ISSN: 0973-7510
E-ISSN: 2581-690X
Most bacteria and fungi are capable of producing biofilms, enabling them to thrive in nature on distinct surfaces. Biofilm formation stands out as one of the most prominent virulence mechanisms that contribute to the infection’s chronicity by functioning as a defense against antimicrobials and host immune systems. Microbial isolates capable of generating biofilms have been discovered to possess higher resistance to frequently administered antifungal drugs. In this research study, 91 Candida isolates from Vulvovaginal Candidiasis (VVC) patients were tested for biofilm development. Candida species were identified, and clinical isolates were tested for antifungal susceptibility (AST). Three methods were used to screen the isolates: the Congo agar method (CRA), the visual tube method (VT), and the Microtitre plate method (MTP). Nearly 60% of the 91 clinical isolates tested were recognized as Non-Albicans Candida (NCAC) species. Itraconazole resistance was shown to be the highest in clinical isolates, followed by Amphotericin B resistance. There were 11(12.09%) isolates that formed strong biofilms, 35(38.46%) isolates that formed moderate biofilms, and 45(49.45%) isolates that formed no biofilm. Because there is a growing incidence of NCAC in the study, it is critical to speciate the Candida species as NCAC are more resistant to routinely used azole medicines. Furthermore, a spike in the prevalence of biofilm producers has been reported, implying greater pathogenicity and antifungal resistance.
Microtitre Plate Method, Congo Red Agar Method, Itraconazole Resistance, Fluconazole Resistance, Antifungal Resistance
More than 70% of women will experience Vulvovaginal candidiasis (VVC), a common gynecological illness, at some point in their life. VVC is usually not fatal; however, recurrence of VVC impairs the quality of life in afflicted women.1 VVC has been reported to be the second-leading cause of vaginitis.2 Candida colonizes roughly 10-15% of asymptomatic patients; almost fifty percent of women who were previously diagnosed with VVC will contract a second episode. Recurrent infections are also caused in about 5-10% of all women.3 Many variables, such as long-term usage of antifungal medications available over-the-counter and one-dose therapy of fungal infections, contribute to the increased incidence of VVC. Although it is thought that C. albicans, an opportunistic dimorphic fungal pathogen, is involved in majority of VVC infections, there is growing evidence of Non-Albicans Candida (NCAC) causing VVC.4,5 NCAC develops innate and acquired drug resistance to routinely given antifungal medications resulting in increasing antifungal resistance. Candida species produce virulence factors such as secreted aspartyl proteinases (SAP), hemolysin, lipases, coagulase, and biofilm formation, which contribute to pathogenicity, allow them to elude host immune responses, and establish themselves as a pathogen causing morbidity.6 Germ tube production, hyphal formation, phenotypic switching, and thigmotropism are other virulence factors that contribute to their pathogenicity.7 Candida forms biofilms by affixing to a wide range of surfaces, both abiotic and biotic. These have three-dimensional structural configuration composed of proteins, polysaccharides, and DNA embedded in the extracellular matrix (ECM) of fungal cell aggregates.8 The biofilm matrix forms a physical barrier that protects the Candida species that are immersed in it from environmental influences while also giving structural integrity to the biofilm. The biofilm matrix also prevents mechanical breakdown of the biofilm. Adherence, proliferation, maturity, and dispersion are the four critical steps in the creation of a biofilm.9 Fungal cells capable of generating biofilms are noted to be highly resistant to the antifungal drugs and to have a different metabolism than their planktonic counterparts. The biofilm works as a barrier, defending against attacks by the host immune system and antifungal medications.10 Candida species cause greater mortality in immunocompromised patients, necessitating the use of broad-spectrum antifungal medicines. However, continuous use of antifungal medicines, as well as abuse of over-the-counter drugs, leads to an increase in antifungal resistance in Candida species and cause greater mortality in immunocompromised patients, necessitating the use of broad-spectrum antifungal medicines. However, continuous use of antifungal medicines, as well as abuse of over-the-counter drugs, leads to an increase in antifungal resistance in Candida species.11 Furthermore, biofilm development is a major contributor to fungal resistance to antimycotic medications such as azoles, polyenes, and echinocandins.12 Several investigations on biofilm formation and antifungal resistance have revealed that they play a substantial part in pathogenicity of the infection.13,14 VVC, in particular, is believed to be a biofilm-mediated infection.15 This highlights the need for us to better our understanding of the infectious agent involved, in order to improve clinical outcomes and develop effective strategies for combating VVC. It is thus critical for researchers to contribute to the existing knowledge on Candida biofilm prevalence and antifungal resistance, as both of these Candidal attributes have been evolving considerably over the years.16-19 Furthermore, these are bound to alter based on the study population and its dynamics.13,18 Although several studies have demonstrated an increase in fluconazole resistance in VVC cases worldwide, there are only a limited number of studies that emphasize the evolving resistance of Candida species to other antifungals such as itraconazole, amphotericin B, and voriconazole.20,21 This study intends to shed light on the rising antifungal resistance and biofilm formation in both pregnant and non-pregnant women belonging to the suburban population. MTP (Microtitre plate method), CRA (Congo red agar), visual tube assay, bioluminescent assay, light or fluorescence microscopy, and other methods are available for biofilm screening.22,23 Although the MTP approach is considered the gold standard for identifying biofilm development, it is tedious and requires instruments such as a UV spectrophotometer, limiting its use in normal laboratory processes. These drawbacks can be overcome by employing simpler alternative procedures such as CRA and VT.23 In this study, we examined 91 non-repetitive Candida vaginal isolates for biofilm development as well as their antifungal susceptibility profile. Biofilm formation was screened using three different techniques: MTP, CRA and VT to examine the efficiency of these methods.
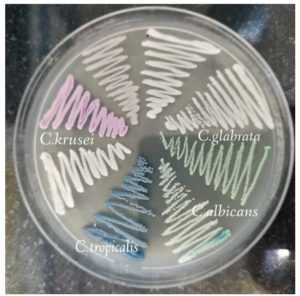
This cross-sectional study involved 91 non-repetitive species of Candida identified from both pregnant and non-pregnant women visiting SRM Medical College Hospital and Research Centre with complaints of curdy white vaginal discharge from May 2022–October 2022. Standard microbiological procedures were used to identify the clinical isolates, such as identification of yeast cells by KOH met mount, germ tube formation, chlamydospore formation, sugar fermentation and sugar assimilation assays. Furthermore, speciation of C. albicans and NCAC was done using HiCrome Candida differential agar (Himedia Mumbai, India). On Candida differential agar (CDA), Green colonies are formed by C. albicans, C. krusei formed pink to purple colonies, C. tropicalis was identified by formation of dark blue colonies, and cream to white colonies were formed by C. glabrata. (Figure 1). The culture was kept viable by subculturing it on Sabouraud’s dextrose broth (SDB) and storing it in the deep freezer until needed.
Phenotypic detection of Biofilm production
Congo red agar (CRA)
CRA is a qualitative evaluation method for spotting microorganisms that produce biofilm. CRA is prepared by adding 0.8g of Congo red along with 36g of sucrose to 37 g/L of Brain heart infusion (BHI) agar. The clinical isolates to be evaluated were streaked over CRA petri dishes and were cultured at 37°C for 24 hours. Biofilm producers were distinguished by the emergence of dark black colonies with a brittle crystalline texture, while non-biofilm formers did not change colour and maintained pink colony morphology. Positive results of the assay is indicated by formation of black coloured colonies with dry crystalline consistency. Weak biofilm formers are normally pink, with intermittent darkening of the colonies’ centres.24,25
Visual Tube method
In 5ml of SDB, a loopful of overnight Candida culture is introduced and cultured at 37°C for 24 hours. After the incubation period, the tubes were decanted and further washed with Phosphate buffered saline (PBS). The tubes were kept in upside down position for drying. The dried tubes were then stained with 0.1% Crystal violet. The excess stains in the tubes were removed and sterile water was used to clean the stained tubes. The tubes were again dried inverted. Formation of visible film lining the tube’s wall and its bottom indicates biofilm formation. Biofilm production in tubes was evaluated and classified as weak/non (+), moderate (++), or strong (+++).24,26
Microtitre plate method (MTP)
Millsap et al., MTP test was used to screen 91 Vulvovaginal Candidiasis isolates for biofilm development. In brief, 100μl of the overnight grown cell suspension of Candida was added in triplicate to the 96 welled MTP plates. The sterility and the non-specific binding of the media was tested by adding merely the broth in the wells of the MTP plate in triplicates followed by incubation at 37°C for 72 hours. Following the incubation period, the contents of the plate were removed by gently by tapping off the plates. To eliminate planktonic (free floating) organisms, the plates were rinsed with PBS. To detect sessile organisms, 0.1% Crystal violet was used to stain the plates. Distilled water was utilized to remove excess stains. Solubilization of the stain was done by adding acetic acid to the wells and a wavelength of 450 nanometers was applied to assess the absorbance using an ELISA reader (Merilyzer, Elisa reader, and washer).26 The mean OD values are used to estimate the Candida species’ adhering capacity (Table 1).
Table (1):
Classification of Candida adherence by MTP Method
Mean OD |
Adherence |
Biofilm formation |
|---|---|---|
>0.320 |
Strongly adherent |
High |
0.120-0.320 |
Moderately adherent |
Moderate |
<0.120 |
Non adherent |
Weak/Strong |
Antifungal Susceptibility Testing
The Antifungal susceptibility profile of the clinical isolates were identified for the most commonly suggested antifungal drugs by the Disc diffusion method according to the CLSI guidelines.6,7 Mueller-Hinton agar (MHA) was used to carry out the susceptibility testing with some minute modifications. Briefly, MHA for Candida was prepared by adding 0.5mg/ml of Methylene blue to the MHA medium, followed by autoclaving. Candidal cell suspensions were prepared to a turbidity to match 0.5 McFarland standards (106 CFU/ml), similar to the standard procedure for testing antibacterial substances. These Candida cell suspensions were then spread onto agar plates. Antifungal discs (fluconazole (25g), amphotericin B (100g), itraconazole (10g), and voriconazole (1 g)) were positioned on the inoculated plates and were subsequently incubated at 37°C for a duration of 24-48 hours. The diameter of the inhibition zone was measured after the incubation period. The results were evaluated in accordance with CLSI.27,28 In this investigation, ATCC 10231 of C. albicans was utilised as the reference strain.
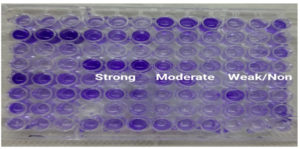
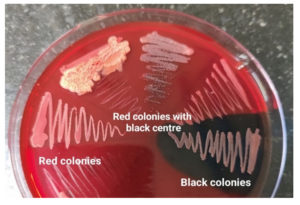
C. albicans was detected in 38(41.76%) of the 91 clinical isolates investigated, C. glabrata in 22(24.17%) isolates, C. krusei in 19(20.88%) isolates, C. tropicalis in 8(8.79%) isolates and C. parasilopsis in 4(4.39%) isolates. In the MTP assay, 11(12.09%) of the isolates were found to be strong formers, while 35(38.46%) isolates were moderate biofilm formers and 45(49.45%) of the clinical strains were either weak or Non-biofilm formers (Figure 2) (Table 2). According to the VT method, 19(20.88%) isolates were strong biofilm producers, 35(38.46%) isolates were moderate biofilm producers, and the remaining 37(40.66%) isolates were weak/non biofilm producers (Figure 3). By the CRA method, the majority of Candida isolates, 82% (90.11%), displayed a red colony shape with no dry crystalline consistency, 4 (4.39%) of the clinical isolates had black colonies with dry crystalline consistency, while 5 (5.49%) had red to pink colonies with dry crystalline consistency (Figure 4) (Table 3). Among the 91 clinical isolates, 33(36.36%) vaginal isolates were from pregnant women with complaints of vaginal discharge and 58(63.73%) isolates were from non-pregnant women. C. albicans was detected in 15(45.45%) of 33 clinical isolates from pregnant women, while NCAC was found in 18(54.54%). C. albicans was found in 23(39.65%) vaginal isolates from non-pregnant women, while NCAC was isolated from 35(60.34%) clinical strains. Antifungal susceptibility profiles of clinical strains were evaluated for fluconazole, amphotericin B, itraconazole, and voriconazole. Fluconazole sensitivity was detected in 86(94.50%) clinical isolates, intermediate sensitivity in 02(2.20%) isolates, and resistance in 03(3.30%) isolates. Amphotericin B sensitivity was found in 49(53.85%) clinical isolates, with 23(25.27%) showing moderate sensitivity and 19(20.88%) being resistant. Itraconazole resistance was found in 51(56.04%) clinical isolates, with 24(26.37%) exhibiting moderate sensitivity and 16(17.58%) being sensitive. Two clinical isolates were discovered to be resistant to voriconazole, whereas 89(97.80%) isolates were found to be susceptible. Table 4 shows the antifungal susceptibility profile of C. albicans and NCAC.
Figure 2. Biofilm screening by Microtitre plate method: A. Strong, B. Moderate and C. Weak/Non biofilm producer
Figure 3. Biofilm screening by visual tube method: A. Strong, B. Moderate and C. Weak/Non biofilm producers
Figure 4. Congo red agar showing black colonies with dry crystalline consistency, red colonies with black center, and red to pink colonies
Table (2):
Strong, moderate and weak/non biofilm producers by MTP method according to Candida species isolated
Species distribution |
Total number of isolates (n=91) |
Strong biofilm producers (n=11) |
Moderate biofilm producers (n=35) |
Weak/non biofilm producers (n=45) |
|---|---|---|---|---|
Candida albicans |
38(41.76%) |
05(13.16%) |
16(42.10%) |
17(44.73%) |
Candida glabrata |
22(24.17%) |
02(9.09%) |
06(27.27%) |
14(63.63%) |
Candida krusei |
19(20.88%) |
02(10.53%) |
08(8.79%) |
09(9.89%) |
Candida tropicalis |
08(8.79%) |
01(16.66%) |
03(3.30%) |
04(4.39%) |
Candida parapsilosis |
04(4.39%) |
01(16.66%) |
02(10.53%) |
01(16.66%) |
Table (3):
Strong, moderate and weak/non biofilm producers by MTP, VT method and CRA method
Strength |
MTP Method |
VT Method |
CRA Method |
|---|---|---|---|
Strong |
11(12.09%) |
19(20.88%) |
4(4.39%) |
Moderate |
35(38.46%) |
35(38.46%) |
5(54.94%) |
Weak/Non |
45(49.45%) |
37(40.66%) |
82(90.11%) |
Table (4):
Antifungal susceptibility profile of Candida species
Sensitive |
Intermediate |
Resistant |
|
|---|---|---|---|
Fluconazole |
86(94.50%) |
02(02.20%) |
03(03.30%) |
Amphotericin B |
49(53.85%) |
23(25.27%) |
19(20.88%) |
Itraconazole |
16(17.58%) |
24(26.37%) |
51(56.04%) |
Voriconazole |
89(97.80%) |
0.00(0.00%) |
02(02.20%) |
C. albicans and NCAC are responsible for a wide range of human diseases leading to morbidity and mortality, particularly in individuals with compromised immunity. It is responsible for an extensive spectrum of diseases, including superficial skin infections, VVC and disseminated Candidiasis.29 A recent study conducted in the year 2023 reported around 15.0% prevalence of VVC.30 C. albicans is being consistently identified as the most predominant species of the genus Candida responsible for causing VVC for many decades with the present emergence of NCAC. C. glabrata, C. parapsilosis, C. tropicalis, and C. krusei are identified as the most common NCAC causatives of VVC. A study conducted in the year 2010 reported more than 70% prevalence of C. albicans and numerous studies have been conducted that reveal a higher prevalence of C. albicans as compared to NCACs.31-34 Another study made on the species distribution and antifungal susceptibility profile of clinical isolates from women with recurrent and non-recurrent VVC revealed C. albicans to be the most predominant species followed by C. glabrata and C.tropicalis.35 However, there has been an upsurge in the prevalence of NCAC in recent years. NCAC species are particularly resistant to the majority of generally prescribed antifungal drug class, azoles.36-38 Various studies performed in recent years on the antifungal resistance in NCAC have proved the same.39 The present study reported 41.76% prevalence of C. albicans. 24.27% of C. glabrata, 20.88% of C. krusei and 8.79% of C. tropicalis and 4.39 % of C. parapsilosis were isolated among NCAC. These findings are consistent with prior research that found an increase in NCAC incidence compared to C. albicans prevalence.29,36,38 Key virulence factors influencing the development of vulvovaginal candidiasis include hemolysin, lipase, protease, germ tube formation, and biofilm formation.27 C. albicans and NCAC can form biofilms on an array of surfaces, including catheters, implanted devices, and the mucosal surface of the oral cavity.28,40 It is also proven that C. albicans biofilm contributes to pathogenicity and increased fungal burden in murine vaginitis ex vivo models.15 Candida species capable of forming biofilm are often associated with less resistance to antifungal medications and less sensitive to the host immunological responses.40 The extracellular matrix of the biofilm plays a major role in making the biofilm considerably resistant to various antifungals and helps them to survive in adverse conditions.41,14 It is also found that in vivo conditions are comparatively more favourable for rapid biofilm production.13 As a result, screening for biofilm formation in instances of VVC is critical, especially in recurring cases of vulvovaginal Candidiasis. A prior study found 44% biofilm formation in VVC, as well as an increased incidence of NCAC.15 By MTP technique, our study found 11 (12.08%) high biofilm producers and 35 (38.46%) intermediate biofilm producers while 45(49.45%) isolates didn’t form any biofilm. These results are similar to the prior studies made on biofilm production in VVC.38,43 More than half of the C. albicans were observed to develop biofilm. The VT technique found 19 (20.88%) strong biofilm formers and 35 (38.46%) intermediate biofilm formers. Furthermore, the VT method was more sensitive than the CRA method. In biofilms produced by certain clinical strains, it proved challenging to visually differentiate among moderate and weak/ non-Biofilm formers applying the VT method. This aligns with the results of a previous investigation that compared various approaches for screening biofilm.24 More than half of the clinical strains were itraconazole resistant, and more than 20% of the isolates were amphotericin B resistant. Similar to the previous investigation, the vaginal Candida strains were identified to be responsive to Fluconazole and Voriconazole although many research have found increased fluconazole resistance in VVC, which contradicts the findings of the current study.38,43
NCAC predominance was found in patients with VVC. Itraconazole resistance was found in more than half of the clinical isolates tested while the clinical strains were found to be sensitive to fluconazole. Information on antifungal susceptibility becomes crucial as prompt treatment is required as VVC causes inflammation and vulvovaginal pruritus. Fluconazole should continue to be the first-line of drugs prescribed by the clinicians because VVC isolates are very responsive to it, followed by Voriconazole, even though some studies show an increase in fluconazole resistance in VVC. Almost half of the clinical isolates tested positive for Strong and Moderate biofilm formers. Furthermore, the prevalence of biofilm producers continues to rise, indicating increased pathogenicity and antifungal resistance, emphasizing the significance of screening for biofilm formation in VVC.
ACKNOWLEDGMENTS
The authors would like to thank SRM Medical College Hospital and Research Centre, Kattankulatur for providing the required lab facilities to carry out the experiments.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Yano J, Sobel JD, Nyirjesy P, et al. Current patient perspectives of vulvovaginal candidiasis: incidence, symptoms, management and post-treatment outcomes. BMC Womens Health. 2019;19(1):48.
Crossref - Anderson MR, Klink K, Cohrssen A. Evaluation of Vaginal Complaints. JAMA. 2004;291(11):1368-79.
Crossref - Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369(9577):1961-1971.
Crossref - Makanjuola O, Bongomin F, Fayemiwo S. An Update on the Roles of Non-albicans Candida Species in Vulvovaginitis. J Fungi. 2018;4(4):121.
Crossref - Deorukhkar SC, Saini S, Mathew S. Non-albicans Candida Infection: An Emerging Threat. Interdiscip Perspect Infect Dis. 2014;615958.
Crossref - Yang YL. Virulence factors of Candida species. J Microbiol Immunol Infect. 2003;36(4):223-228.
- Sardi JCO, Scorzoni L, Bernardi T, Fusco-Almeida AM, Mendes Giannini MJS. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol. 2013;62(Pt 1):10-24.
Crossref - Al-Fattani MA, Douglas LJ. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J Med Microbiol. 2006;55(Pt 8):999-1008.
Crossref - Blankenship JR, Mitchell AP. How to build a biofilm: a fungal perspective. Curr Opin Microbiol. 2006;9(6):588-594.
Crossref - Tobudic S, Kratzer C, Lassnigg A, Presterl E. Antifungal susceptibility of Candida albicans in biofilms. Mycoses. 2012;55(3):199-204.
Crossref - Dawson CC, Intapa C, Jabra-Rizk MA. “Persisters”: Survival at the Cellular Level. PLoS Pathog. 2011;7(7):e1002121.
Crossref - Taff HT, Mitchell KF, Edward JA, Andes DR. Mechanisms of Candida biofilm drug resistance. Future Microbiol. 2013;8(10):1325-1337.
Crossref - Malinovska Z, Conkova E, Vaczi P. Biofilm Formation in Medically Important Candida Species. J Fungi. 2023;9(10):955.
Crossref - Ajetunmobi OH, Badali H, Romo JA, Ramage G, Lopez-Ribot JL. Antifungal therapy of Candida biofilms: Past, present and future. Biofilm. 2023;5:100126.
Crossref - Harriott MM, Lilly EA, Rodriguez TE, Fidel PL, Noverr MC. Candida albicans forms biofilms on the vaginal mucosa. Microbiology. 2010;156(Pt 12):3635-3644.
Crossref - Atiencia-Carrera MB, Cabezas-Mera FS, Tejera E, Machado A. Prevalence of biofilms in Candida spp. bloodstream infections: A meta-analysis. PLoS One. 2022;17(2):e0263522.
Crossref - Stover KR, Hawkins BK, Keck JM, Barber KE, Cretella DA. Antifungal resistance, combinations and pipeline: oh my! Drugs Context. 2023;12:1-23.
Crossref - Czajka KM, Venkataraman K, Brabant-Kirwan D, et al. Molecular Mechanisms Associated with Antifungal Resistance in Pathogenic Candida Species. Cells. 2023;12(22):2655.
Crossref - Gerges MA, Fahmy YA, Hosny T, et al. Biofilm Formation and Aspartyl Proteinase Activity and Their Association with Azole Resistance Among Candida albicans Causing Vulvovaginal Candidiasis, Egypt. Infect Drug Resist. 2023;16:5283-5293.
Crossref - Daneshnia F, de Almeida Junior JN, Ilkit M, et al. Worldwide emergence of fluconazole-resistant Candida parapsilosis: current framework and future research roadmap. Lancet Microbe. 2023;4(6):e470-e480.
Crossref - Turan D, Aksaray S. Increase in Antifungal Resistance Due to Variability in Candida Species: Experience from the Central Mycology Laboratory. Haydarpasa Numune Training and Research Hospital Medical Journal. 2023. ;63(4):457-464.
Crossref - Triveni AG, Suresh Kumar M, Manjunath C, Shivannavar CT, Gaddad SM. Biofilm formation by clinically isolated Staphylococcus aureus from India. The Journal of Infection in Developing Countries. 2018;12(12):1062-1066.
Crossref - Kırmusaoglu S. The Methods for Detection of Biofilm and Screening Antibiofilm Activity of Agents. In: Antimicrobials, Antibiotic Resistance, Antibiofilm Strategies and Activity Methods. IntechOpen. 2019.
Crossref - Mathur T, Singhal S, Khan S, Upadhyay D, Fatma T, Rattan A. Detection of biofilm formation among the clinical isolates of Staphylococci: An evaluation of three different screening methods. Indian J Med Microbiol. 2006;24(1):25-9.
Crossref - Freeman DJ, Falkiner FR, Keane CT. New method for detecting slime production by coagulase negative staphylococci. J Clin Pathol. 1989;42(8):872-874.
Crossref - Dhanasekaran D, Vinothini K, Latha S, Thajuddin N, Panneerselvam A. Human dental biofilm: Screening, characterization, in vitro biofilm formation and antifungal resistance of Candida spp. Saudi J Dent Res. 2014;5(1):55-70.
Crossref - Paiva LCF, Vidigal PG, Donatti L, Svidzinski TIE, Consolaro MEL. Assessment of in vitro biofilm formation by Candida species isolates from vulvovaginal candidiasis and ultrastructural characteristics. Micron. 2012;43(2-3):497-502.
Crossref - Williams DW, Jordan RPC, Wei XQ, et al. Interactions of Candida albicans with host epithelial surfaces. J Oral Microbiol. 2013;5(1).
Crossref - Tan TY, Tan AL, Tee NWS, Ng LSY. A retrospective analysis of antifungal susceptibilities of Candida bloodstream isolates from Singapore hospitals. Ann Acad Med Singap. 2008;37(10):835-840.
Crossref - Martinez-Garcia E, Martinez-Martinez JC, Martin-Salvador A, et al. Epidemiological Profile of Patients with Vulvovaginal Candidiasis from a Sexually Transmitted Infection Clinic in Southern Spain. Pathogens. 2023;12(6):756.
Crossref - Krishnasamy L, Rubini D, Senthilganesh J, et al. Phylogenetic characterization of biofilm forming multidrug resistant Candida albicans and Non albicans Candida causing vulvovaginal candidiasis. Gene Rep. 2020;19:100644.
Crossref - Maraki S, Mavromanolaki VE, Stafylaki D, Nioti E, Hamilos G, Kasimati A. Epidemiology and antifungal susceptibility patterns of Candida isolates from Greek women with vulvovaginal candidiasis. Mycoses. 2019;62(8):692-697.
Crossref - Shi Y, Zhu Y, Fan S, Liu X, Liang Y, Shan Y. Molecular identification and antifungal susceptibility profile of yeast from vulvovaginal candidiasis. BMC Infect Dis. 2020;20(1):287.
Crossref - Dharmik PG, Gomashe A V, Upadhyay VG. Susceptibility pattern of various azoles against Candida species causing vulvovaginal candidiasis. J Obstet Gynaecol India. 2013;63(2):135-137.
Crossref - Rolo J, Faria-Gonçalves P, Barata T, et al. Species Distribution and Antifungal Susceptibility Profiles of Isolates from Women with Nonrecurrent and Recurrent Vulvovaginal Candidiasis. Microbial Drug Resistance. 2021;27(8):1087-1095.
Crossref - Hedayati MT, Taheri Z, Galinimoghadam T, Aghili SR, Yazdani Cherati J, Mosayebi E. Isolation of Different Species of Candida in Patients With Vulvovaginal Candidiasis From Sari, Iran. Jundishapur J Microbiol. 2015;8(4):e15992.
Crossref - Abdullahi Nasir I, Uchenna E, Onyia J, Ifunanya AL. Prevalence of vulvovaginal candidiasis among nonpregnant women attending a tertiary health care facility in Abuja, Nigeria. Res Rep Trop Med. 2015;6:37-42.
Crossref - Tulasidas S, Rao P, Bhat S, Manipura R. A study on biofilm production and antifungal drug resistance among Candida species from vulvovaginal and bloodstream infections. Infect Drug Resist. 2018;11:2443-2448.
Crossref - Husni R, Bou Zerdan M, Samaha N, et al. Characterization and susceptibility of non-albicans Candida isolated from various clinical specimens in Lebanese hospitals. Front Public Health. 2023;11.
Crossref - Mathe L, Van Dijck P. Recent insights into Candida albicans biofilm resistance mechanisms. Curr Genet. 2013;59(4):251-264.
Crossref - Massey J, Zarnowski R, Andes D. Role of the extracellular matrix in Candida biofilm antifungal resistance. FEMS Microbiol Rev. 2023;47(6).
Crossref - Mohammadi F, Hemmat N, Bajalan Z, Javadi A. Analysis of Biofilm-Related Genes and Antifungal Susceptibility Pattern of Vaginal Candida albicans and Non-Candida albicans Species. Biomed Res Int. 2021;1-9.
Crossref - Sobel JD. Resistance to Fluconazole of Candida albicans in Vaginal Isolates: a 10-Year Study in a Clinical Referral Center. Antimicrob Agents Chemother. 2023;67(5):e00181-23.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.