ISSN: 0973-7510
E-ISSN: 2581-690X
Heavy metal pollution is a growing environmental concern that affects ecosystem, human health and biodiversity. Cadmium is one of the most hazardous heavy metal, exhibiting high toxicity in plants, animals and humans. It is widely distributed in environment due to industrial activities, urbanization and improper waste disposal. This necessitates the removal of toxic metals from the wastewater and soil sediments. Bioremediation using microorganisms promises to be an excellent choice for the detoxification of heavy metals from wastewater. Molasses, a cost effective by product of sugar industry serves as an excellent carbon source for bacterial EPS production. Integrating molassess based EPS production with microbial bioremediation holds promise for sustainable pollution control and waste management. The main objective of this study was to evaluate the potential of three extracellular polymeric substances (EPS) producing bacteria EPS-1, EPS-2 and EPS-3, to remove cadmium from sewage wastewater using molasses as a source of carbon. The bioremediation of cadmium from sewage water was examined under in vitro conditions. The removal efficiency (%) of Cd2+ by the isolates was determined by using Atomic Absorption Spectrometry (AAS). Results showed that EPS-1 had maximum removal efficiency of 51%, while the removal by EPS-2 and EPS-3 was found to be 37% and 49%, respectively after 72 h of incubation under in vitro conditions in sewage water amended with molasses. If combined with inexpensive carbon sources, bioremediation of sewage water using EPS producing bacteria might be a more economical and sustainable option than chemical treatments. The results signify the potential of bacterial strains for their application in large scale removal of Cd2+ present in polluted water.
Bioremediation, Sewage Water, Bacterial Strains, Molasses, EPS
A huge volume of wastewater is produced due to rapid industrialization and urbanization in India. An approximately 38354 million liters per day (MLD) sewage is produced in major cities of India, but due to limited sewage treatment plant capacity, only 11786 MLD of sewage water is treated.1 Faridabad industrial complex is a major player in India’s manufacturing landscape. Being an industrial hub, the pollution level in city is always a matter of concern and has been documented as the 18th most polluted city by Central Pollution Control Board (CPCB) with a notably high Comprehensive Environmental pollution Index (CEPI).2 Faridabad houses a large number of electroplating, textile manufacturing, smelting, dyeing and printing units, all of discharge substantial volume of untreated industrial effluent containing various hazardous heavy metals. These effluents, when released directly into water systems, contribute to the contamination of sewage water, which is further compounded by the use of chemical fertilizers. Sewage water, in this context, refers to the wastewater produced from domestic activities and industrial operations, often carrying a high load of pollutants, including heavy metals, due to insufficient treatment. Metal toxicity in sewage water exposes the extent of industrial area served by local sewage system. All wastewater is collected at sewage disposal sites, where the sewage water treatment begins. Unfortunately, many of sewage disposal sites lack sufficient equipment and therefore most of the untreated sewage water is released into the rivers and waterways without any treatment. Prolonged irrigation of agricultural land with sewage water containing heavy metals at concentrations exceeding the permissible limits established by Indian Standards can lead to soil contamination, posing significant risks to soil health and crop safety.3 Sewage effluent is a major vector for the dissemination of toxic heavy metals into the environment, facilitating their bioaccumulation and biomagnification within the trophic levels of the food chain. These metals ultimately accumulate in various biotic entities, including microorganisms, fauna, and humans, leading to a range of metabolic and physiological disruptions.
Cadmium is recognized as highly toxic and carcinogenic element posing significant risks to living beings. It is classified as one of the priority pollutants by U.S. regulatory agencies.4 Cadmium enters the environment through various pathways, including industrial activities such as electroplating, mining, and smelting. Additionally, it is released during the incineration of plastics and batteries, as well as from the burning of fossil fuels. Other significant sources include the use of phosphate fertilizers and the application of sewage sludge.5 Acute exposure to cadmium may result in symptoms like abdominal discomfort, nausea and vomiting. Chronic exposure to cadmium can have severe long-term effects on health, as it accumulates in the body, particularly in the kidneys and bones. Over time, it can cause kidney damage, osteoporosis and increase the risk of fractures. Additionally, cadmium exposure leads to respiratory problems, including lung cancer, and neurological disorders.
Cadmium is also known to affect the functioning for many enzymes and proteins. It may interfere with the incorporation of essential trace metals such as zinc and selenium into metalloproteins, potentially impairing their activity. Additionally, cadmium can competitively inhibit calcium binding on regulatory proteins, including calmodulin, which plays a pivotal role in modulating intracellular signalling pathways and cellular homeostasis.6 This has raised a global concern for effective removal of cadmium from sewage wastewater to permissible limits before discharging into natural waterways. Many physico-chemical processes including reverse osmosis, ion exchange, solvent extraction and chemical precipitation are used for the metal removal.7 The main problem with physico-chemical treatment is the generation of secondary pollutants, which can again enter into the environment with the passage of time and the problem remains unsettled. In addition, these processes are usually unsuccessful at low metal concentrations. Consequently, there is a growing need to develop cost-effective, environmentally friendly solutions to reduce toxic heavy metals to safe levels. One promising alternative that has gained increasing attention in recent years is the use of bacterial biomass for the removal of toxic chemicals. Certain bacterial species can populate in metal contaminated environments, exhibiting significant resistance to toxic heavy metals through various adaptive mechanisms. These microorganisms have developed plethora of mechanisms of metal tolerance to avoid cellular damage caused by heavy metals and find their application in wastewater and sewage treatment. These bacteria may sequester heavy metals inside the cells by certain metal binding proteins which renders the toxic heavy metals unavailable,8 precipitate metals as metal sulphides through hydrogen sulphide production,9,10 binds heavy metals to cell wall components and EPS.11-15 EPS are of vital importance not only because they can form protective layer to guard the cell against the harsh external environments but also for remediation of metals in natural environments. EPS are generally composed of a variety of decomposable organic substances, such as polysaccharide (exopolysaccharides) and proteins, but also contain other macro-molecules such as lipids, DNA and humic substances, and hence implicated as promising biosorbents for heavy metal removal.16,17 The primary aim of this study was to assess the efficacy of three EPS-producing bacterial isolates in removing cadmium ions from sewage wastewater. In this context, molasses waste was utilized as a carbon source to support bacterial growth and enhance their metal removal capacity. Molasses, a by-product of sugar processing, is rich in organic compounds and has been identified as a cost-effective and sustainable carbon source for microbial applications. Its use not only provides an economical alternative for bacterial cultivation but also contributes to the recycling of industrial waste, making it an environmentally beneficial choice for bioremediation processes chemicals. Sewage samples were collected from sewage wastewater dumping site in sector-21, Faridabad, which receives domestic as well as industrial wastewater. The removal efficiency (%) of Cd2+ by the isolates was determined in sewage water alone as well as amended with nutrients by using Atomic Absorption Spectrometry (AAS).
Sample collection and physico-chemical characterization of sewage water
Sewage samples were collected from sewage disposal site from Faridabad (Figure 1). Samples were collected from sewage disposal sites in plastic containers that were pre-treated with dilute nitric acid, rinsed with distilled water, and stored in a refrigerator at 4 °C until they were analysed. Physicochemical characteristics of sewage sample were determined. The parameters included odor of water, pH, colour and turbidity. Microbial load in sewage water was also determined. The concentration of cadmium ions in sewage water was examined by atomic absorption spectrometer (AAS).
Figure 1. (a) Sewage disposal site in sector-21 A, Faridabad (b) sewage water sample collection site
Bacterial strains
The three EPS-producing bacterial isolates EPS-1, EPS-2 and EPS-3 used in this study has previously been characterized by biochemical tests and 16S rDNA sequence analysis and were found to be 99% similar to Bacillis sp.263ZY1, Bacterium YC-LK-LKJ45 and Bacillus subtilis strain DHXJ07 respectively.18
EPS production on Congo red agar (CRA) medium
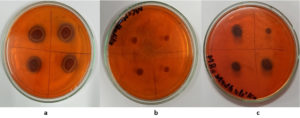
The isolates were screened for the production of exopolysaccharides qualitatively on CRA media (Peptone 0.5%, Yeast extract 0.1%, NaCl 0.1%, Glucose 1%, Agar 1% and Congo Red 0.08%). 10 µL of the culture broth of the selected isolates containing approximately 108 cells/mL was spotted on CRA plates and incubated for 48 h. The isolates were observed for the colour and physical appearance of the colonies.
Transmission electron microscopy (TEM) analysis
Bacterial cells were grown in LB media supplemented with 100 pm cadmium as cadmium chloride. Cells were observed with a transmission electron microscope (TEM) after 48 hrs of incubation.
Bioremediation of sewage water using selected isolates
The bioremediation of cadmium from sewage water was examined under in vitro conditions using EPS-1, EPS-2 and EPS-3, respectively. Two distinct experiments were performed to check the ability of bacterial strains to detoxify cadmium from both unsterilized sewage water as well as sterilized sewage water. In the first experiment, freshly collected sewage water was autoclaved at 121 °C for 15 mins to make them free from other microorganisms before inoculating them with selected bacterial isolates. The following treatments were done:
- Sterilized Sewage water (SSW) – 500 mL (Control)
- SSW (490 mL) + 10 mL EPS-1
- SSW (490 mL) + 10 mL EPS-2
- SSW (490 mL) + 10 mL EPS-3
- SSW (490 mL) + peptone (0.5%) + Glucose (1%) + 10 mL EPS-1
- SSW (490 mL) + peptone (0.5%) + Glucose (1%) + 10 mL EPS-2
- SSW (490 mL) + peptone (0.5%) + Glucose (1%) + 10 mL EPS-3
The second experiment was setup with similar treatments with unsterilized sewage water (USW) along with the following additional treatments:
- USW (450 mL) + Peptone (0.5%) + 40 mL Molasses + 10 mL EPS-1
- USW (450 mL) + Peptone (0.5%) + 40 mL Molasses + 10 mL EPS-2
- USW (450 mL) + Peptone (0.5%) + 40 mL Molasses + 10 mL EPS-3
- Molasses added in the medium was procured from sugar industry, Modinagar. The molasses was diluted with distilled water in the ratio of 1:1
All experiments were carried out at 37 °C for 96 h and samples were taken out from each flask after 72, 84 and 96 h of incubation. Bacterial cells were harvested by centrifugation at 5000 rpm for 5 min and bacterial supernatants were filtered through membrane filters (0.22 µm). Cadmium concentration was measured from the supernatant by the atomic absorption spectrophotometry. The efficiency of cadmium removal was determined by calculating the difference in metal concentration in culture at the time of inoculation and the concentration at the time of sampling.
Physico-chemical characterization of sewage water
The pH of sewage water sample was measured immediately after its collection using a pH meter and it was around 7.0. It was brownish in color and turbid. The turbidity was due to a wide variety of suspended materials that range in size from colloidal to coarse. Unpleasant odor was noted in sewage sample. Total microbial load of sewage water was 3×1010 cfu/ml at the time of sampling. Cd2+ concentration as determined by AAS was 6.8 ppm. The physicochemical properties of sewage water are shown in Table 1.
Table (1):
Physico-chemical characteristics of effluent sample
Parameters |
CFU |
pH |
Turbidity |
Odor |
Color |
Cadmium |
|---|---|---|---|---|---|---|
Sewage water |
3 × 1010 |
7 |
Turbid |
Pungent |
Brownish |
6.8 ppm |
Abbreviations: CFU: Colony forming unit
EPS production by selected isolates and TEM analysis
The mucoid colonies suggested EPS production aiding cadmium removal. Culturing on CRA medium plates confirmed EPS production with black, mucoid colonies after 24 hours (Figure 2).
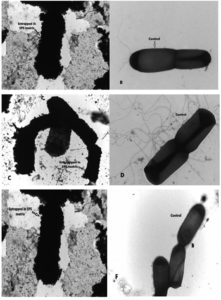
TEM analysis after 24 hours showed cadmium entrapment in EPS matrix of isolates EPS-1, EPS-2, and EPS-3 (Figure 3).
Figure 3. Transmission electron micrographs of isolates EPS-1, EPS-2 and EPS-3 (A): EPS-1 grown in the presence of 100 µg/mL Cd after 24 h of incubation, (B): EPS-1 control, (C): EPS-2 grown in the presence of Cd, (D): EPS-2 control, (E): EPS-3 grown in the presence of Cd, (F): EPS-3 control
Bioremediation of sewage water using selected isolates
In the sterilized sewage wastewater, all three strains showed the lower reduction of cadmium as compared to unsterilized one. Moreover, the reduction in cadmium concentration was more when sewage water was supplemented with peptone and glucose. The results are compiled in Table 2. The percentage reduction of cadmium was 36%, 31%, 34% by EPS-1, EPS-2 and EPS-3, respectively after 84 h of incubation and thereafter it showed a decrease (Table 3).
Table (2):
Cadmium concentration in sterilized sewage water after inoculation with three bacterial strains
| Bacterial isolates | Reduction of cadmium (µg/mL) | |||||
|---|---|---|---|---|---|---|
| With nutrients | Without nutrients | |||||
| 72 h | 84 h | 96 h | 72 h | 84 h | 96 h | |
| EPS-1 | 4.54hi | 4.35j | 4.74f | 5.24c | 4.86e | 4.88e |
| EPS-2 | 4.65fgh | 4.70fg | 5.11d | 5.60g | 5.21c | 5.47b |
| EPS-3 | 4.55hi | 4.47i | 4.86e | 4.86e | 4.61gh | 4.92e |
Values followed by different letters within the same column are significantly different from each other (p < 0.05). Values sharing the same letter are not significantly different
Table (3):
Cadmium reduction (%) from sterilized sewage water by three bacterial strains
| Bacterial isolates | Percentage reduction of cadmium | |||||
|---|---|---|---|---|---|---|
| With nutrients | Without nutrients | |||||
| 72 h | 84 h | 96 h | 72 h | 84 h | 96 h | |
| EPS-1 | 33.23 | 36.02 | 30.29 | 22.94 | 28.29 | 28.23 |
| EPS-2 | 31.61 | 30.88 | 24.85 | 17.64 | 23.23 | 19.55 |
| EPS-3 | 33.08 | 34.26 | 28.52 | 28.52 | 32.20 | 27.64 |
The experimental data were subjected to analysis of variance (ANOVA), followed by Duncan’s New Multiple Range Test (DMRT), with a significance level set at 5%. The mean values sharing the same letter are not significantly different.
The cadmium removal was enhanced in unsterilized sewage water supplemented with glucose (39%, 31% and 36% for EPS-1, EPS-2 and EPS-3, respectively) (Table 4). This may be attributed to the presence of microbes that bind the cadmium to their cell wall constituents. Although the studies pertaining to the removal of heavy metals from aqueous solutions under in vitro conditions are plenty, the studies employing the bacterial isolates for the removal of heavy metals from sewage water or industrial effluents are scarce.
Table (4):
Cadmium concentration in unsterilized sewage water after inoculation with three bacterial strains
| Bacterial isolates | Reduction of cadmium (µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Without nutrient media | With nutrient media | With Molasses | |||||||
| 72 h | 84 h | 96 h | 72 h | 84 h | 96 h | 72 h | 84 h | 96 h | |
| EPS-1 | 5.35c | 4.51fg | 5.18d | 4.65ef | 4.14j | 4.37h | 4.27i | 3.32m | 3.44im |
| EPS-2 | 5.76a | 5.16d | 5.42c | 5.41c | 4.72e | 5.15d | 5.14d | 4.28i | 4.43gh |
| EPS-3 | 5.56b | 4.67e | 5.18d | 5.09d | 4.32h | 4.64ef | 4.53fg | 3.48kl | 3.59k |
Values followed by different letters within the same column are significantly different from each other (p < 0.05). Values sharing the same letter are not significantly different
In second set of experiment (with unsterilized sewage water) glucose was replaced with molasses, which was used as a carbon source for its low cost and availability. Cadmium concentration was determined after inoculation with the strains (Table 4). All three strains showed higher cadmium removal in the presence of molasses as compared to glucose amended sewage water. The efficacy of cadmium removal in increasing order was EPS-2 (37%) EPS-1 (49%) and EPS-3 (51%) (Table 5).
Table (5):
Cadmium reduction (%) from unsterilized sewage water by three bacterial strains
| Bacterial isolates | Percentage of Cadmium Reduction | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Without nutrient media | With nutrient media | With Molasses | |||||||
| 72 h | 84 h | 96 h | 72 h | 84 h | 96 h | 72 h | 84 h | 96 h | |
| EPS-1 | 21.32 | 33.67 | 23.82 | 31.61 | 39.12 | 35.73 | 37.20 | 51.18 | 49.41 |
| EPS-2 | 15.29 | 24.12 | 20.29 | 20.44 | 30.59 | 24.26 | 24.41 | 37.05 | 34.85 |
| EPS-3 | 18.23 | 31.32 | 24.18 | 25.15 | 36.47 | 31.76 | 33.38 | 48.82 | 47.20 |
The experimental data was analysed by analysis of variance (ANOVA) and Duncan’s New Multiple Range (DMRT) at 5% significance level. The mean values sharing the same letter are not significantly different.
Bioremediation of heavy metal-containing wastes using microbes can be accelerated by adding organic carbon sources as amendments. Molasses is a preferred substrate for this purpose as it provides dual benefits: it serves as a carbon source for the growth of the introduced bacteria and, being rich in sugars like sucrose, it promotes the production of exopolysaccharides. This is particularly important in bioremediation processes involving microbes that remove heavy metals mainly by sequestering them in extracellular polymeric substances rather than through metabolism-dependent binding proteins. The strains used in this study were previously isolated and characterized in the same laboratory. All isolates showed resistance to cadmium and were capable of removing significant amounts of cadmium (78% by EPS-1, 72% by EPS-2, and 74% by EPS-3).18 In this study, a biotechnological approach was employed for treating sewage water by adding molasses as an organic carbon source instead of nutrient media, aiming to enhance process efficiency and reduce wastewater treatment costs. Strains EPS-1, EPS-2, and EPS-3 exhibited high cadmium removal efficiencies of 51%, 37%, and 49%, respectively, after 82 hours in sewage water supplemented with molasses. However, the removal efficiency decreased after 82 hours, possibly due to polysaccharide degradation by glycohydrolases during the stationary growth phase, leading to reduced EPS yields and cadmium adsorption. Previous studies have also reported a similar decrease in EPS production over time.19,20
Molasses has been proven to be an excellent carbon source for EPS production to facilitate heavy metal removal by numerous researchers. Donmez and Aksu found molasses to be a suitable alternative to carbon sources like glucose, and sucrose for removing Cu2+ from wastewater using Kluyveromyces marxianus.21 Smyk and Ignatowicz demonstrated more effective nitrogen removal when molasses was used as an external carbon source during wastewater treatment.22 Stenotrophomonas maltophilia showed significant removal efficiencies of 32%, 75.7%, and 51.1% for nickel, copper, and chromium ions, respectively, in a molasses-based medium. Kilic et al. reported that bacterial cultures from Ankara Stream could remove heavy metals through EPS production.23 Pseudomonas fluorescens produced more EPS using sugarcane molasses compared to sucrose.24 Sengupta et al. also found molasses extract to be a cost-effective substrate for exopolysaccharide secretion in Ochrobactrum pseudintermedium C1 during heavy metal biosorptions studies.25 Cheah et al. observed increased EPS yield and metal removal by Bacillus cereus with the addition of 1% molasses to the nutrient broth.26 Trichoderma showed significant cadmium removal from tannery effluent supplemented with 1% molasses at pH 4.27 These studies suggest that this strategy can effectively scale up metal removal from wastewater.
Interest in sustainable bacterial EPS production is growing for applications in pharmaceuticals, medicine, and industrial biotechnology. Using sugarcane molasses waste as a substrate offers a cost-effective production method suitable for various industries. In the present study, all three bacterial strains were capable of removing upto 51% of cadmium from sewage water, signifying that these selected isolates can be efficiently used for the large-scale metal removal from wastewater with minimum cost and high efficiency. The advantage of using these bacterial strains is that they produce significant amounts of EPS that was responsible for their ability to accumulate heavy metals and protect themselves. All three strains were able to remove cadmium ions present in wastewater within incubation period of 84 h. This highlights bioremediation as a highly effective alternative for ensuring efficient treatment and optimal performance, particularly when dealing with metal pollution in industrial effluents.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the Department of Soil Science, Chaudhary Charan Singh Haryana Agricultural University (CCS HAU), Hisar, for providing access to the Atomic Absorption Spectroscopy (AAS) facility, which was instrumental in carrying out the analytical part of this research work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Kaur R, Wani SP, Singh AK, Lal K. Wastewater production, treatment and use in India. In National Report presented at the 2nd regional workshop on Safe Use of Wastewater in Agriculture. 2012;16:1-13.
- Annual Report 2022-2023, Central Pollution control board. https://cpcb.nic.in/annual-report/. Accessed 2 March, 2025.
- Alengebawy A, Abdelkhalek ST, Qureshi SR, Wang MQ. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics. 2021;9(3):42.
Crossref - Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy Metal Toxicity and the Environment. In: Luch, A. (eds) Molecular, Clinical and Environmental Toxicology. Experientia Supplementum, vol 101. Springer, Basel.
Crossref - Kubier A, Wilkin RT, Pichler T. Cadmium in soils and groundwater: A review. Appl Geochem. 2019;108:1-16.
Crossref - Choong G, Liu Y, Templeton DM. Interplay of calcium and cadmium in mediating cadmium toxicity. Chem-Biol Interact. 2014;211:54-65.
Crossref - Rafique M, Hajra S, Tahir MB, Gillani SSA, Irshad M. A review on sources of heavy metals, their toxicity and removal technique using physico-chemical processes from wastewater. Environ Sci Pollut Res. 2022;29:16772-16781.
Crossref - Diep P, Mahadevan R, Yakunin AF. Heavy metal removal by bioaccumulation using genetically engineered microorganisms. Front Bioeng Biotechnol. 2018;29(6):157.
Crossref - Jong T, Parry DL. Removal of sulfate and heavy metals by sulfate reducing bacteria in short-term bench scale upflow anaerobic packed bed reactor runs. Water Res. 2003;37(14):3379-3389.
Crossref - Muyzer G, Stams AJ. 2008. The ecology and biotechnology of sulphate-reducing bacteria. Nat Rev Microbiol. 2008;6:441-454.
Crossref - Vijayaraghavan K, Yun Y-S. Bacterial biosorbents and biosorption. Biotechnol Adv. 2008;26(3):266-291.
Crossref - Yang J, Wei W, Pi S, et al. Competitive adsorption of heavy metals by extracellular polymeric substances extracted from Klebsiella sp. J1. Bioresour Technol. 2015;196:533-239.
Crossref - Yue ZB, Li Q, Li CC, Chen TH, Wang J. Component analysis and heavy metal adsorption ability of extracellular polymeric substances (EPS) from sulfate reducing bacteria. Bioresour Technol. 2015;194:399-402.
Crossref - Shamim S. Biosorption of heavy metals. Biosorption. 2018;2:21-49.
Crossref - Cao R, Zhang Y, Ju Y, et al. Exopolysaccharide-producing bacteria enhanced Pb immobilization and influenced the microbiome composition in rhizosphere soil of pakchoi (Brassica chinensis L.). Front Microbiol. 2023;14:1117312.
Crossref - Yin Y, Hu Y, Xiong F. Biosorption properties of Cd (II), Pb (II), and Cu (II) of extracellular polymeric substances (EPS) extracted from Aspergillus fumigatus and determined by polarographic method. Environ Monit Assess. 2013;185(8):6713-6718.
Crossref - Costa OYA, Raaijmakers JM, Kuramae EE. Microbial extracellular polymeric substances: ecological function and impact on soil aggregation. Front Microbiol. 2018;23(9):1636.
Crossref - Chauhan M, Solanki M. Isolation and molecular characterization of three Bacillus strains for their tolerance against various heavy metals. J Microbiol Biotechnol Food Sci. 2017;6(4):1065-1069.
Crossref - Pham PL, Dupont I, Roy D, Lapointe G. Production of exopolysaccharide by Lactobacillus rhamnosus R and analysis of its enzymatic degradation during prolonged fermentation. Appl Environ Microbiol. 2000;(6):2302-2310.
Crossref - Moghannem SAM, Farag MM, Shehab AM, Azab MS. Exopolysaccharide production from Bacillus velezensis KY471306 using statistical experimental design. Braz J Microbiol. 2018;49(3):452-462.
Crossref - Donmez G, Aksu Z. The effect of copper (II) ions on the growth and bioaccumulation properties of some yeasts. Process Biochem. 1999;35(1-2):135-142.
Crossref - Smyk J, Ignatowicz K. The influence of molasses on nitrogen removal in wastewater treatment with activated sludge. J Ecol Eng. 2017;18(4):199-203.
Crossref - Kilic NK, Kurkcu G, Kumruoglu D, Donmez G. EPS production and bioremoval of heavy metals by mixed and pure bacterial cultures isolated from Ankara Stream. Water Sci and Technol. 2015;72(9):1488-1494.
Crossref - Sirajunnisa A, Vijayagopal V, Viruthagiri T. Effect of synthetic carbon substrates and cane molasses, an agro waste on exopolysaccharide production by P. fluorescens. Int J Eng Appl. 2012;1(1):60-66.
Crossref - Sengupta D, Datta S, Biswas D, Banerjee S, Das S. Prospective bioremediation of toxic heavy metals in water by surfactant exopolysaccharide of Ochrobactrum pseudintermedium using cost-effective substrate. Intr Microbiol. 2021;24(3):441-453.
Crossref - Cheah C, Cheow YL, Ting ASY. Co-cultivation, metal stress and molasses: strategies to improving exopolymeric yield and metal removal efficacy. Sustain Environ Res. 2022;32:9.
Crossref - Tello-Galarreta FA, Durand-Paz JH, Rojas-Villacorta W, et al. In Vitro Effect of Molasses Concentration, pH, and Time on Chromium Removal by Trichoderma spp. from the Effluents of a Peruvian Tannery. Processes. 2023;11(5):1557.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.