, which may lead to device-related infections, which pose new challenges in their management. Current research is focussed on competitive interaction strategies,which can lead to prevention and control of such concerns33,34.
Although various new molecules with the anti- biofilm activity are being researched and commercially used there is still dearth of eco-friendly anti- biofilm compounds. Biosurfactants can serve as a viable and effective alternative especially for anti-biofilm activity in the medical prosthetics and implants. Catheterization is a common medical intervention for urine passage in most surgical and debilitating hospitalization patients. Since the biosurfactant producing isolate L. fermentum is a normal flora of female urinary tract, the purified lipopeptide biosurfactant is a strong candidate for application as an effective anti-biofilm agent against the C. albicans biofilms formation and inhibition.
ACKNOWLEDGMENTS
Not Applicable.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All the authors designed the experiments. AS and MKD performed the experiments and analyzed the data. RNP and MKD prepared the manuscript. All the authors read the manuscript and corrections were made as required.
FUNDING
None.
ETHICS STATEMENT
Not Applicable.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
- Pennell KD, Abriola LM. Surfactant-enhanced aquifer remediation Fundamental processes and practical applications. Bioremediation: Principles and practice. Technomic Publishing, 1998: 693-743.
- Fakruddin M. Biosurfactants: Production and Application. J Pet Environ Biotechnol. 2012;3-124.
Crossref - Satpute SK, Kulkarni GR, Banpurkar AG, et al. Biosurfactant from Lactobacilli species: Properties, challenges and potential biomedical applications. J Basic Microbiol. 2016;56(11):1140-1158.
Crossref - Khameneh B, Zarei H, Bazzaz FS. The effect of silver nanoparticles on Staphylococcus epidermidis biofilm biomass and cell viability. Nanomed J. 2015;1(5):302-307.
- KulkarniVD, Kulkarni PS. Green Synthesis of Copper Nanoparticles Using Ocimum sanctum Leaf Extract. International Journal of Chemical Studies. 2013;1(3):1-4.
- Rodrigues L, Moldes A, Teixeria J, Oliveira R. Kinetic study of fermentative biosurfactant production by Lactobacillus strains. Biochem Eng J. 2006;28:109-116.
Crossref - Sharma D, Singh SB, Shailly K. Biosurfactants of lactic acid bacteria. ISBN. 978-3-319-26215-4. Springer Briefs in Microbiology. (Springer International Publishing), Switzerland. 2016;17-29.
Crossref - Cornea CP, Israel R, Sicuia OA, Voaides C, Zamfir M, Simona G. Biosurfactant production by Lactobacillus spp. Strains isolated from Romanian traditional fermented food products. Rom Biotechnol Lett. 2014;21(2):11312-11320.
- Sirsikar A,Hadawale S, Gupta A, Jaysree RC, Rajendran N. Comparative study of biosurfactant producing microorganisms using different energy sources. Int J Pharm Sci Rev Res. 2014;26(1):101-105.
- Morikawa M, Hirata Y, Imanaka T. A study on the structure function relationship of lipopeptide iosurfactants. BBA-Mol Cell Biol L. 2000;1488(3):211-218.
Crossref - Krepsky N, Silva FS, Fontana LF, Crapez MA. Alternative methodology for isolation of biosurfactant producing bacteria. Braz. J. Biol. 2007;67(1):117-24.
Crossref - Lai CC, Huang YC, Wei YH, Chang JS. Biosurfactant-enhanced removal of total petroleum hydrocarbons from contaminated soil. J Hazard Mater. 2009;167 (1-3): 609-614.
Crossref - Sperber WH, Swan J. Hot-loop test for the determination of carbon dioxide production from glucose by lactic acid bacteria. Appl Environ Microbiol. 1976;31(6):990-991.
Crossref - Suresh Chander CR, Lohitnath T, Mukesh KDJ, Kalaichelvan PT. Production and characterization of biosurfactant from Bacillus subtilis MTCC441 and its evaluation to use as bioemulsifier for food bio – preservative. Adv in Appl Sci Res. 2012;3(3):1827-1831.
- Yin H, Qiang Y, Jia J, et al. Characteristics of biosurfactant produced by Pseudomonas aeruginosa S6 isolated from oil –containing wastewater. Proc Biochem. 2008;44:302-308.
Crossref - Varadavenkatesan T, Ramachandra MV. Production of a lipopeptide biosurfactant by a novel Bacillus sp. And its applicability to enhanced oil recovery. ISRN Microbiol. 2013:621519. 2013; 3: 1-8.
Crossref - Fracchia L, Cavallo M, Allegrone G, Martinottie M. A Lactobacillus-derived biosurfactant inhibits biofilmformation of human pathogenic Candida albicans biofilm producers, Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology. Mendez Vilas A Ed. 2010;2:827-837
- Merritt JH, Kadouri DE, O’Toole GA. Growing and Analyzing Static Biofilms. Curr Protoc Microbiol. 2005;1:1B.1.
- Ghasemi A, Moosavi-Nasab M, Setoodeh P, Mesbahi G, Yousefi G. Biosurfactant production by Lactic acid bacterium Pediococcus dextrinicus SHU1593 Grown on different carbon sources: strain screening followed by product characterization. Scientific Reports. 2019;9:5287, 1-12.
Crossref - Abdalsadiq N, Hassan Z, Lani M, Bulgasem B. Identification of biosurfactant produced by Lactobacillus sp. Using mass spectrometry. Res J Life Sci Boinform Pharm Chem Sci. 2018;4:247-258.
Crossref - Kaur S, Kaur R. Biosurfactant from Lactobacillus sp. as an anti-biofilm agent. J of Biotech, Computational Biol and Bionanotech. 2019;100(3):335–343.
Crossref - Ramani K, Jain SC, Mandal A, Sekaran G. Microbial induced lipoprotein biosurfactant from slaughterhouse lipid waste and its application to the removal of metal ions from aqueous solution. Colloids Surfaces B: Biointerfaces. 2012;97:254-63.
Crossref - Ceresa C, Tessarolo F, Maniglio D, et al.Inhibition of Candida albicans biofilm by lipopeptide AC7 coated medical-grade silicone in combination with farnesol. AIMS Bioengineering. 2018;5(3):192–208.
Crossref - Sambanthamoorthy K, Feng X, Patel R, Patel S, Paranavitara C. Antimicrobial and anti-biofilm potential of biosurfactants isolated from Lactobacillus against multidrug- resistant pathogens. BMC Microbiol. 2014;14:197.
Crossref - Sharma D, Saharan BS, Chauhan N, Procha S, Lal S. Isolation and functional characterization of novel biosurfactant produced by Enterococcus faecium. Springer Plus. 2015;4(4):1-14.
Crossref - Gudina EJ, Rocha V, Teixeira J, Rodrigues L. Antimicrobial and antiadhesive properties of a biosurfactant isolated from Lactobacillus paracasei ssp. paracaseiA20. Lett Appl Microbiol.2010;50(4):419-24.
Crossref - Maldonado NC, Silva DR, Cecilia M, Nader MME. A simple technique to detect Klebsiella biofilm forming strains. Inhibitory potential of Lactobacillus fermentum CRL 1058 whole cells and products. Commun Curr Res Edu Topics Trends Appl Microbiol. Mendez Vilas (Ed.). 2007;52-59.
- Taheur BF, Kouidhi B, Fdhila K, et al. Anti-bacterial and anti-biofilm activity of probiotic bacteria against oral pathogens. Microb Path.2016;97:213-20.
Crossref - Pradeepa SAD, Matthews K, Hegde AR, et al. Multidrug resistant pathogenic bacterial biofilm inhibition by Lactobacillus plantarum exopolysaccharide. Bioactive Carbohydrates and Dietary Fibre. 8(1):7-14.
Crossref - Khiralla GM, Mohamed EAH, Farag AG, Elhariry H. Anti-biofilm effect of Lactobacillus pentosus and Lactobacillus plantarum cell-free supernatants against some bacterial pathogens. J Biotech Res. [ISSN: 1944-3285]. 2015;6:86-95.
- Percival SL, Suleman L, Vuotto C, Donelli G. Healthcare- associated infections, medical devices and biofilms: risk, tolerance and control. J Med Microbiol.2015;64(Pt 4):323-334.
Crossref - Bossa L, Kline K, McDougald D, Lee BB, Rice SA. Urinary catheter-associated microbiota changes in accordance with treatment and infection status. PLoS One.2017;12(6):e0177633.
Crossref - Singhai M, Malik A, Shahid M, Malik MA, Goyal R. A study on device-related infections with special reference to biofilm production and antibiotic resistance. J Glob Infect Dis.2012;4(4):193-8.
Crossref - Gominet M, Compain F, Beloin C, Lebeaux D. Central Venous catheters and biofilm: where do we stand in 2017? Acta Pathologica, Microbiologica, EtImmunologica, Scandinavica, APMIS. 2017;125(4):365-375.
Crossref
© The Author(s) 2020. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.